Abstract
The function of indigenous lactobacilli in the control of other intestinal microbial species is not clear. Still more controversial is the effect of dietary bacterial supplements containing lactobacilli or other species. This situation is unlikely to change unless the mechanisms that control the colonization of ingested bacteria are better understood, and until more detailed information becomes available on the mechanisms by which certain populations of indigenous bacteria can affect the population sizes of other species. We used gnotobiotic mice and a continuous-flow culture system to study the interactions between Escherichia coli and (i) clostridia (in chloroform-treated cecal suspensions from conventional mice) and (ii) three strains of lactobacilli isolated from conventional mice. In gnotobiotic mice, the lactobacilli suppressed E. coli multiplication in the stomach and the small intestine, but had no demonstrable effect on E. coli multiplication in the large intestine. In contrast, clostridia were most effective in controlling E. coli multiplication in the large intestine. In the presence of both lactobacilli and clostridia, E. coli populations in the various regions of the gastrointestinal tract resembled those found in conventionalized control animals. The control of E. coli populations was not related to changes in pH or intestinal motility. In vitro stimulation of the above-described in vivo interactions required a two-stage continuous-flow culture in which the effluent from the first stage represented the influx to the second. The first stage was inoculated with lactobacilli, and the second stage was inoculated with either a pure culture of E. coli or E. coli and clostridia. In these instances, the E. coli populations in the second stage of the culture resembled in size those found in the large intestine of gnotobiotic mice harboring a similar flora. Although there are some current shortcomings of this in vitro model, we expect that a multistage continuous-flow culture can be developed to satisfactorily model the interactions among bacterial populations along the entire gastrointestinal tract.
Full text
PDF

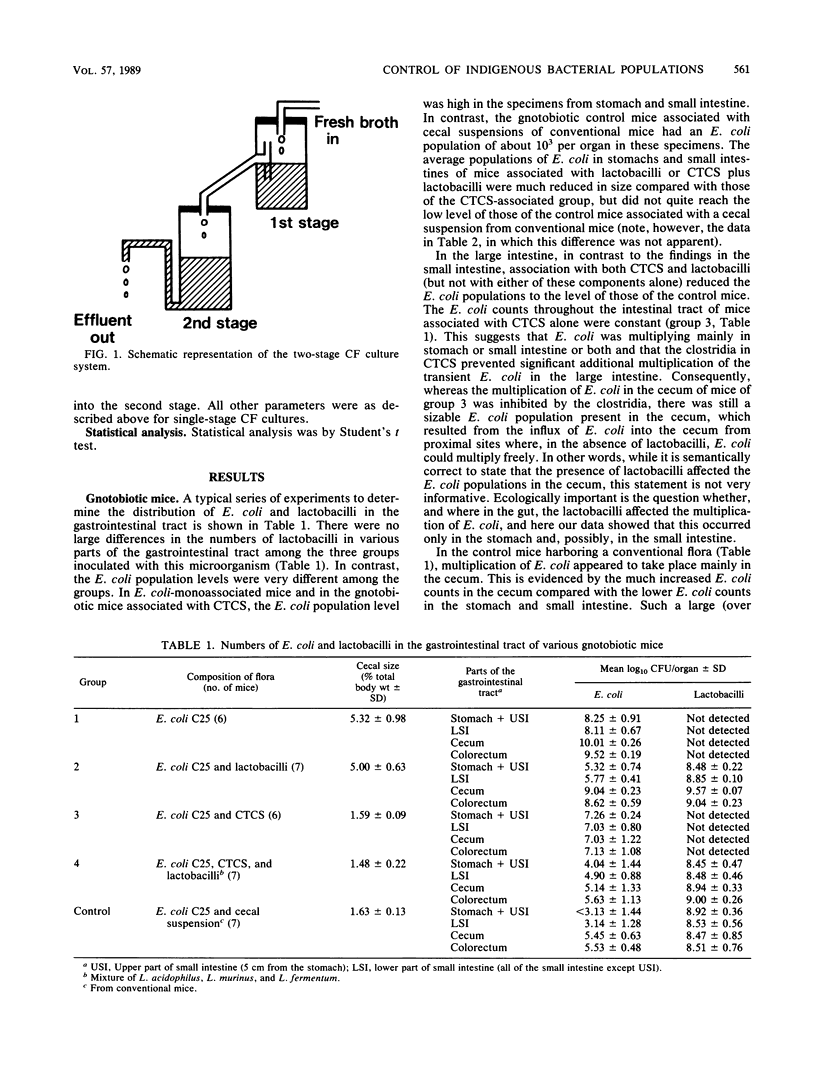
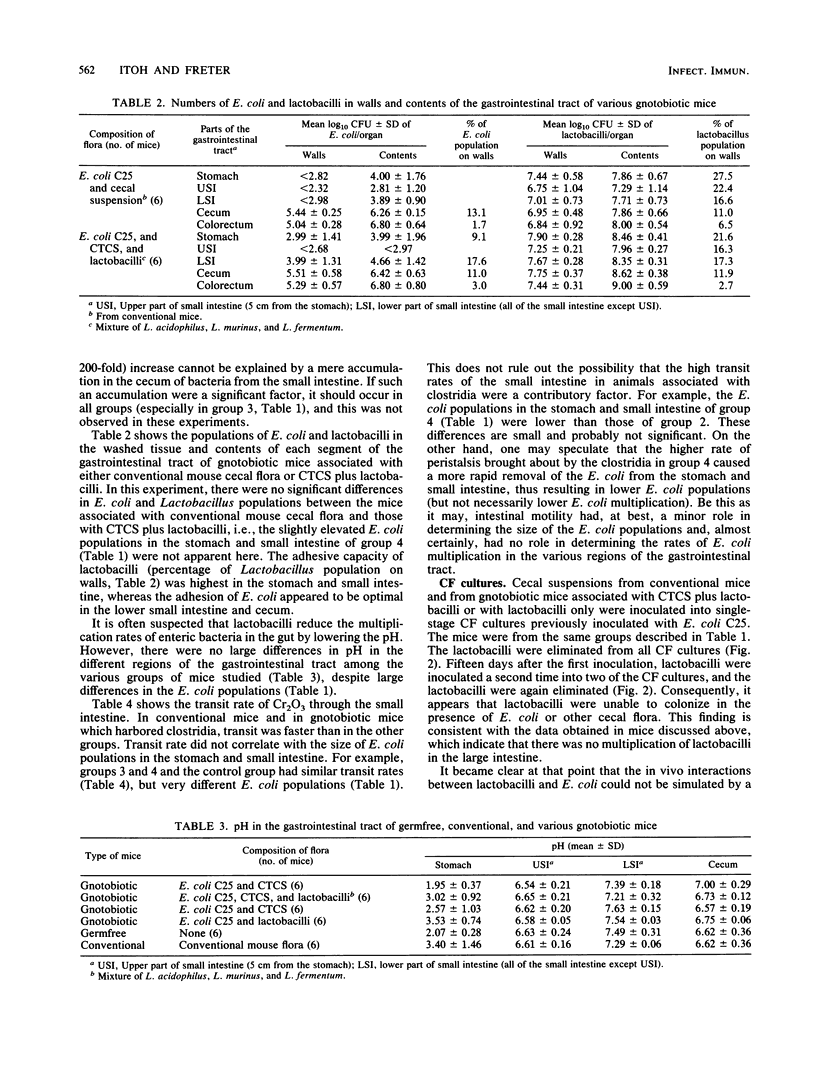
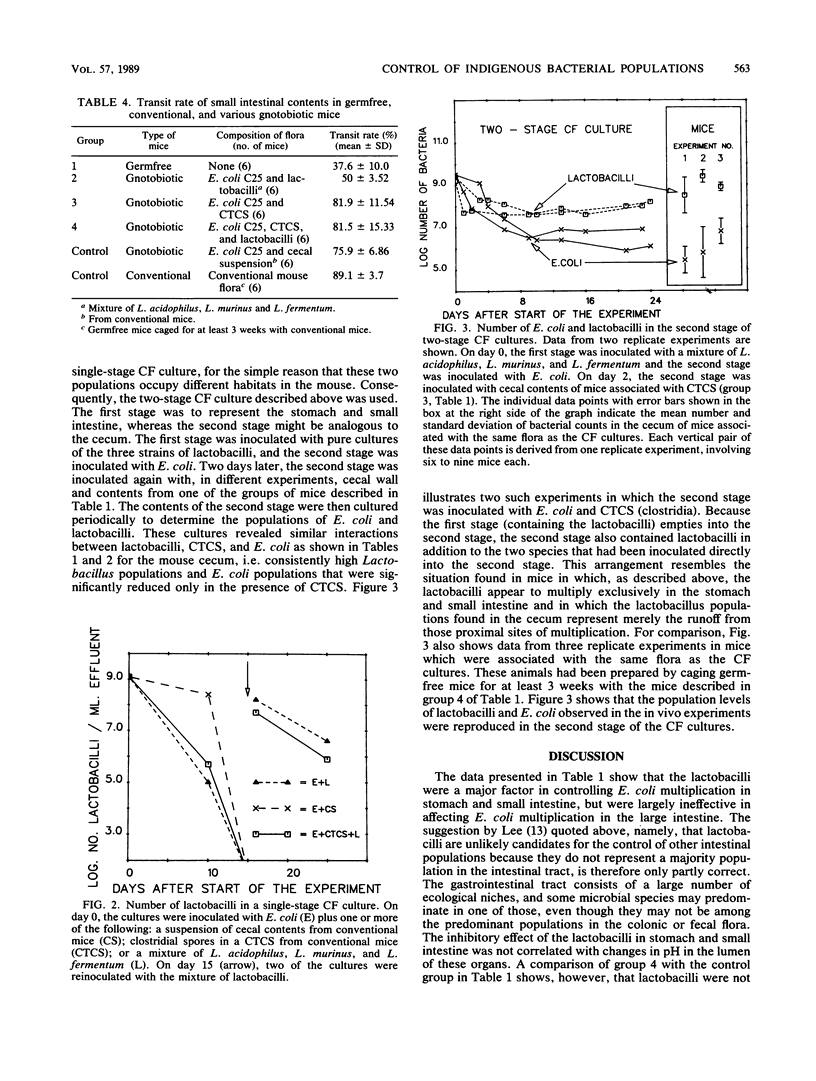


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aranki A., Freter R. Use of anaerobic glove boxes for the cultivation of strictly anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1329–1334. doi: 10.1093/ajcn/25.12.1329. [DOI] [PubMed] [Google Scholar]
- Conway P. L., Gorbach S. L., Goldin B. R. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J Dairy Sci. 1987 Jan;70(1):1–12. doi: 10.3168/jds.S0022-0302(87)79974-3. [DOI] [PubMed] [Google Scholar]
- Freter R., Brickner H., Botney M., Cleven D., Aranki A. Mechanisms that control bacterial populations in continuous-flow culture models of mouse large intestinal flora. Infect Immun. 1983 Feb;39(2):676–685. doi: 10.1128/iai.39.2.676-685.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Stauffer E., Cleven D., Holdeman L. V., Moore W. E. Continuous-flow cultures as in vitro models of the ecology of large intestinal flora. Infect Immun. 1983 Feb;39(2):666–675. doi: 10.1128/iai.39.2.666-675.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin B. R., Swenson L., Dwyer J., Sexton M., Gorbach S. L. Effect of diet and Lactobacillus acidophilus supplements on human fecal bacterial enzymes. J Natl Cancer Inst. 1980 Feb;64(2):255–261. doi: 10.1093/jnci/64.2.255. [DOI] [PubMed] [Google Scholar]
- Hazenberg M. P., Custers-van Leshout L. M. Conversion of germ-free mice to the normal state by Clostridia. Z Versuchstierkd. 1976;18(4):185–190. [PubMed] [Google Scholar]
- Itoh K., Mitsuoka T. Characterization of clostridia isolated from faeces of limited flora mice and their effect on caecal size when associated with germ-free mice. Lab Anim. 1985 Apr;19(2):111–118. doi: 10.1258/002367785780942589. [DOI] [PubMed] [Google Scholar]
- Itoh K., Mitsuoka T. Production of gnotobiotic mice with normal physiological functions. I. Selection of useful bacteria from feces of conventional mice. Z Versuchstierkd. 1980;22(3):173–178. [PubMed] [Google Scholar]
- Itoh K., Mitsuoka T., Sudo K., Suzuki K. Comparison of fecal lactobacilli in mice of different strains under different housing conditions. Z Versuchstierkd. 1983;25(4):193–200. [PubMed] [Google Scholar]
- Kleeman E. G., Klaenhammer T. R. Adherence of Lactobacillus species to human fetal intestinal cells. J Dairy Sci. 1982 Nov;65(11):2063–2069. doi: 10.3168/jds.S0022-0302(82)82462-4. [DOI] [PubMed] [Google Scholar]
- Shahani K. M., Ayebo A. D. Role of dietary lactobacilli in gastrointestinal microecology. Am J Clin Nutr. 1980 Nov;33(11 Suppl):2448–2457. doi: 10.1093/ajcn/33.11.2448. [DOI] [PubMed] [Google Scholar]
- Veilleux B. G., Rowland I. Simulation of the rat intestinal ecosystem using a two-stage continuous culture system. J Gen Microbiol. 1981 Mar;123(1):103–115. doi: 10.1099/00221287-123-1-103. [DOI] [PubMed] [Google Scholar]
- Wilson K. H., Freter R. Interaction of Clostridium difficile and Escherichia coli with microfloras in continuous-flow cultures and gnotobiotic mice. Infect Immun. 1986 Nov;54(2):354–358. doi: 10.1128/iai.54.2.354-358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


