Abstract
Nucleic acid polymerases have evolved elaborate mechanisms that prevent incorporation of the non-cognate substrates, which are distinguished by both the base and the sugar moieties. While the mechanisms of substrate selection have been studied in single-subunit DNA and RNA polymerases (DNAPs and RNAPs, respectively), the determinants of substrate binding in the multisubunit RNAPs are not yet known. Molecular modeling of Thermus thermophilus RNAP-substrate NTP complex identified a conserved β′ subunit Asn737 residue in the active site that could play an essential role in selection of the substrate ribose. We utilized the Escherichia coli RNAP model system to assess this prediction. Functional in vitro analysis demonstrates that the substitutions of the corresponding β′ Asn458 residue lead to the loss of discrimination between ribo- and deoxyribonucleotide substrates as well as to defects in RNA chain extension. Thus, in contrast to the mechanism utilized by the single-subunit T7 RNAP where substrate selection commences in the inactive pre-insertion site prior to its delivery to the catalytic center, the bacterial RNAPs likely recognize the sugar moiety in the active (insertion) site.
Copying (replication) and read-out (transcription) of genetic information contained within cellular genomes are carried out by an array of DNAPs1 and RNAPs, respectively. These enzymes operate within the same compartment, accessed by both types of substrates, r- and dNTPs, and control their fidelity at both co- and post-synthetic steps to avoid the so-called “error catastrophe,” when the amount of mistakes exceeds redundancy of the system. The first mechanism relies on the discrimination against the “wrong” nucleotide, whereas the second mechanism is invoked after the incorporation of a mismatched nucleotide.
The initial selection of the cognate substrate is comprised of two parts: the selection of the nucleotide complementary to the template DNA base and the selection of the correct sugar. The mechanism for sugar selection has been extensively studied in several DNAPs and in the single-subunit T7 RNAP. DNAPs actively discriminate against the rNTP via a “steric gate” formed by the Glu and Phe side chains (1–5), which sandwich the substrate sugar moiety and exclude the 2′-OH, while the substrate is positioned in the insertion site that has “closed” (active) configuration. In single-subunit T7 RNAP, Tyr639 hydroxyl has been implicated in the positive selection of the ribose via formation of a hydrogen with the 2′-OH group (6–8). In contrast to DNAPs, T7 RNAP commences the substrate selection in the inactive “open” conformation, while the substrate and the Tyr639 side chain are located in the so called pre-insertion site far away from the catalytic center (9, 10). This conclusion is consistent with the mutational analysis of T7 RNAP where amino acid changes that affect transcription fidelity cluster around the pre-insertion site (6, 7, 11). By contrast, no mutations affecting the fidelity or sugar discrimination by multisubunit RNAPs have been characterized.
Single- and multisubunit RNAPs possess no sequence or apparent structural similarity yet carry out the transcription cycle in a nearly identical manner and could utilize analogous structural elements during catalysis (12–14). These similarities suggest that the basic substrate selection mechanism might also be conserved and that a residue analogous to the Tyr639 would play a critical role in maintaining the ribose specificity in multisubunit RNAPs. However, modeling of the substrate NTP bound to the Thermus thermophilus RNAP active site (15) suggests that β′ Asn458 (Escherichia coli numbering is used throughout) within a highly conserved sequence motif 458NADFDGD464 that includes the catalytic Asp triad (β′ Asp460, Asp462, Asp464) (16–18) could mediate specific recognition of the O2′ ribose atom (Fig. 1A). Thus, Asn458 is likely not a structural analog of Tyr639, as the latter initially recognizes the ribose in the pre-insertion site.
Fig. 1. A model of the substrate bound to the T. thermophilus RNAP active (insertion) site.

The numbering corresponds to the E. coli RNAP. The bridge helix (orange) is shown in the uniform conformation that has been observed in the yeast RNAP (16); in an alternative distorted state the central portion of the helix (dark blue) is flipped out (17). The DNA template strand and the RNA nucleotides in the RNA/DNA hybrid are shown in red and yellow, respectively. Two catalytic Mg2+ ions (cMg1 and cMg2, magenta spheres) are coordinated (white dashed lines) by the four catalytic RNAP Asp residues (white) and by the substrate phosphates. β′ Asn458 (cyan) located in the β′ active site loop (white ribbon) and β′ Arg425 (cyan) forming internal hydrogen-bonding network in the crystal structure (cyan dashed lines) make putative contacts (cyan dashed lines) with the substrate ribose. A, overall view of the substrate-binding site. B, close-up view of the active site.
To evaluate the role of β′ Asn458 in substrate selection by a bacterial RNAP, we prepared and tested the conservative substitutions of this residue for their effects on utilization of the non-cognate substrates by the E. coli RNAP. Here we demonstrate that, in accordance with the structural predictions, these substitutions lead to a loss of discrimination of sugar moiety.
EXPERIMENTAL PROCEDURES
In Vitro Transcription Reactions
The wild-type and altered RNAPs were expressed in pIA299 background and purified as described previously. All templates for transcription reactions were generated by PCR amplification. To form halted transcription elongation complexes (TECs), linear DNA template (40 nM), RNAP (50 nM), ApU (100 μM), and starting NTPs (2.5 μM ATP and GTP, 1 μM CTP, 10 μCi of [α-32P]CTP (3000 Ci/mmol)) were mixed on ice in 50 μl of TGA buffer (20 mM Tris acetate, 20 mM sodium acetate, 10 mM magnesium acetate, 5% glycerol, 14 mM 2-mercaptoethanol, 0.1 mM EDTA, pH 8.0). Reactions were incubated at 37 °C for 15 min and purified from nucleotides by gel filtration through G50 spin columns (GE Health) equilibrated in TGA buffer. Transcription was restarted by addition of substrates indicated in figure legends, and samples were removed at selected times and quenched by the addition of an equal volume of STOP buffer (10 M urea, 20 mM EDTA, 45 mM Tris borate, pH 8.3).
RESULTS
Experimental Set-up
To determine whether β′ Asn458 residue is critical for the interaction with the 2′-OH, we changed Asn458 to Asp and Ser; these are the conservative changes that would not be expected to substantially alter the structure of the protein but would change either the chemical properties (Asp) or the size (Ser) of the discriminating Asn side chain. The altered enzymes were overexpressed from a polycistronic vector that allows assembly of the core α2ββ′ RNAP in vivo (19), purified, and tested for the ability to discriminate between the cognate and non-cognate substrates. Similarly purified wild-type (WT) RNAP was used as a control. We assembled TECs on pIA349 template that encodes a T7A1 promoter (21). On this template, TECs can be initially halted at position 37 by withholding UTP from the reaction mix (Fig. 2A), purified by gel filtration to remove the unincorporated substrates and then “walked” to the next template position during addition of a subset of NTPs. We used a similar approach to measure rC/dC selectivity using pIA171 template (20) instead.
Fig. 2. Assay for the incorporation of the r/dNMPs.
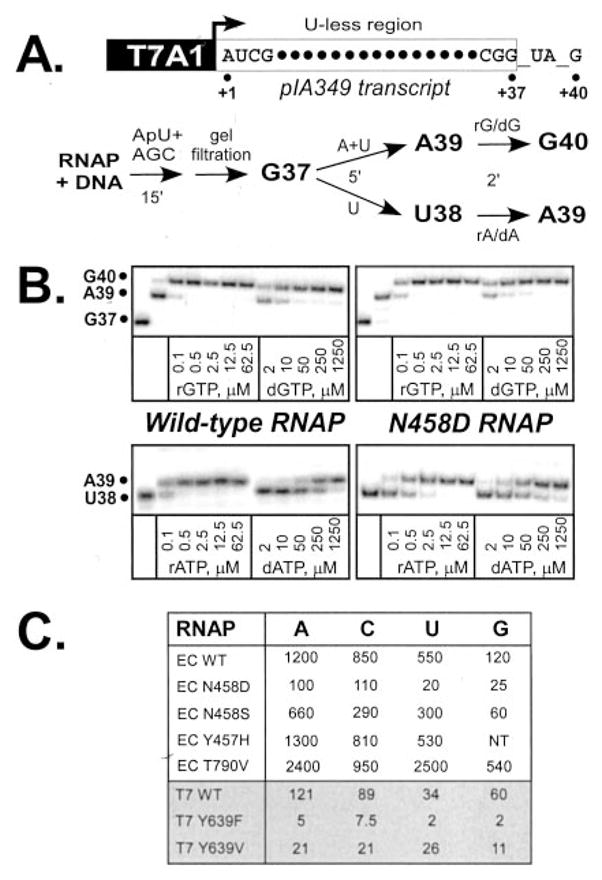
A, linear pIA349 template used to generate radiolabeled TECs halted at position G37 with the start site indicated by an arrow) (top) and the schematic representation of the assays used to measure the utilization of r/dNTP substrates (bottom). B, representative gel panels illustrating the extension of the nascent RNA in complexes halted at A39 or U38 upon addition of the increasing concentrations of r/dGTP and r/dATP substrates. Selection of rUTP versus dTTP was measured by extension of G37 RNA; selection of r/dCTP was assayed on a similar T7A1 promoter template, pIA171. C, comparison of the discrimination efficiencies between four r/d nucleotide combinations. The assays were repeated two to four times for each enzyme-r/dNTP combination; the discrimination efficiencies varied within 20%; NT, not tested. In E. coli RNAP, all substitutions were in the β′ subunit; the effects of selected substitutions in T7 RNAP (6) are presented for comparison.
Substitution of Asn458 Leads to Increased dNTP Utilization
We formed a halted TEC on an appropriate template and tested extension of 32P-labeled RNA upon addition of different unlabeled substrates. For each r/dNTP pair assayed, selected substrate was added to a concentration ranging between 0.1–62.5 μM for NTPs and 2–1250 μM for dNTPs, reactions were allowed to proceed for 2 min at 37 °C and quenched with STOP buffer (Fig. 2B). The WT RNAP exhibits strong preference toward rNTP substrates, while in the N458D variant these preferences are relaxed. For example, a 2-min incubation with 0.1 μM rGTP led to efficient extension of the nascent RNA by the WT RNAP, whereas an ~100-fold higher concentration of dGTP was required to achieve the same degree of extension. For the purpose of quantitative comparison we selected the ratio between concentrations of dNTP and rNTP, when half of the transcripts are extended by one nucleotide, as the discrimination quotient. The selectivity of incorporation of different r/dNTP pairs by WT RNAP ranges from 120- to 1200-fold (Fig. 2C) suggesting that the substrate selection depends not only on the identity of the sugar moiety but also on the base structure, even when the Watson-Crick base pairing is maintained. Similar variations have been reported for T7 RNAP (6) and DNAP I (2).
As expected, substitution of Asn458 for Asp led to substantially relaxed sugar discrimination ranging from 4.8-fold loss of discrimination for rG/dG pair to 26-fold loss in the case of the rU/dT pair. Substitution of Asn458 for Ser led to a smaller loss of preference for the ribose, whereas substitution of an adjacent Tyr457 residue did not alter sugar selection (Fig. 2C). The maximal observed effect for N458D RNAP was somewhat less than that reported for the T7 Y639F RNAP (6, 8); the base-specific order of r/dNTP selectivity was also different between the bacterial and T7 enzymes (Fig. 2C and Ref. 6). The direct comparison of these data is not straightforward, however, as we have used pre-steady state assays of substrate incorporation by the TEC, whereas Sousa and co-workers (6, 8) used multiround assays that include both the initiation and elongation steps, which resulted in different discrimination efficiencies for the same RNAP depending on the assay design. In T7 RNAP Tyr to Phe substitution likely completely disrupts polar discriminative interactions with the substrate ribose, whereas Asn for Asp and Ser substitutions in the bacterial enzyme might still maintain specific hydrogen bonding with rNTP sugar moiety. In addition, Asn458 might be not the only residue participating in the ribose recognition in the active site. Indeed, according to the modeling guanidinium group of a highly conserved in bacteria and eukaryotes β′ Arg425 appears proximal to the substrate sugar (Fig. 1B). The experiments are now under way to test the role of β′ Arg425 in substrate selection.
Molecular modeling of the multisubunit enzyme pre-insertion site (10) suggested that E. coli β′ Thr790 residue could play a role in ribose selection analogous to that of the T7 Tyr639 residue. However, the T790V RNAP not only did not loose preference for rNTP substrates, it actually exhibited more stringency in substrate selection relative to the WT, ranging from 1.1-fold effect in case of rC/dC to 4.8-fold effect for rU/dT (Fig. 2C). These data underscore the importance of the Thr790 residue in substrate selection but argue against involvement of its hydroxyl group in positive selection of the substrate ribose; less conservative substitutions of Thr to Ala and Leu led to gross defects in catalysis (data not shown).
A greater loss of discrimination upon the Asn to Asp substitution suggests that charge distribution on the discriminating protein group is more important than the side chain size. In the absence of the experimental crystallographic data on the RNAP-substrate complex structure, we propose a model that is certainly too tentative to predict the exact scheme of the hydrogen-bonding with the substrate but nonetheless allows to zero in on the three protein groups that likely interact with the substrate ribose: Asn458 side chain, Asn458 main chain carbonyl oxygen, and Arg425 guanidinium group. In the high resolution crystal structure of the T. thermophilus RNAP holoenzyme (17) these three groups form an internal hydrogen-bonding network (Fig. 1B). Interestingly, the main chain conformation of Asn458 does not fall in the most favorable region on the Ramachandran plot (φ = −68°; ψ = 77°). The stabilization of this unfavorable conformation likely comes from the hydrogen-bonding of the Asn458 main chain carbonyl with Arg425 and with its own side chain (Fig. 1B). The latter interaction would prevent flipping of the Asn458 side chain amido group that might be crucial for the proper sensing of the substrate ribose. This network of interaction would be enhanced upon binding of the rNTP substrate. In the model, Arg425 as well as the main chain carbonyl oxygen and the side chain amide of Asn458 make contacts with the 3′-OH group, whereas the Asn458 side chain oxygen likely recognizes 2′-OH group of the substrate ribose. The modeling of N458S substitution showed that although the interactions with the sugar would be weakened due to the smaller size of the Ser side chain, its hydroxyl group may preserve the framework of protein-protein and protein-substrate contacts similar to that of Asn (data not shown). In contrast, the negatively charged Asp side chain would lack the interactions with the main chain carbonyl potentially affecting both the main chain and side chain conformations and thus distorting the optimal orientation of the discriminating residue. In addition, the acidic Asp458 side chain might also form a salt bridge with the adjacent Arg425 that would further perturb the Asp interactions with the substrate ribose.
Substitution of Asn458 Leads to Profound Defects in Transcript Elongation
RNAP variants with altered substrate selection properties could be used to determine the contribution of individual transcript bases to recognition of regulatory signals. This approach is particularly important for the functional analysis of transient kinetic intermediates, such as those occurring during transcription termination and relies on the ability of nucleotide analogs to affect RNAP response to a particular signal (nucleotide analogs interference mapping (NAIM)). This analysis requires that RNAP is able to incorporate substrate analogs bearing modifications at various positions (22) but does not have defects in recognition of the transcription signals intended for study.
We studied the elongation properties of the N458D RNAP on pIA349 template that encodes several well characterized pause sites (Fig. 3). We found that N458D substitution confers a strong elongation defect; the rate of transcription elongation was dramatically reduced (more than 20-fold), and the enzyme paused strongly early in the transcribed sequence. We conclude that N458D RNAP, albeit able to efficiently incorporate dNTPs into the nascent RNA, is not suitable for NAIM due to its profound elongation defects. N458S RNAP also displayed a reduced elongation rate, but the defect was less pronounced (5-fold; Fig. 3 and data not shown). These results are also in a good agreement with our modeling. In the RNAP holoenzyme structure β′ Arg425 makes strong hydrogen bonds with β′ Asp464 from the catalytic triad. We presume that these interactions are crucial for the proper positioning of the Asp464 side chain that is likely required to optimize the coordination of the major catalytic Mg2+ ion (cMg1, Fig. 1B). In N458D enzyme side chains of Arg425 and Asp458 may form a salt bridge (see above), thereby disrupting functionally important Arg425/Asp464 contacts and subsequently violating proper Mg2+ coordination and compromising catalysis. Consistently, the smaller polar Ser residue would not affect strongly the orientation of the Arg425 side chain and would not exhibit dramatic effect on catalysis.
Fig. 3. Transcription elongation by the Asn458 mutant enzymes.

Top, transcript generated from the T7A1 promoter on a linear pIA349 template; transcription start site (+1), transcript end (run-off) and positions of ops (U43) and Phis (U145) pause sites are indicated. Bottom, halted G37 TECs were challenged with heparin at 50 μg/ml and NTPs at low (20 μM GTP, 100 μM ATP, CTP, and UTP) or high (100 μM GTP, 500 μM ATP, CTP, and UTP) concentrations. Aliquots were withdrawn at times indicated above each lane, followed by the high NTP chase (1 mM each NTP; C) and quenched as above. Positions of the DNA size markers are shown on the right (in nt).
Asn458 is located on the catalytic loop next to the active site. Thus the alternative possible explanation of the observed alterations in the substrate selection and catalysis exhibited by the mutant enzymes would be allosteric effects on the active site configuration. However, substitutions of an adjacent Tyr457 residue for His or Val did not confer either altered substrate selection or defects in transcription elongation (Fig. 2C and data not shown).
DISCUSSION
The major conclusion of this work is that the β′ Asn458 residue in the E. coli RNAP provides the recognition of sugar moiety on the incoming NTP substrate. No significant changes in misincorporation of non-templated NTP substrates arose from Asn458 substitutions (see supplemental data), indicating that the determinants for the sugar and base selection are non-overlapping, as was observed for the single-subunit T7 RNAP (11). The proposed ribose selection mechanism would also be likely valid for eukaryotic RNAPs that contain a highly conserved counterpart of β′ Asn458; indeed this role for the corresponding Asn479 residue in Rpb1 had been proposed earlier (14).
For both T7 and E. coli RNAPs the selectivity is far below the values reported for the DNAPs that exclude the rNTP substrates with several thousand- to a million-fold efficiency (1, 2). These differences could be explained by the fact that the levels of rNTPs are at least 10-fold higher in the cell than the levels of the corresponding dNTPs (25). Thus, to prevent incorporation of rNTPs DNAPs must impose the strict discrimination mechanism, which is achieved by the steric exclusion of the ribose 2′-hydroxyl. On the other hand, RNAPs might face the opposite problem: the relatively inefficient (e.g. via a single H bond) discrimination between r- and dNTPs could result not only in the synthesis of compromised messages but also in “draining” of the dNTPs pool. Cellular RNAPs would be expected to evolve tighter control mechanisms as compared with the phage ones, since at the time phage RNAP becomes engaged in transcription the host cell is usually moribund.
In DNAPs, the substrate recognition is thought to occur exclusively in the insertion site (23). In contrast, in the T7 TECs substrate can be bound in either the pre-insertion or in the insertion sites located 10 Å apart, suggesting that RNAPs may select substrates in two rather than in a single site (9, 10). The presence of a pre-insertion site in which the substrate can be “sampled” prior to catalysis was also proposed recently for multisubunit RNAPs (10, 24). Our present data on Asn458, which is adjacent to the active site, suggest that in bacterial RNAPs substrate selection, at least in part the rNTP/dNTP selection, occurs in the insertion site. Thus the sugar selection may principally occur in the pre-insertion site in T7 but in the insertion site in bacterial RNAPs. If discrimination in favor of rNTP binding were to occur predominantly in the insertion site, persistence of these interactions after catalysis might hinder the movement of the incorporated nucleotide from the n to the n − 1 site, thereby slowing translocation and the rate of polymerization. In T7 RNAP, where the pre-insertion site binding seems to be preferable, the Tyr639-2′-OH contact might be compromised during the transition to the closed form. This might explain a faster rate of T7 RNAP transcription as compared with the multisubunit cellular enzymes (25).
One cannot, however, rule out the possibility that rNTP/dNTP discrimination occurs both in pre-insertion and insertion sites by the different sets of residues. Indeed, substitution of the E. coli β′ Thr790 that is located in the bridge helix and likely belongs to the putative pre-insertion site for Val not only did not decrease the enzyme preference for the ribose but instead increased discrimination up to ~5-fold (Fig. 2C), implying the direct interactions of β′ Thr790 (or an adjacent) residue with the substrate. Since β′ Thr790 is located 18 Å away from the active (insertion) site and ~8 Å from the modeled substrate ribose, this result provides strong support to the hypothesis of existence of the substrate pre-insertion site in multisubunit RNAPs. It is therefore possible that some other residue from the pre-insertion site may play a ribose discriminating role similar to that of Asn458 in the insertion site. Alternatively, upon the substrate binding to the insertion site the structural elements that we are now assigning to the pre-insertion site might move toward the RNAP active center to constitute a single closed insertion site. We are currently dissecting the roles of residues in the pre-insertion site of the bacterial RNAP in selection of sugar and the base moieties of the incoming substrate.
Supplementary Material
Footnotes
This work was supported in part by a grant from the National Institutes of Health (to I. A.) and by the RIKEN Institute (to D. G. V.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental data, Fig. S1, and Refs. 1–5.
The abbreviations used are: DNAP, DNA polymerase; RNAP, RNA polymerase; nt, nucleotide; TEC, transcription elongation complex; WT, wild-type; NAIM, nucleotide analogs interference mapping.
References
- 1.Joyce CM. Proc Natl Acad Sci U S A. 1997;94:1619 –1622. doi: 10.1073/pnas.94.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astatke M, Ng K, Grindley ND, Joyce CM. Proc Natl Acad Sci U S A. 1998;95:3402–3407. doi: 10.1073/pnas.95.7.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer PL, Sarafianos SG, Arnold E, Hughes SH. Proc Natl Acad Sci U S A. 2000;97:3056 –3061. doi: 10.1073/pnas.97.7.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao G, Orlova M, Georgiadis MM, Hendrickson WA, Goff SP. Proc Natl Acad Sci U S A. 1997;94:407–411. doi: 10.1073/pnas.94.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang G, Franklin M, Li J, Lin TC, Konigsberg W. Biochemistry. 2002;41:10256 –10261. doi: 10.1021/bi0202171. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y, Eckstein F, Padilla R, Sousa R. Biochemistry. 1997;36:8231–8242. doi: 10.1021/bi962674l. [DOI] [PubMed] [Google Scholar]
- 7.Brieba LG, Sousa R. Biochemistry. 2000;39:919 –923. doi: 10.1021/bi992324+. [DOI] [PubMed] [Google Scholar]
- 8.Sousa R, Padilla R. EMBO J. 1995;14:4609 –4621. doi: 10.1002/j.1460-2075.1995.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin YW, Steitz TA. Cell. 2004;116:393–404. doi: 10.1016/s0092-8674(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 10.Temiakov D, Patlan V, Anikin M, McAllister WT, Yokoyama S, Vassylyev DG. Cell. 2004;116:381–391. doi: 10.1016/s0092-8674(04)00059-5. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Brieba LG, Sousa R. Biochemistry. 2000;39:11571–11580. doi: 10.1021/bi000579d. [DOI] [PubMed] [Google Scholar]
- 12.Yin YW, Steitz TA. Science. 2002;298:1387–1395. doi: 10.1126/science.1077464. [DOI] [PubMed] [Google Scholar]
- 13.Tahirov TH, Temiakov D, Anikin M, Patlan V, McAllister WT, Vassylyev DG, Yokoyama S. Nature. 2002;420:43–50. doi: 10.1038/nature01129. [DOI] [PubMed] [Google Scholar]
- 14.Gnatt AL, Cramer P, Fu J, Bushnell DA, Kornberg RD. Science. 2001;292:1876 –1882. doi: 10.1126/science.1059495. [DOI] [PubMed] [Google Scholar]
- 15.Artsimovitch I, Patlan V, Sekine S, Vassylyeva MN, Hosaka T, Ochi K, Yokoyama S, Vassylyev DG. Cell. 2004;117:299 –310. doi: 10.1016/s0092-8674(04)00401-5. [DOI] [PubMed] [Google Scholar]
- 16.Cramer P, Bushnell DA, Kornberg RD. Science. 2001;292:1863–1876. doi: 10.1126/science.1059493. [DOI] [PubMed] [Google Scholar]
- 17.Vassylyev DG, Sekine S, Laptenko O, Lee J, Vassylyeva MN, Borukhov S, Yokoyama S. Nature. 2002;417:712–719. doi: 10.1038/nature752. [DOI] [PubMed] [Google Scholar]
- 18.Zhang G, Campbell EA, Minakhin L, Richter C, Severinov K, Darst SA. Cell. 1999;98:811–824. doi: 10.1016/s0092-8674(00)81515-9. [DOI] [PubMed] [Google Scholar]
- 19.Artsimovitch I, Svetlov V, Murakami KS, Landick R. J Biol Chem. 2003;278:12344 –12355. doi: 10.1074/jbc.M211214200. [DOI] [PubMed] [Google Scholar]
- 20.Artsimovitch I, Landick R. Proc Natl Acad Sci U S A. 2000;97:7090 –7095. doi: 10.1073/pnas.97.13.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artsimovitch I, Landick R. Cell. 2002;109:193–203. doi: 10.1016/s0092-8674(02)00724-9. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz A, Rahmouni AR, Boudvillain M. EMBO J. 2003;22:3385–3394. doi: 10.1093/emboj/cdg310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunkel TA, Bebenek K. Annu Rev Biochem. 2000;69:497–529. doi: 10.1146/annurev.biochem.69.1.497. [DOI] [PubMed] [Google Scholar]
- 24.Holmes SF, Erie DA. J Biol Chem. 2003;278:35597–35608. doi: 10.1074/jbc.M304496200. [DOI] [PubMed] [Google Scholar]
- 25.Uptain S, Kane C, Chamberlin M. Annu Rev Biochem. 1997;66:117–172. doi: 10.1146/annurev.biochem.66.1.117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


