Abstract
Hydrophobic Actinobacteria are commonly associated with the stabilization of foams in activated sludge systems. One possible attractive approach to control these foam-stabilizing organisms is the use of specific bacteriophages. We describe the genome characterization of a novel polyvalent DNA phage, GTE2, isolated from activated sludge. This phage is lytic for Gordonia terrae, Rhodococcus globerulus, Rhodococcus erythropolis, Rhodococcus erythropolis, Nocardia otitidiscaviarum, and Nocardia brasiliensis. Phage GTE2 belongs to the family Siphoviridae, possessing a characteristic icosahedral head encapsulating a double-stranded DNA linear genome (45,530 bp) having 10-bp 3′-protruding cohesive ends. The genome sequence is 98% unique at the DNA level and contains 57 putative genes. The genome can be divided into two components, where the first is modular and encodes phage structural proteins and lysis genes. The second is not modular, and the genes harbored there are involved in DNA replication, repair, and metabolism. Some have no known function. GTE2 shows promising results in controlling stable foam production by its host bacteria under laboratory conditions, suggesting that it may prove useful in the field as a biocontrol agent.
INTRODUCTION
Activated sludge systems are used globally to treat both domestic and industrial wastewaters. The process is driven by a complex microbial community that degrades a wide diversity of organic substrates for their growth, and in some systems they also remove nitrogen and phosphorus (32). Many systems suffer from the serious operational problems of bulking and foaming, both arising from the unwanted growth of certain filamentous bacterial populations (8, 26). Their proliferation can disrupt the critical process of solid- and liquid-phase separation in clarifiers and cause the process to fail (8, 39).
Persistent foams occur in the activated sludge process when certain Actinobacteria strains found in the mixed liquor accumulate and stabilize the bubbles created during aeration (8, 33). Strains commonly found in foams include those whose cell walls contain the strongly hydrophobic mycolic acids (the mycolata) and include members of the genera Corynebacterium, Dietzia, Gordonia, Millisia, Skermania, Mycobacterium, Nocardia, Rhodococcus, Tsukamurella, and Williamsia (8, 33). Currently, the control and prevention of foaming are difficult, and there is a need for novel approaches (8, 33). One potentially attractive control strategy is to reduce mycolata cell numbers in the mixed liquor below the foaming threshold value by applying suitable lytic phages (38, 41). This environmentally safe approach offers the ability to target the foam-stabilizing filamentous bacterial strains specifically without affecting the important populations required for the successful treatment of wastewater.
Relatively little has been published on actinobacterial phages other than those that target the Mycobacteria (14, 15), although phages lytic for Rhodococcus were recently described (36). Thomas et al. (38) previously isolated 17 phages from activated sludge specific for members of the mycolata, 12 of which could propagate on members of more than one genus, and we have recently characterized a Tsukamurella phage, TPA2 (29). The aim of this study was to characterize a novel Gordonia phage isolated from activated sludge, GTE2, and test its potential as a biocontrol agent in laboratory-scale foaming experiments.
MATERIALS AND METHODS
Bacterial strains used in study.
The mycolata bacterial strains used in this study are listed in Table S1 in the supplemental material. All strains were grown in peptone yeast calcium (PYCa) broth or agar (29). All chemicals were obtained from Sigma, Australia, unless otherwise noted.
Phage purification, host range determination, and single-step growth curves.
Phage GTE2 was isolated from the Merrimac wastewater treatment plant (Queensland, Australia), as detailed previously by Thomas et al. (38), using Gordonia terrae (Gter34) as the host. Phage recovery and purification were performed by using G. terrae as described previously by Petrovski et al. (29). Ten rounds of phage dilution and single-plaque isolation were performed before further studies were undertaken, to ensure that the final GTE2 phage suspension resulted from a single virion.
After purification, a dilution series of GTE2 (∼1010 PFU/ml) was spotted onto swabbed lawn plates of each bacterial strain listed in Table S1 in the supplemental material, incubated for 2 days, and inspected for the presence of plaques. Single-step growth curves were conducted as described previously by Petrovski et al. (29).
Electron microscopy.
Virus particles were allowed to adsorb onto Formvar-coated 200-mesh copper grids for 5 min. These grids were washed twice for 1 min in double-distilled water (ddH2O) and negatively stained with 2% (wt/vol) uranyl acetate for 2 min. Excess liquid was absorbed using filter paper, and the grids were allowed to air dry before being examined under a Jeol JEM-100cx transmission electron microscope at an accelerating voltage of 100 kV.
DNA techniques, sequencing, and annotation.
Prior to DNA isolation, phage GTE2 was precipitated by using polyethylene glycol (PEG), and DNA was isolated from the precipitated phage using SDS-proteinase K as described previously by Petrovski et al. (29).
The genome of GTE2 was DNA sequenced by using the Roche GS FLX genome sequencer and titanium chemistry by Genoseq (University of California at Los Angeles, Los Angeles, CA). The pyrosequencing reads were assembled by using gsAssembler (Roche Applied Science, Indianapolis, IN). The resulting single contig obtained had a minimum of 40-fold read coverage.
To determine the single-stranded DNA cos sites at each end of the phage DNA, a combination of PCR amplification and direct sequencing was used. Phage DNA was PCR amplified by using primers SP1 (5′-CAGCGCCATTGCTTCTTG) and SP2 (5′-CATGCGGTTAGCTGGATAC) in reaction mixtures containing 10% (vol/vol) dimethyl sulfoxide and AmpliTaq Gold reaction buffer (Applied Biosystems). The reaction was subjected to 30 cycles as follows: 92°C for 3 min (first cycle only), 92°C for 60 s, 52°C for 30 s, 72°C for 70 s, and 72°C for 5 min (last cycle only). Sanger DNA sequencing of the PCR products or sequencing directly from the phage DNA (300 ng) was performed by the Australian Genome Research Facility (AGRF) (Brisbane, Australia) by using primers SP1 and SP2.
Geneious 4.0.4 software (9) was used to identify all open reading frames (ORFs) longer than 100 nucleotides. The putative proteins encoded by each ORF were screened for identity with other sequences deposited within the GenBank database using the BlastP interface provided by the NCBI. The conserved domain database (CDD) (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml) and Pfam database (http://pfam.sanger.ac.uk) were used to make predicted protein family allocations (12). The presence of tRNA and tmRNA was screened for by using ARAGORN (http://www.acgt.se/online.html) (25). Transmembrane domains were predicted by using DAS (dense alignment surface method) transmembrane prediction (http://www.sbc.su.se/∼miklos/DAS/) (7).
Effect of phage GTE2 on foam stability.
Triplicate 20-ml aliquots of each bacterial host (A600 adjusted to 1.0, equal to ∼108 CFU/ml) were incubated at room temperature overnight with or without the addition of phage GTE2 (multiplicity of infection [MOI] of 0.3). Foaming potential was assessed by using a laboratory-scale foaming apparatus, described previously by Stratton et al. (34), at an aeration rate of 100 ml/min for 1 min and criteria described previously by Blackall and Marshall (4), but modified as described previously by Petrovski et al. (28).
Nucleotide sequence accession number.
The nucleotide sequence for GTE2 has been deposited in the GenBank database under accession number HQ403646.
RESULTS AND DISCUSSION
General features of phage GTE2.
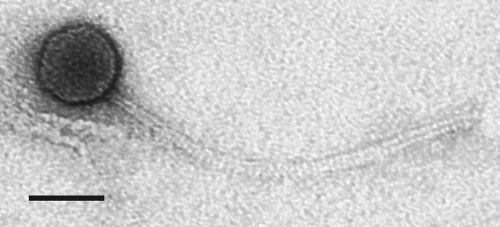
Phage GTE2 was isolated originally from the Merrimac wastewater treatment plant (Queensland, Australia) in 1999 by J. Thomas in our laboratory, based on its ability to form lytic plaques (∼1-mm diameter) on a lawn plate of G. terrae strain Gter34 (38). Transmission electron microscopy (TEM) revealed that GTE2 belongs to the family Siphoviridae, possessing their characteristic long noncontractile tail (∼273 nm) and isometric capsid (∼57 nm) (Fig. 1). The burst size of GTE2 was determined to be 126 ± 8 PFU/ml, with a latency period of 2.5 h in PYCa broth at 30°C.
Fig. 1.
Electron micrograph of GTE2. Scale bar, 50 nm.
Phages are considered attractive antibacterial agents in mixed microbial populations because of their narrow host specificities (35). Interestingly, phages isolated from activated sludge have been reported to display unusually broad host ranges, with some being reported to lyse both Gram-negative and Gram-positive bacteria (20, 21, 38). To date, none of these polyvalent phages have had their genomes completely sequenced. It is always possible that phages reported to be polyvalent are in fact contaminated and contain more than one phage; put simply, a polyvalent phenotype can be mimicked by the presence of two or more unrelated narrow-host-range phages in a phage stock. To ensure that the apparent broad-host-range phenotype of GTE2 was not a laboratory artifact resulting from its inadequate purification, it was purified 10 times (see Materials and Methods) from single plaques before being grown to produce a high-titer (∼1010 PFU/ml) stock. TEM micrographs of this preparation revealed only a single virion morphotype (Fig. 1). Furthermore, when pyrosequenced, all contigs were fragments from a single GTE2 phage. No orphan contigs were obtained, further supporting that GTE2 was a single phage. To ensure further that the GTE2 stock was free from contaminating phages, all host range determinations were performed by using phage drop dilutions. Only after these precautions were implemented was GTE2 screened for its ability to lyse 65 different mycolata strains (listed in Table S1 in the supplemental material).
GTE2 generated lytic plaques on lawn plates of strains from six mycolata genera: Gordonia terrae (Gter34), Rhodococcus globerulus (Rglo35), Rhodococcus erythropolis (Rery19), Rhodococcus erythropolis (Rery29), Nocardia otitidiscaviarum (Noti14), and Nocardia brasiliensis (Nbra42). Interestingly, this phage lysed only one of the five G. terrae strains screened. The reason for this is unknown, but it may be the result of restriction-modification systems interfering with phage infectivity (13). Restriction-modification can be overcome by host infection with a high phage titer and isolation for the rare infective event that escapes DNA restriction, as the phage progeny will be insensitive to the restriction-modification system in the host (24). Despite numerous attempts using very high PFU titers (≥1010), we were unable to obtain GTE2 plaques on any of the four other G. terrae strains. Since a phage host range can change depending on the host used for its replication from differences in host restriction methylation (13), GTE2 was grown in each of the six bacterial hosts that it infected and was then reassessed against all 65 of the mycolata. No changes in host range were observed regardless of the bacterium in which GTE2 replicated initially.
One possible explanation for these host range data for GTE2 is that only certain strains possess the appropriate receptor(s) for phage attachment. If so, the same receptor(s) appears to be found in several different mycolata genera but not in all strains of each genus. Alternatively, it is possible that all strains in each genus possess the phage receptor but that only some can support subsequent phage replication. Several mycolata genera have been reclassified (e.g., Nocardia amarae is now Gordonia amarae, and Rhodococcus maris is now Dietzia maris [30, 31]). Host range data from phages like GTE2 may reveal novel insights into mycolata systematics. Phages for Streptomyces, a member of the mycolata, have been used in systematic studies (23) where phage typing was reported to allow the unequivocal identification of individual species (2). Other characterized phages targeting members of the mycolata, like Mycobacterium, Tsukamurella, and Rhodococcus, target members of each genus only (16, 29, 36). Whether the GTE2 host range data are exposing novel systematic information remains to be elucidated.
Genomic features of GTE2.
The relatively few complete genome sequences of phages currently available emphasize how little is known about them (15). Four Rhodococcus phage genome sequences are now available (36), but to our knowledge, no genome sequences of lytic phages for Gordonia and Nocardia strains have been reported, although putative prophages have been detected in whole-genome sequences of both Nocardia farcinica (17) and Gordonia bronchialis (19). Phage GTE2 has a linear double-stranded DNA genome consisting of 45,530 bp with a G+C content of 60.3 mol%. The cos site was identified as a 10-bp 3′ overhang sequence, 5′-CGGTAGGCTT-3′. Most of the GTE2 genome (98%) shares no significant match to DNA sequences in the GenBank database.
A total of 57 putative open reading frames were identified in the GTE2 genome, and no tRNAs could be located. The ORFs were numbered consecutively from the end closest to the gene encoding the large terminase. Thirty-five ORFs are encoded on one strand, and 22 are encoded on the opposite strand (Fig. 2). A total of 19 ORFs (33%) could be annotated functionally by comparisons with known protein sequences and N-terminal sequencing. The possible functions of some gene products can also be deduced based on their locations within the genome and from comparative analyses with other phage genomes. However, in the case of GTE2 this approach was generally unhelpful. Many of the putative genes showed statistically significant levels of similarity only to genes of unknown function and so could not be assigned a possible function(s) (Table 1).
Fig. 2.
Genetic map of the GTE2 genome. The arrows represent the putative genes and the direction in which they are transcribed. The first half of the genome has a modular arrangement, and modules are indicated by the line below. The second half of the genome appears to be nonmodular. Genes are shaded similarly if their protein products have similar functions.
Table 1.
ORFs and gene products of GTE2
| ORFa | aa (% identity)b | Protein function (motif)c | Matchd | E valuee |
|---|---|---|---|---|
| 1 | 159 (42) | Unknown | VWBp32, Streptomyces phage | 1e−22 |
| 2 | 560 (48) | Terminase (pfam03354) | SSPB78_13581, Streptomyces sp. strain SPB78 | 5e−154 |
| 3 | 477 (40) | Putative portal (pfam05133) | Hypothetical protein, Corynebacterium urealyticum DSM 7109 | 4e−81 |
| 4 | 235 (34) | Unknown | Gp5, Mycobacterium phage Ramsey | 7e−23 |
| 5 | 154 (36) | Unknown | Hypothetical protein, Micrococcus luteus SK58 | 2e−07 |
| 6 | 322 (55) | Main capsid (pfam05065) | Putative structural phage protein, Corynebacterium urealyticum DSM 7109 | 2e−59 |
| 7 | 170 (40) | Unknown | Gp9, Mycobacterium phage Halo | 2e−18 |
| 8 | 114 (31) | Unknown | Gp10, Mycobacterium phage Che9d | 2e−04 |
| 9 | 91 | Unknown | ||
| 10 | 121 (29) | Unknown | Gp13, Mycobacterium phage Che9d | 1e−07 |
| 11 | 282 (32) | Major tail (pfam05345) | RER_22600, Rhodococcus erythropolis PR4 | 6e−38 |
| 12 | 98 (38) | Unknown | MAB_1794, Mycobacterium abscessus ATCC 19977 | 6e−07 |
| 13 | 88 (48) | Unknown | Jden_2318, Jonesia denitrificans DSM 20603 | 3e−06 |
| 14 | 1548 (34) | Tape measure protein (COG5412) | Gp21, Mycobacterium phage CrimD | 8e−46 |
| 15 | 309 (33) | Tail protein | Gp18, Mycobacterium phage Che9d | 2e−34 |
| 16 | 532 (47) | Tail protein | Gp19, Mycobacterium phage Che9d | 2e−142 |
| 17 | 130 (42) | Unknown (pfam10910) | Gp20, Mycobacterium phage Che9d | 6e−19 |
| 18 | 182 (58) | Putative lysin | Nfa15420, Nocardia farcinica IFM 10152 | 3e−36 |
| 19 | 317 (49) | Unknown | Nfa15430, Nocardia farcinica IFM 10152 | 2e−71 |
| 20 | 163 (30) | Unknown | Nfa600, Nocardia farcinica IFM 10152 | 6e−09 |
| 21 | 103 | Unknown | ||
| 22 | 372 (26) | Unknown | Hypothetical protein, Rhodococcus equi ATCC 33707 | 7e−10 |
| 23 | 296 (59) | Tail fiber | gp4, Mycobacterium phage Bxb1 | 2e−16 |
| 24 | 213 (45) | Unknown | GTE5p025, Gordonia terrae phage GTE5 | 4e−12 |
| 25 | 214 (31) | Unknown | Namu_2691, Nakamurella multipartita DSM 44233 | 4e−11 |
| 26 | 193 (68) | Unknown (pfam10263) | TM4_gp80, Mycobacterium phage TM4 | 2e−51 |
| 27 | 211 | Unknown | ||
| 28 | 147 | Unknown | ||
| 29 | 130 | Unknown | ||
| 30 | 96 (42) | Archeal Holliday junction resolvase (pfam01870) | VRR-NUC domain-containing protein, Alkaliphilus oremlandii OhILAs | 1e−12 |
| 31 | 181 | Unknown | ||
| 32 | 163 (33) | Unknown | Gp59, Mycobacterium phage PLot | 4e−04 |
| 33 | 370 (26) | Unknown | Hypothetical protein, Desulfovibrio aespoeensis Aspo-2 | 2e−04 |
| 34 | 128 | Unknown | ||
| 35 | 796 (31) | DNA Pol I (COG0749) | DNA-directed DNA polymerase I, Chryseobacterium gleum ATCC 35910 | 8e−72 |
| 36 | 153 (43) | dCMP deaminase, putative (pfam00383) | dCMP deaminase, Archaeoglobus fulgidus DSM 4304 | 1e−29 |
| 37 | 280 | Cutinase (pfam01083) | ||
| 38 | 289 (30) | Thymidylate synthase (pfam00303) | Thymidylate synthase, Methanosaeta thermophila PT | 2e−06 |
| 39 | 298 | Unknown | ||
| 40 | 82 (55) | Unknown | Hypothetical protein, Aeromicrobium marinum DSM 15272 | 8e−04 |
| 41 | 119 (44) | Phage-encoded dCTP pyrophosphatase (pfam08761) | Phage-encoded dCTP pyrophosphatase, Anoxybacillus flavithermus WK1 | 2e−06 |
| 42 | 171 | Thymidine monophosphate kinase (TMPK) (cd01672) | ||
| 43 | 107 | Unknown | ||
| 44 | 462 (30) | Helicase (Pfam00271, Pfam00176) | Hypothetical protein, Holdemania filiformis | 1e−45 |
| 45 | 118 | Unknown | ||
| 46 | 148 | Unknown | ||
| 47 | 666 (23) | Putative primase (COG3598) | Gp46, Rhodococcus phage ReqiPine5 | 4e−10 |
| 48 | 128 | Unknown | ||
| 49 | 138 | Unknown | ||
| 50 | 98 | Unknown | ||
| 51 | 66 | Unknown | ||
| 52 | 63 | Unknown | ||
| 53 | 156 | Unknown | ||
| 54 | 139 | Unknown | ||
| 55 | 103 | Unknown | ||
| 56 | 121 | Unknown | ||
| 57 | 131 (39) | Homing endonuclease (pfam01844) | Gp49, Burkholderia phage KS9 | 8e−07 |
ORFs were numbered consecutively.
The percent identity was based on the best match when a BlastP analysis was performed. aa, amino acid.
Predicted function is based on amino acid identity, conserved motifs, N-terminal sequencing, and gene location within functional modules.
The most closely related gene (only if named) and the name of the organism are given.
The probability of obtaining a match by chance as determined by BLAST analysis. Only values less than 10−4 were considered significant.
DNA packaging, structural proteins, and host lysis.
Analysis of the GTE2 genome sequence revealed that terL encodes a large terminase based on its sequence identity and the presence of the large terminase-specific pfam03354 motif. No obvious small terminase could be recognized. Small terminases are encoded typically upstream of large terminase genes and function together with the large terminase in the packaging of phage DNA (6). The putative orf1 gene upstream of the large terminase shares a low level of amino acid similarity with the hypothetical phage protein from Streptomyces phage VWB and some Mycobacterium phages. Given its location, it is possible that orf1 encodes a novel class of small terminases, although this remains to be confirmed.
The cluster of genes within the module of orf3 to orf24 is predicted to encode the phage structural proteome and host cell lysis functions. These gene modules, together with the packaging genes (i.e., orf1 and terL), are transcribed in the same direction. The first gene product of this cluster, orf3, contains a pfam05133 motif characteristic of phage portal proteins. The portal protein connects the head and tail proteins, creating an avenue for DNA injection into the host cell during the DNA packaging process (18). Other genes within this cluster are likely to encode other structural proteins, although they show no statistically significant sequence identity with any previously characterized phage proteins.
A previously reported SDS-PAGE analysis of GTE2 revealed the presence of five structural proteins (37). Two of the bands were present in larger amounts than the others, indicating that they were more likely to be the major structural proteins (37). These two proteins, estimated to be 223 kDa and 37 kDa in size, were N-terminally sequenced previously by Thomas (37) and correspond to the gene products of orf6 (ATKSVLQPPASGSAGIVGGT) and orf11 (ASKVENVFAAMPRATGALLAR) of phage GTE2. In silico analysis of these genes revealed that Orf6 and Orf11 are the major capsid and tail proteins, respectively, based on the presence of highly conserved motifs (Table 1). The predicted size of Orf11 is 29.7 kDa and is in reasonable agreement with the properties of the major tail protein determined by SDS-PAGE. The size of the major capsid protein assessed by SDS-PAGE is strikingly different from its theoretical size. It is known from previous work with other phages that the major capsid protein can be covalently self-linked, and this may explain the behavior of this protein determined by SDS-PAGE (40). Other genes identified within this cluster were orf14, encoding the tape measure protein, and the orf15, orf16, and orf23 genes, encoding phage tail proteins.
An interesting feature of some phages (including lambda and Mu) is a conserved programmed translational frameshift (42). This process involves two overlapping genes, where the second gene has no obvious start codon and is expressed following translational slippage. The two genes located between the major tail and tape measure proteins are thought to encode proteins involved in tail assembly (42). These (orf12 and orf13) were further investigated. Orf13 shares identity with the end portion of Gp15 in mycobacteriophage Halo that is translated by a programmed translational slippage mechanism. We further investigated the DNA sequence upstream of orf13 to determine the slippage sequence. A sequence 28 bp before the end of orf12 (i.e., GGGGGAA) was identical to the predicted slippage sequence in Mycobacterium phage L5 (42). The occurrence of this feature in a wide range of seemingly unrelated bacteriophages is intriguing, especially since its functional purpose is unclear (42).
The putative protein encoded by orf18 is most closely related to hypothetical proteins in Nocardia farcinica (17) and Rhodococcus erythropolis (GenBank accession number AY_002765699). More distantly related but of equal importance, the Orf18 protein shares identity with the lysin protein of Mycobacterium phages Lockley, Jasper, and Myrna. Although no holin protein could be identified for GTE2, the adjacent orf17 gene encodes a protein of unknown function but which contains a conserved motif characteristic of viral proteins. Orf17 has two predicted transmembrane regions, a topology similar to that observed for other phage holin proteins (27). Given its location, it is speculated that this gene may encode a novel holin, but again, this remains speculative.
DNA metabolism, modification, and repair.
Examination of the proteins encoded by orf25 to orf57 suggests that this region is involved in DNA metabolism, modification, and repair. Unlike the region encoding the phage structural proteins, this DNA region does not appear to be modular. Most of the genes (i.e., 23 out of 33) are transcribed in the opposite direction of the genes encoding phage structural proteins (Fig. 2). While the functions of many genes in this cluster can be assigned only speculatively, a number of them contain conserved motifs that suggest their possible functions. The predicted genes appear to encode proteins involved in DNA synthesis and repair and include orf26, which contains the pfam10263 conserved motif predicted to be involved in transcriptional elongation, and orf35, which is predicted to encode DNA polymerase I (Pol I). Included in this gene cluster encoding putative enzymes involved in DNA synthesis are orf38, encoding a pfam00303 conserved motif characteristic of a thymidylate synthase; orf41, a phage dCTP pyrophosphatase with the pfam08761 motif; and orf42, a guanylate kinase. The putative proteins Orf44 and Orf47 contain conserved domains suggestive of helicase- and primase-like proteins. Orf44 contains the pfam00176 motif at its N-terminal end and the pfam00271 motif at its C-terminal end. The N-terminal region is typical of enzymes involved in transcription, DNA repair, DNA recombination, and chromatin unwinding (11), while the C-terminal region is characteristic of DNA helicase (5). The orf47 gene appears to encode a primase protein based on the presence of conserved motifs.
Proteins thought to be involved in DNA modification include those encoded by orf36 (a deaminase) and orf57, which appears to encode a homing endonuclease possibly involved in the DNA digestion of the host bacterium DNA.
Cryptic genes.
The DNA sequence of GTE2 is very different from other phage genome sequences deposited in public databases. Analysis revealed that this genome is divided into two large segments. Within these segments, unusual putative orthologs were detected. For example, orf37 encodes a conserved pfam01083 motif, which is characteristic of cutinase enzymes, degrading plant cell wall cutin (3). At the amino acid level no significant amino acid similarity with any other protein sequences in public databases was detected, although insignificant similarity with a Mycobacterium cutinase was suggested. This cutinase enzyme is synthesized frequently by phytopathogenic fungi but has also been detected in some phytopathogenic bacteria (10). On the basis of bioinformatic analyses, Belbahri et al. (3) hypothesized previously that the cutinase gene was horizontally acquired by fungi from members of the Actinobacteria. This is the first report of a possible cutinase-encoding gene detected in a phage, and although the function of this gene is unknown, its presence suggests that GTE2 or similar phages may have acted as a vector for its distribution among the Actinobacteria. Since GTE2 has a GC mol% content similar to that of its hosts, it is not possible to determine the origin of this gene on the basis of GC mol% content.
The protein encoded by orf30 contains the pfam01870 motif characteristic of the Holliday junction resolvases of the Archaea (22). Whether the protein encoded by orf30 performs the same function in this GTE2 phage is unknown, but given its significant identity to known Holliday junction resolvases, this would seem probable. The presence of this gene in GTE2 suggests that such resolvases may not be exclusive to the Archaea.
Evolution of GTE2.
The increasing availability of complete phage genome sequences is revealing clues as to their ancestry. The largest group of completely sequenced phages infecting the same host bacteria are those that infect Mycobacterium species (16). These genomic data reveal that phages are genetically diverse and often demonstrate mosaic gene architectures. Mycobacterium phages have been divided into several clusters and subclusters based on similarities between their DNA and protein sequences (16). We characterized a Tsukamurella phage (TPA2) whose proteins share some similarity with Mycobacterium phage proteins that fall into cluster B (29). Despite the low DNA genome sequence similarity to other phages, proteins encoded by the Tsukamurella phage TPA2 genome could be annotated functionally and categorized with those from the Mycobacterium phages. Equally, Rhodococcus phage ReqiPine5 was classified into the same cluster as TPA2 in Mycobacterium phage cluster B despite no DNA sequence similarity (36). With the sequence of phage GTE2 now available, we attempted to place this phage into the Mycobacterium phage taxonomy but could not categorize it because it shares very little similarity to the genome sequence of any other phage. However, an examination of each of the 57 GTE2 ORFs showed that the first gene module (orf1 to orf24) encodes proteins with most having some similarity to Mycobacterium phage proteins, while the second module (orf25 to orf57) contains only three ORFs with similarity to those from Mycobacterium phages. Furthermore, the clustering pattern varied with each putative GTE2 protein (Table 2). Thus, phage GTE2 should be viewed as a singleton according to the classification system described by Hatfull et al. (16).
Table 2.
GTE2 genes and the clusters to which they belongd
| Genea | Clusterb |
|---|---|
| orf1 | I |
| orf2 | A |
| orf3 | A |
| orf4 | F |
| orf6 | F |
| orf7 | G |
| orf8 | F |
| orf9 | F |
| orf10 | F |
| orf11 | G |
| orf13 | G |
| orf14 | Gc |
| orf15 | F |
| orf16 | F |
| orf17 | F |
| orf18 | A |
| orf19 | E |
| orf20 | Corndog |
| orf22 | B |
| orf23 | A |
| orf24 | I |
| orf26 | TM4 |
| orf33 | H |
| orf47 | B |
The designated gene in GTE2. Genes not listed share no similarity with genes of the Mycobacterium phages.
The cluster in which the protein sequence would be categorized based on closest similarity.
The similarity of this protein sequence spans less than 50% of the protein.
Based on the classification system described previously by Hatfull et al. (16).
GTE2 as a biocontrol agent.
The isolation of GTE2 described previously by Thomas et al. (38) was conducted for the purposes of the biocontrol of the foam-stabilizing bacteria in activated sludge. Phage GTE2 was of particular interest because of its ability to target multiple bacterial hosts, all implicated in foaming episodes (28). Since each of the six hosts that GTE2 infects has the ability to stabilize foam in laboratory-scale foaming tests, we determined whether GTE2 could be used to control foaming in a laboratory-scale system. The optical density (OD) of the bacterial cultures was adjusted to an A600 of ∼1.0 prior to the addition of phage (MOI = 0.3) (or uninoculated broth for controls), and these cultures were incubated at room temperature for 24 h. The data showed that the foaming ability of each of these bacterial cultures decreased markedly in the presence of phage GTE2, and for some strains no stable foam was generated (Tables 3 and 4). The phage had no effect on the foam stabilization displayed by Gordonia aichiensis (Raic22), which is not a host for GTE2, confirming that foaming control is host specific. Further analyses of the cultures exposed to phage GTE2 revealed that the number of CFU ml−1 was reduced by greater than 100-fold (data not shown). Thus, the phage GTE2-mediated cell number reduction clearly eliminates preexisting stable foam under laboratory conditions (28) and encourages its application in the control of foaming-activated sludge mixed liquor in full-scale plants.
Table 3.
Foaming thresholds with and without GTE2
Table 4.
Foaming assay scalea
| Rating | Description |
|---|---|
| 0 | As for pure water; no foam |
| 1 | 1.0–3.0 cm of foam with fragile ill-formed bubbles; insufficient stability to form films; immediate collapse upon cessation of aeration |
| 1ab | Flotation of clumped bacterial cells to the surface of the air-water interface; clumped cells remain afloat upon cessation of aeration, producing a scum layer |
| 2 | Intermitted films sufficiently stable; usually generated from a fragile foam structure of limited ht; films unstable upon cessation of aeration |
| 3 | Substantial foaming (i.e., bubbles about 10 cm in diam) to ht of 3–8 cm; infrequent or regular film formation, with both film and foam semistable upon cessation of aeration |
| 4 | Initially 8–15 cm of foam (about 1-cm-diam bubbles), with stable films being formed at regular intervals; body of the foam and films stable for 3–5 min once aeration ceases |
| 5 | Stable foam 5–10 cm in ht in 2 min, after which it collapses to ht of 3–5 cm; foam is stable when aeration ceases; no films |
| 6 | Stable foam 15–30 cm in ht with no films; bubble size is about 0.5 cm during aeration and increases to 2.0–3.0 cm in diam in 3–5 min from the time when aeration ceases |
Adapted from reference 28.
This key has been added to represent the “scummers.”
Conclusions.
Broad-host-range phages appear to be uncommon, and most phages have very narrow host ranges (1). The basis for phage polyvalency is unclear, and little is still known regarding the molecular mechanism(s) that controls host specificity. GTE2 is the first actinobacterial multigenus polyvalent bacteriophage to be completely sequenced and annotated. This sequence is highly novel, sharing almost no DNA sequence similarity with all other known phage genomes. The genome sequence of GTE2 will now allow its presence to be monitored in wastewater treatment plants and its population dynamics to be examined.
This study has demonstrated that GTE2 could be used potentially as a biocontrol agent for foaming problems in activated sludge. Our laboratory-scale tests showed that GTE2 can prevent stable foam formation by its host bacteria. Further work is required to determine if GTE2 and other phages can control foaming in wastewater treatment plants, but the results presented here are encouraging.
Supplementary Material
ACKNOWLEDGMENTS
The research was supported by Australian Research Council Linkage grant LP0774913, together with Melbourne Water (David Gregory) and South East Water (Graham Short), whom we thank for their financial support. S.P. was funded by ARC Linkage and La Trobe University grants.
Footnotes
Present address: Department of Microbiology, La Trobe University, Bundoora, Victoria 3086, Australia.
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 15 April 2011.
REFERENCES
- 1. Alonso M. D. C., Rodriguez J., Borrego J. J. 2002. Characterization of marine bacteriophages isolated from the Alboran Sea (Western Mediterranean). J. Plankton Res. 24:1079–1087 [Google Scholar]
- 2. Anderson A. S., Wellington E. M. H. 2001. The taxonomy of Streptomyces and related genera. Int. J. Syst. Evol. Microbiol. 51:797–814 [DOI] [PubMed] [Google Scholar]
- 3. Belbahri L., Calmin C., Mauch F., Anderson J. O. 2008. Evolution of the cutinase gene family: evidence for lateral gene transfer of a candidate Phytophthora virulence factor. Gene 408:1–8 [DOI] [PubMed] [Google Scholar]
- 4. Blackall L. L., Marshall K. C. 1989. The mechanism of stabilization of actinomycete foams and the prevention of foaming under laboratory conditions. J. Ind. Microbiol. 4:181–188 [Google Scholar]
- 5. Bork P., Koonin E. V. 1993. An expanding family of helicases within the DEAD/H superfamily. Nucleic Acids Res. 21:751–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Catalano C. E. 2000. The terminase enzyme from bacteriophage lambda: a DNA-packaging machine. Cell. Mol. Life Sci. 57:128–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cserzo M., Wallin E., Simon I., von Heijne G., Elofsson A. 1997. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 10:673–676 [DOI] [PubMed] [Google Scholar]
- 8. de los Reyes F. L. 2010. Foaming, p. 215–259 In Seviour R. J., Nielsen P. H. (ed.), Microbial ecology of activated sludge. IWA Publishing, London, United Kingdom [Google Scholar]
- 9. Drummond A. J., et al. 2010. Geneious v5.1. Biomatters Ltd., Auckland, New Zealand [Google Scholar]
- 10. Dutta K., Sen S., Veeranki V. D. 2009. Production, characterization and application of microbial cutinases. Process Biochem. 44:127–134 [Google Scholar]
- 11. Eisen J. A., Sweder K. S., Hanawalt P. C. 1995. Evolution of the SNF2 family of proteins: subfamilies with distinct sequences and functions. Nucleic Acids Res. 23:2715–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finn R. D., et al. 2010. The Pfam protein families database. Nucleic Acids Res. 38:D211–D222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gregory R., Saunders V. A., Saunders J. R. 2010. Rule-based simulation of temperate bacteriophage infection: restriction-modification as a limiter to infection of bacterial populations. Biosystems 100:166–177 [DOI] [PubMed] [Google Scholar]
- 14. Hatfull G. F., et al. 2006. Exploring the mycobacteriophage metaproteome: phage genomics as an educational platform. PLoS Genet. 2:e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hatfull G. F., Cresawn S. G., Hendrix R. W. 2008. Comparative genomics of the mycobacteriophages: insights into bacteriophage evolution. Res. Microbiol. 159:332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hatfull G. F., et al. 2010. Comparative genomic analysis of 60 mycobacteriophage genomes: genome clustering, gene acquisition and gene size. J. Mol. Biol. 397:119–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ishikawa J., et al. 2004. The complete genome sequence of Nocardia farcinica IMF 10152. Proc. Natl. Acad. Sci. U. S. A. 101:14925–14930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Isidro A., Henriques A. O., Tavares P. 2004. The portal protein plays essential roles at different steps of SPP1 DNA packaging process. Virology 322:253–263 [DOI] [PubMed] [Google Scholar]
- 19. Ivanova N., et al. 2010. Complete genome sequence of Gordonia bronchialis type strain (3410T). Stand. Genomic Sci. 2:19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jensen E. C., et al. 1998. Prevalence of broad-host-range lytic bacteriophages of Sphaerotilus natans, Escherichia coli, and Pseudomonas aeruginosa. Appl. Environ. Microbiol. 64:575–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khan S. T., Sataoh H., Katayama H., Kurisu F., Mino T. 2002. Bacteriophages isolated from activated sludge processes and their polyvalency. Water Res. 36:3364–3370 [DOI] [PubMed] [Google Scholar]
- 22. Komori K., Sakae S., Shinagawa H., Morikawa K., Ishino Y. 1999. A Holliday junction resolvase from Pyrococcus furiosus: functional similarity to Escherichia coli RuvC provides evidence for conserved mechanism of homologous recombination in bacteria, eukarya and archaea. Proc. Natl. Acad. Sci. U. S. A. 96:8873–8878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Korn-Wendisch F., Schneider J. 1992. Phage typing—a useful tool in actinomycete systematics. Gene 115:243–247 [DOI] [PubMed] [Google Scholar]
- 24. Labrie S. J., Samson J. E., Moineau S. 2010. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8:317–327 [DOI] [PubMed] [Google Scholar]
- 25. Laslett D., Canback B. 2004. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 32:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nielsen P. H., Kragelund C., Seviour R. J., Nielsen J. L. 2009. Identity and ecophysiology of filamentous bacteria in activated sludge. FEMS Microbiol. Lett. 33:969–998 [DOI] [PubMed] [Google Scholar]
- 27. Park T., Struck D. K., Deaton J. F., Young R. 2006. Topological dynamics of holins in programmed bacterial lysis. Proc. Natl. Acad. Sci. U. S. A. 103:19713–19718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petrovski S., et al. 2011. An examination of the mechanisms for stable foam formation in activated sludge systems. Water Res. 45:2146–2154 [DOI] [PubMed] [Google Scholar]
- 29. Petrovski S., Seviour R. J., Tillett D. 2011. Genome sequence and characterization of the Tsukamurella phage TPA2. Appl. Environ. Microbiol. 77:1389–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rainey F. A., Klatte S., Kroppenstedt R. M., Stackebrandt E. 1995. Dietzia, a new genus including Dietzia maris comb. nov., formerly Rhodococcus maris. Int. J. Syst. Bacteriol. 45:32–36 [DOI] [PubMed] [Google Scholar]
- 31. Ruimy R., Boiron P., Boivin V., Christen R. 1994. A phylogeny of the genus Nocardia deduced from the analysis of small subunit ribosomal DNA sequences, including transfer of Nocardia amarae to the genus Gordona as Gordona amarae comb. nov. FEMS Microbiol. Lett. 123:261–268 [DOI] [PubMed] [Google Scholar]
- 32. Seviour R. J., Nielsen P. H. (ed.). 2010. Microbial ecology of activated sludge. IWA Publishing, Norfolk, United Kingdom [Google Scholar]
- 33. Soddell J. A., Seviour R. J. 1990. Microbiology of foaming in activated sludge plants—a review. J. Appl. Bacteriol. 69:145–176 [Google Scholar]
- 34. Stratton H. M., Brooks P. R., Griffiths P. C., Seviour R. J. 2002. Cell surface hydrophobicity and mycolic acid composition of Rhodococcus strains isolated from activated sludge foams. J. Ind. Microbiol. Biotechnol. 28:264–267 [DOI] [PubMed] [Google Scholar]
- 35. Summers W. C. 2001. Bacteriophage therapy. Annu. Rev. Microbiol. 55:437–451 [DOI] [PubMed] [Google Scholar]
- 36. Summers E. J., et al. 2011. Genomic and functional analyses of Rhodococcus equi phages ReqiPepy6, ReqiPoco6, ReqiPine5, and ReqiDocB7. Appl. Environ. Microbiol. 77:669–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thomas J. A. 2005. Actinophages in activated sludge. Ph.D. thesis. La Trobe University, Victoria, Australia [Google Scholar]
- 38. Thomas J. A., Soddell J. A., Kurtböke D. I. 2002. Fighting foam with phages? Water Sci. Technol. 46(1–2):511–518 [PubMed] [Google Scholar]
- 39. Wanner J., Kragelund C., Nielsen P. H. 2010. Microbiology of bulking, p. 191–214 In Seviour R. J., Nielsen P. H. (ed.), Microbial ecology of activated sludge. IWA Publishing, London, United Kingdom [Google Scholar]
- 40. Wikoff W. R., et al. 2000. Topologically linked protein rings in the bacteriophage HK97 capsid. Science 289:2129–2133 [DOI] [PubMed] [Google Scholar]
- 41. Withey S., Cartmell E., Avery L. M., Stephenson T. 2005. Bacteriophages—potential for application in wastewater treatment processes. Sci. Total Environ. 339:1–18 [DOI] [PubMed] [Google Scholar]
- 42. Xu J., Hendrix R. W., Duda R. L. 2004. Conserved translational frameshift in dsDNA bacteriophage tail assembly genes. Mol. Cell 16:11–21 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.