Abstract
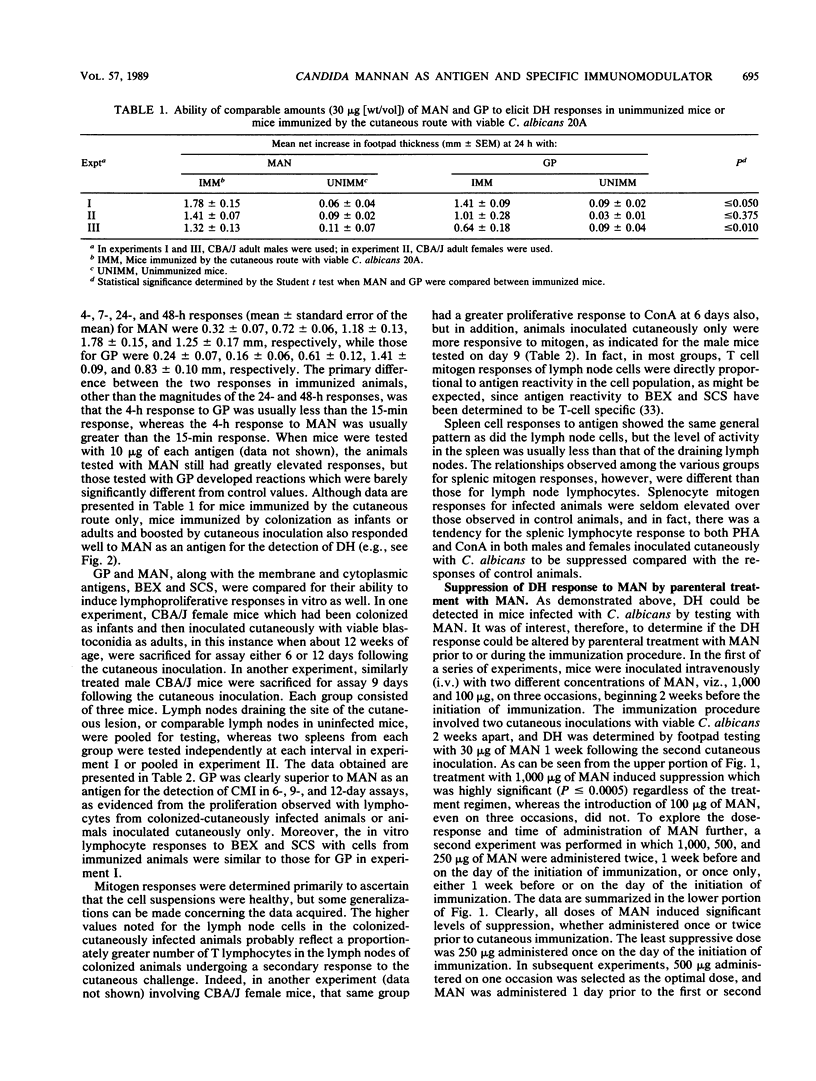
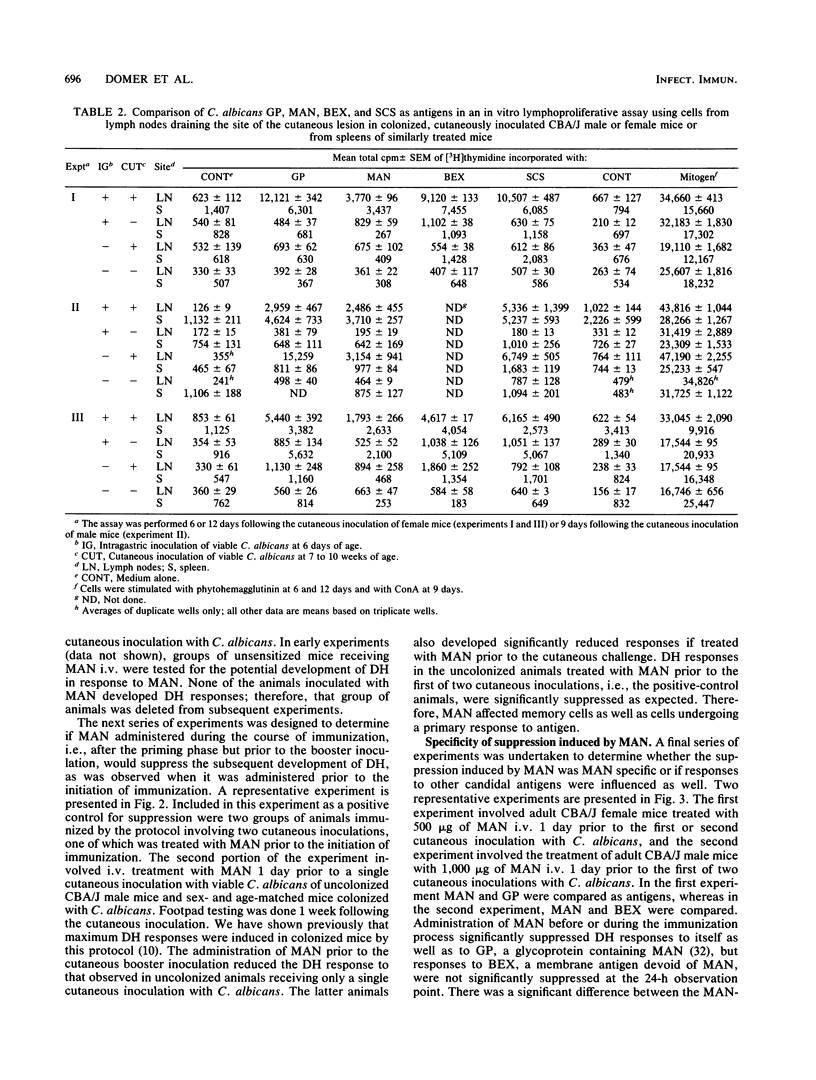
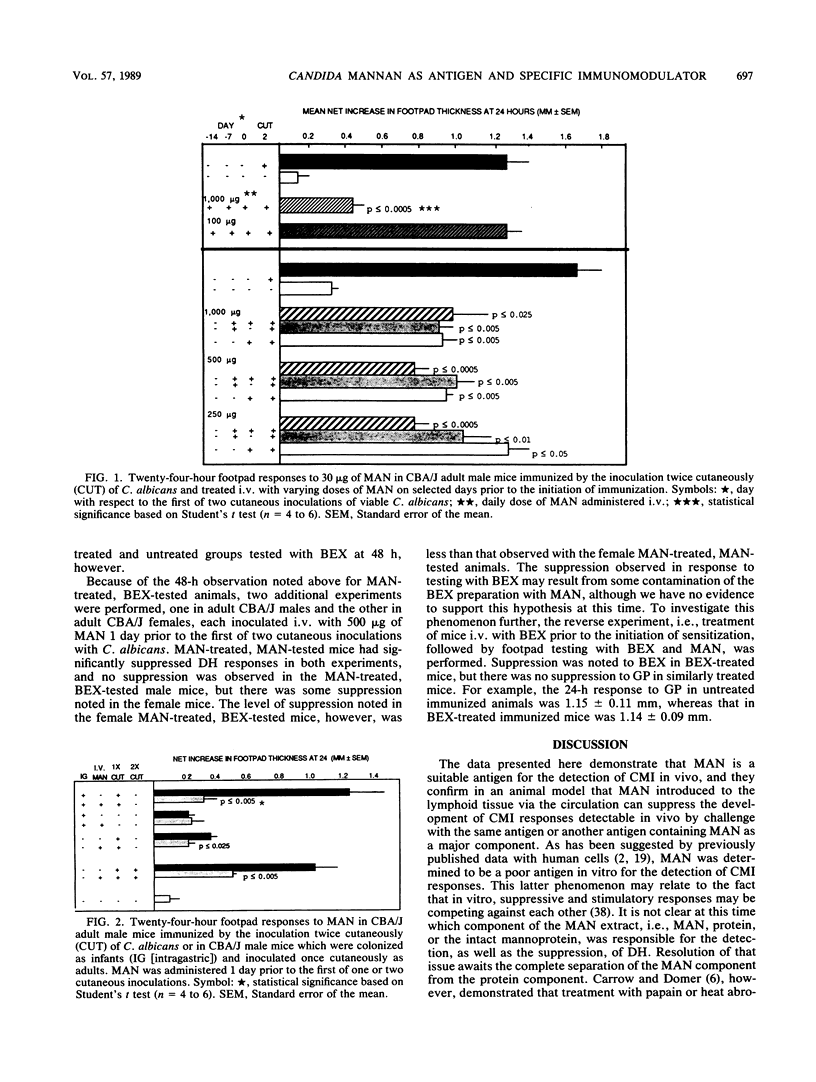
Mannan (MAN) extracted from Candida albicans 20A was investigated for its potential as an antigen in the detection of cell-mediated immunity (CMI) in vivo and in vitro and for its ability to modulate CMI when administered intravenously (i.v.). CBA/J mice were either immunized as adults by the cutaneous inoculation of 10(6) viable blastoconidia or colonized as infants (primed) and then boosted cutaneously as adults. When immunized animals were footpad tested with MAN, highly significant delayed-type hypersensitivity (DH) responses were detected. The DH responses to MAN were of a greater magnitude than those noted with the same quantity of cell wall glycoprotein (GP), an ethylenediamine extract of the cell wall which contains both glucan and MAN. In contrast, GP was a better antigen for the detection of CMI responses in an in vitro lymphoproliferative assay with either spleen or lymph node cell suspensions. Mice treated with MAN i.v. prior to the initiation of immunization or between priming and secondary inoculations developed significantly suppressed DH reactions when tested with either MAN or GP. The lowest effective dose of MAN was 250 micrograms, maximum suppression occurred with 500 micrograms, and either dose given 1 week prior to immunization was suppressive. The suppression by MAN was specific for MAN or the MAN-containing GP. Responses to another unrelated candidal antigen, a membrane extract designated BEX, were relatively unaffected. MAN, therefore, was an effective antigen for the detection of CMI in vivo, and its administration i.v. created what appeared to be a MAN-specific suppression since it could be detected with both MAN and a MAN-containing extract from the cell wall. Caution must be exercised in the interpretation of these data, however, since the protein component of each of these extracts has not been characterized with respect to its potential role in the phenomena observed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson I. K., Rieger C. H., Soltani K., Tkalcevic V., Chan W. C., Lorincz A. L., Matz G. Late onset chronic mucocutaneous candidiasis with lymphoma and specific serum inhibitory factor. Cancer. 1979 Jan;43(1):101–108. doi: 10.1002/1097-0142(197901)43:1<101::aid-cncr2820430116>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Ausiello C. M., Spagnoli G. C., Boccanera M., Casalinuovo I., Malavasi F., Casciani C. U., Cassone A. Proliferation of human peripheral blood mononuclear cells induced by Candida albicans and its cell wall fractions. J Med Microbiol. 1986 Nov;22(3):195–202. doi: 10.1099/00222615-22-3-195. [DOI] [PubMed] [Google Scholar]
- Borkowsky W., Valentine F. T. The proliferative response of human lymphocytes to antigen is suppressed preferentially by lymphocytes precultured with the same antigen. J Immunol. 1979 May;122(5):1867–1873. [PubMed] [Google Scholar]
- Bull F. G., Turner N. R. A serum mannan-binding protein and candidiasis. Sabouraudia. 1984;22(4):347–350. doi: 10.1080/00362178485380571. [DOI] [PubMed] [Google Scholar]
- Canales L., Middlemas R. O., 3rd, Louro J. M., South M. A. Immunological observations in chronic mucocutaneous candidiasis. Lancet. 1969 Sep 13;2(7620):567–571. doi: 10.1016/s0140-6736(69)90264-5. [DOI] [PubMed] [Google Scholar]
- Carrow E. W., Domer J. E. Immunoregulation in experimental murine candidiasis: specific suppression induced by Candida albicans cell wall glycoprotein. Infect Immun. 1985 Jul;49(1):172–181. doi: 10.1128/iai.49.1.172-181.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cates K. L., Grady P. G., Shapira E., Davis A. T. Cell-directed inhibition of polymorphonuclear leukocyte chemotaxis in a patient with mucocutaneous candidiasis. J Allergy Clin Immunol. 1980 Jun;65(6):430–435. doi: 10.1016/0091-6749(80)90235-3. [DOI] [PubMed] [Google Scholar]
- Colizzi V., Asherson G. L., James B. M. The role of I-J in the suppressor T-cell circuit which influences the effector stage of contact sensitivity: antigen together with syngeneic I-J region determinants induces and activates T suppressor cells. Immunology. 1983 May;49(1):191–199. [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Kennell W. Suppression of T-lymphocyte response by Coccidioides immitis antigen. Infect Immun. 1988 Jun;56(6):1424–1429. doi: 10.1128/iai.56.6.1424-1429.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E. Intragastric colonization of infant mice with Candida albicans induces systemic immunity demonstrable upon challenge as adults. J Infect Dis. 1988 May;157(5):950–958. doi: 10.1093/infdis/157.5.950. [DOI] [PubMed] [Google Scholar]
- Domer J. E., Moser S. A. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1978 Apr;20(1):88–98. doi: 10.1128/iai.20.1.88-98.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E., Stashak P. W., Elkins K., Prescott B., Caldes G., Baker P. J. Separation of immunomodulatory effects of mannan from Candida albicans into stimulatory and suppressive components. Cell Immunol. 1986 Sep;101(2):403–414. doi: 10.1016/0008-8749(86)90153-x. [DOI] [PubMed] [Google Scholar]
- Durandy A., Fischer A., Griscelli C. Specific in vitro antimannan-rich antigen of Candida albicans antibody production by sensitized human blood lymphocytes. J Clin Invest. 1983 Jun;71(6):1602–1613. doi: 10.1172/JCI110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durandy A., Fischer A., Le Deist F., Drouhet E., Griscelli C. Mannan-specific and mannan-induced T-cell suppressive activity in patients with chronic mucocutaneous candidiasis. J Clin Immunol. 1987 Sep;7(5):400–409. doi: 10.1007/BF00917018. [DOI] [PubMed] [Google Scholar]
- Ellsworth J. H., Reiss E., Bradley R. L., Chmel H., Armstrong D. Comparative serological and cutaneous reactivity of candidal cytoplasmic proteins and mannan separated by affinity for concanavalin A. J Clin Microbiol. 1977 Jan;5(1):91–99. doi: 10.1128/jcm.5.1.91-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Ballet J. J., Griscelli C. Specific inhibition of in vitro Candida-induced lymphocyte proliferation by polysaccharidic antigens present in the serum of patients with chronic mucocutaneous candidiasis. J Clin Invest. 1978 Nov;62(5):1005–1013. doi: 10.1172/JCI109204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Pichat L., Audinot M., Griscelli C. Defective handling of mannan by monocytes in patients with chronic mucocutaneous candidiasis resulting in a specific cellular unresponsiveness. Clin Exp Immunol. 1982 Mar;47(3):653–660. [PMC free article] [PubMed] [Google Scholar]
- Gatenby P., Basten A., Adams E. Thymoma and late onset mucocutaneous candidiasis associated with a plasma inhibitor of cell-mediated immune function. J Clin Lab Immunol. 1980 May;3(3):209–216. [PubMed] [Google Scholar]
- Gettner S. M., Mackenzie D. W. Responses of human peripheral lymphocytes to soluble and insoluble antigens of Candida albicans. J Med Microbiol. 1981 Aug;14(3):333–340. doi: 10.1099/00222615-14-3-333. [DOI] [PubMed] [Google Scholar]
- Giger D. K., Domer J. E., McQuitty J. T., Jr Experimental murine candidiasis: pathological and immune responses to cutaneous inoculation with Candida albicans. Infect Immun. 1978 Feb;19(2):499–509. doi: 10.1128/iai.19.2.499-509.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector R. F., Domer J. E., Carrow E. W. Immune responses to Candida albicans in genetically distinct mice. Infect Immun. 1982 Dec;38(3):1020–1028. doi: 10.1128/iai.38.3.1020-1028.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Finkel B. E., Murphy J. W. Characterization of efferent T suppressor cells induced by Paracoccidioides brasiliensis-specific afferent T suppressor cells. Infect Immun. 1988 Apr;56(4):744–750. doi: 10.1128/iai.56.4.744-750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Finkel B. E., Murphy J. W. Induction of antigen-specific T suppressor cells by soluble Paracoccidioides brasiliensis antigen. Infect Immun. 1988 Apr;56(4):734–743. doi: 10.1128/iai.56.4.734-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. M. Quantitation of antibody against cell wall mannan and a major cytoplasmic antigen of Candida in rabbits, mice, and humans. Infect Immun. 1980 Oct;30(1):78–89. doi: 10.1128/iai.30.1.78-89.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C. T., Valdimarsson H., Hay R. J. Chronic mucocutaneous candidiasis with a serum-dependent neutrophil defect: response to ketoconazole. J R Soc Med. 1981 Feb;74(2):158–162. doi: 10.1177/014107688107400213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocourek J., Ballou C. E. Method for fingerprinting yeast cell wall mannans. J Bacteriol. 1969 Dec;100(3):1175–1181. doi: 10.1128/jb.100.3.1175-1181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laforce F. M., Mills D. M., Iverson K., Cousins R., Everett E. D. Inhibition of leukocyte candidacidal activity by serum from patients with disseminated candidiasis. J Lab Clin Med. 1975 Oct;86(4):657–666. [PubMed] [Google Scholar]
- Lee W. M., Holley H. P., Jr, Stewart J., Galbraith G. M. Refractory esophageal candidiasis associated with a low molecular weight plasma inhibitor of T-lymphocyte function. Am J Med Sci. 1986 Jul;292(1):47–52. doi: 10.1097/00000441-198607000-00010. [DOI] [PubMed] [Google Scholar]
- Lehner T. The relationship between human helper and suppressor factors to a streptococcal protein antigen. J Immunol. 1982 Nov;129(5):1936–1940. [PubMed] [Google Scholar]
- Lowy A., Tominaga A., Drebin J. A., Takaoki M., Benacerraf B., Greene M. I. Identification of an I-J+ antigen-presenting cell required for third order suppressor cell activation. J Exp Med. 1983 Jan 1;157(1):353–358. doi: 10.1084/jem.157.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon F. L., Domer J. E. Chemical and enzymatic variation in the cell walls of pathogenic Candida species. Can J Microbiol. 1985 Jul;31(7):590–597. doi: 10.1139/m85-112. [DOI] [PubMed] [Google Scholar]
- Moser S. A., Domer J. E., Mather F. J. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1980 Jan;27(1):140–149. doi: 10.1128/iai.27.1.140-149.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz M., Estes G., Kilpatrick M., Di Salvo A., Virella G. Purification of cytoplasmic antigens from the mycelial phase of Candida albicans: possible advantages of its use in Candida serology. Mycopathologia. 1980 Aug 29;72(1):47–53. doi: 10.1007/BF00443050. [DOI] [PubMed] [Google Scholar]
- Murphy J. W., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. J Immunol. 1982 Jan;128(1):276–283. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. II. Characterization of first-order T suppressor cells (Ts1) and induction of second-order suppressor cells. J Immunol. 1983 Jun;130(6):2876–2881. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L. Regulation of cell-mediated immunity in cryptococcosis. III. Characterization of second-order T suppressor cells (Ts2). J Immunol. 1985 Jan;134(1):577–584. [PubMed] [Google Scholar]
- Nelson R. D., Herron M. J., McCormack R. T., Gehrz R. C. Two mechanisms of inhibition of human lymphocyte proliferation by soluble yeast mannan polysaccharide. Infect Immun. 1984 Mar;43(3):1041–1046. doi: 10.1128/iai.43.3.1041-1046.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson P. Y., Semo R., Blumenschein G., Swelstad J. Mucocutaneous candidiasis, anergy and a plasma inhibitor of cellular immunity: reversal after amphotericin B therapy. Clin Exp Immunol. 1971 Nov;9(5):595–602. [PMC free article] [PubMed] [Google Scholar]
- Piccolella E., Lombardi G., Morelli R. Generation of suppressor cells in the response of human lymphocytes to a polysaccharide from Candida albicans. J Immunol. 1981 Jun;126(6):2151–2155. [PubMed] [Google Scholar]
- Piccolella E., Lombardi G., Morelli R. Human lymphocyte-activating properties of a purified polysaccharide from Candida albicans: B and T cell cooperation in the mitogenic response. J Immunol. 1980 Nov;125(5):2082–2088. [PubMed] [Google Scholar]
- Pope L. M., Cole G. T., Guentzel M. N., Berry L. J. Systemic and gastrointestinal candidiasis of infant mice after intragastric challenge. Infect Immun. 1979 Aug;25(2):702–707. doi: 10.1128/iai.25.2.702-707.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss E., Stone S. H., Hasenclever H. F. Serological and cellular immune activity of peptidoglucomannan fractions of Candida albicans cell walls. Infect Immun. 1974 May;9(5):881–890. doi: 10.1128/iai.9.5.881-890.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo-Moreno A., Schneidau J. D., Jr Nature of the skin-reactive principle in culture filtrates prepared from Paracoccidioides brasiliensis. J Bacteriol. 1967 Jun;93(6):1741–1748. doi: 10.1128/jb.93.6.1741-1748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Hayashi Y. Skin reaction and macrophage migration inhibition tests for polysaccharides from Aspergillus fumigatus and Candida albicans. Jpn J Microbiol. 1975 Oct;19(5):355–362. doi: 10.1111/j.1348-0421.1975.tb00892.x. [DOI] [PubMed] [Google Scholar]
- Twomey J. J., Waddell C. C., Krantz S., O'reilly R., L'esperance P., Good R. A. Chronic mucocutaneous candidiasis with macrophage dysfunction, a plasma inhibitor, and co-existent aplastic anemia. J Lab Clin Med. 1975 Jun;85(6):968–977. [PubMed] [Google Scholar]
- Verhaegen H., de Cock W., de Cree J. In vitro phagocytosis of Candida albicans by peripheral polymorphonuclear neutrophils of patients with recurrent infections:Case reports of serum-dependent abnormalities. Biomedicine. 1976 Jun;24(3):164–170. [PubMed] [Google Scholar]
- Walker S. M., Urbaniak S. J. A serum-dependent defect of neutrophil function in chronic mucocutaneous candidiasis. J Clin Pathol. 1980 Apr;33(4):370–372. doi: 10.1136/jcp.33.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin S. S., Yu I. R., Ledger W. J. Inhibition of Candida albicans--induced lymphocyte proliferation by lymphocytes and sera from women with recurrent vaginitis. Am J Obstet Gynecol. 1983 Dec 1;147(7):809–811. doi: 10.1016/0002-9378(83)90044-3. [DOI] [PubMed] [Google Scholar]
- Wright C. D., Herron M. J., Gray G. R., Holmes B., Nelson R. D. Influence of yeast mannan on human neutrophil functions: inhibition of release of myeloperoxidase related to carbohydrate-binding property of the enzyme. Infect Immun. 1981 May;32(2):731–738. doi: 10.1128/iai.32.2.731-738.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


