Abstract
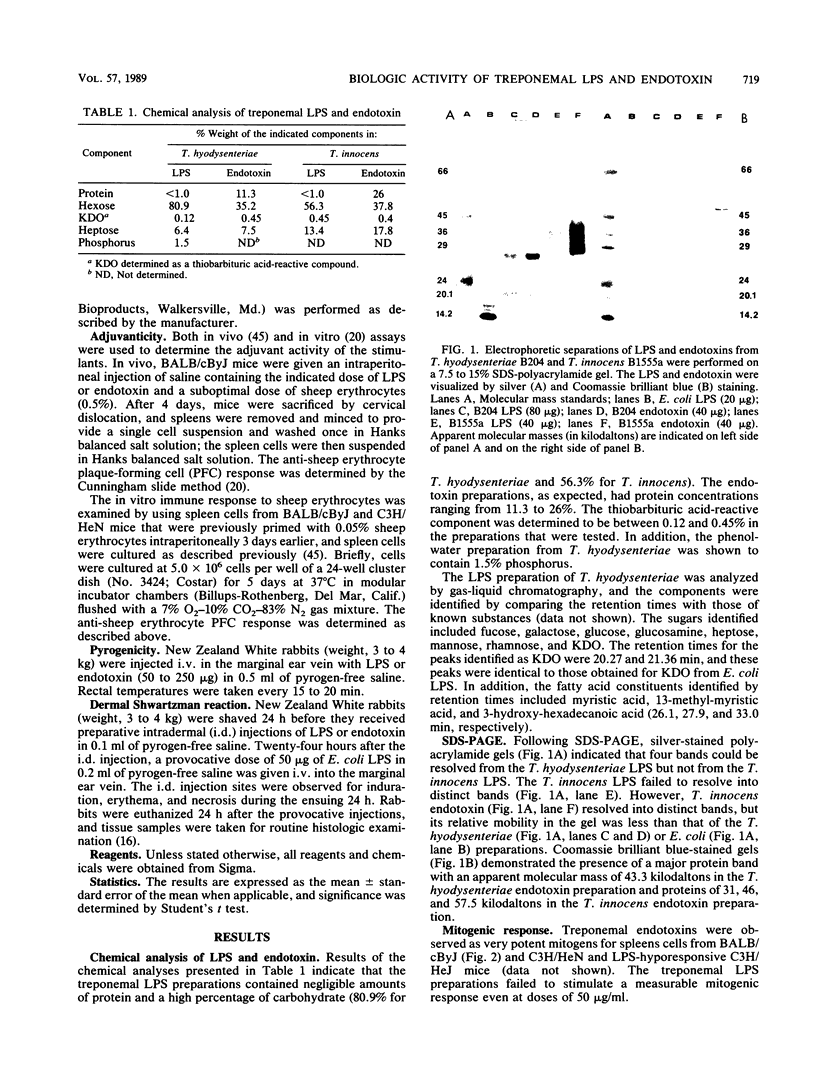
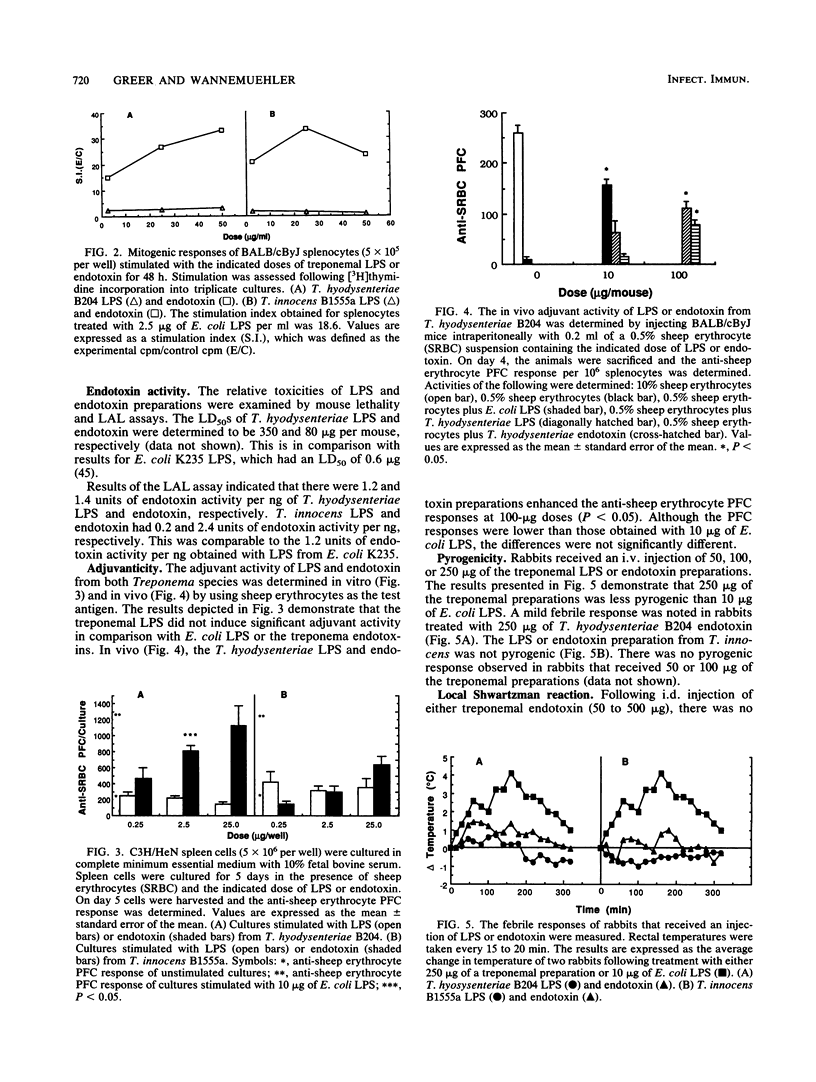
The chemical composition and classical biologic activities of lipopolysaccharide (LPS; phenol-water) and endotoxin (butanol-water) preparations from virulent Treponema hyodysenteriae and avirulent Treponema innocens were examined. The LPS and endotoxin preparations from T. hyodysenteriae B204 contained approximately 80.9 and 35.2% hexose, 0.12 and 0.45% thiobarbituric acid-reactive compound, and less than 1 and 11.3% protein, respectively. The LPS and endotoxin preparations of T. innocens B1555a contained approximately 56.3 and 37.8% hexose, 0.45 and 0.4% thiobarbituric acid-reactive compound, and less than 1 and 26% protein, respectively. A silver-stained 7.5 to 15% sodium dodecyl sulfate-polyacrylamide gel showed four bands for the T. hyodysenteriae preparations, while the T. innocens preparations failed to resolve into discrete bands on electrophoresis. We determined by the Limulus amebocyte lysate assay that the treponemal preparations had comparable amounts of endotoxin activity when Escherichia coli LPS was used as a standard. The 50% lethal doses of LPS and endotoxin from T. hyodysenteriae for BALB/cByJ mice were 380 and 80 micrograms, respectively. The treponemal preparations were poor adjuvants, failed to induce a dermal Shwartzman reaction, and were not pyrogenic. The treponemal LPS preparations, unlike the endotoxin preparations, were not mitogenic for murine spleen cells. Differences in virulence between the two treponemal species could not be associated with the biologic activities of the respective LPS or endotoxin moieties, but the endotoxin preparations were consistently more active than the purified LPS preparations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Beck G., Habicht G. S., Benach J. L., Coleman J. L. Chemical and biologic characterization of a lipopolysaccharide extracted from the Lyme disease spirochete (Borrelia burgdorferi). J Infect Dis. 1985 Jul;152(1):108–117. doi: 10.1093/infdis/152.1.108. [DOI] [PubMed] [Google Scholar]
- Billiau A. Gamma-interferon: the match that lights the fire? Immunol Today. 1988 Feb;9(2):37–40. doi: 10.1016/0167-5699(88)91256-X. [DOI] [PubMed] [Google Scholar]
- Cinco M., Banfi E., Panfili E. Heterogeneity of lipopolysaccharide banding patterns in Leptospira spp. J Gen Microbiol. 1986 Apr;132(4):1135–1138. doi: 10.1099/00221287-132-4-1135. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Hofstad T. The distribution of heptose and 2-keto-3-deoxy-octonate in Bacteroidaceae. J Gen Microbiol. 1974 Dec;85(2):314–320. doi: 10.1099/00221287-85-2-314. [DOI] [PubMed] [Google Scholar]
- Hogan M. M., Vogel S. N. Lipid A-associated proteins provide an alternate "second signal" in the activation of recombinant interferon-gamma-primed, C3H/HeJ macrophages to a fully tumoricidal state. J Immunol. 1987 Dec 1;139(11):3697–3702. [PubMed] [Google Scholar]
- Isogai E., Isogai H., Kurebayashi Y., Ito N. Biological activities of leptospiral lipopolysaccharide. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Feb;261(1):53–64. doi: 10.1016/s0176-6724(86)80062-1. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., McAdam K. P., Kasper D. L. Lipopolysaccharides from Bacteroides fragilis are mitogenic for spleen cells from endotoxin responder and nonresponder mice. Infect Immun. 1982 Jun;36(3):1139–1145. doi: 10.1128/iai.36.3.1139-1145.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Kinyon J. M., Harris D. L., Glock R. D. Enteropathogenicity of various isolates of Treponema hyodysenteriae. Infect Immun. 1977 Feb;15(2):638–646. doi: 10.1128/iai.15.2.638-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Odaka C., Moro I., Fujiwara T., Nishihara T., Okahashi N., Hamada S. Local Shwartzman activity of lipopolysaccharides from several selected strains of suspected periodontopathic bacteria. J Periodontal Res. 1987 Mar;22(2):103–107. doi: 10.1111/j.1600-0765.1987.tb01547.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehmann V., Freudenberg M. A., Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med. 1987 Mar 1;165(3):657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERGENHAGEN S. E., HAMPP E. G., SCHERP H. W. Preparation and biological activities of endotoxins from oral bacteria. J Infect Dis. 1961 May-Jun;108:304–310. doi: 10.1093/infdis/108.3.304. [DOI] [PubMed] [Google Scholar]
- Matthews H. M., Yang T. K., Jenkin H. M. Treponema innocens lipids and further description of an unusual galactolipid of Treponema hyodysenteriae. J Bacteriol. 1980 Sep;143(3):1151–1155. doi: 10.1128/jb.143.3.1151-1155.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Betz S. J., Jacobs D. M. Isolation of a lipid A bound polypeptide responsible for "LPS-initiated" mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976 Sep 1;144(3):840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Nuessen M. E., Birmingham J. R., Joens L. A. Biological activity of a lipopolysaccharide extracted from Treponema hyodysenteriae. Infect Immun. 1982 Jul;37(1):138–142. doi: 10.1128/iai.37.1.138-142.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuessen M. E., Joens L. A., Glock R. D. Involvement of lipopolysaccharide in the pathogenicity of Treponema hyodysenteriae. J Immunol. 1983 Aug;131(2):997–999. [PubMed] [Google Scholar]
- Nuessen M. E., Joens L. A. Serotype-specific opsonization of Treponema hyodysenteriae. Infect Immun. 1982 Dec;38(3):1029–1032. doi: 10.1128/iai.38.3.1029-1032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn C. W., Cockayne A., Bailey M. J. The outer membrane of Treponema pallidum: biological significance and biochemical properties. J Gen Microbiol. 1985 Sep;131(9):2349–2357. doi: 10.1099/00221287-131-9-2349. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Markham R. B., Eardley D. Correlation of the biologic responses of C3H/HEJ mice to endotoxin with the chemical and structural properties of the lipopolysaccharides from Pseudomonas aeruginosa and Escherichia coli. J Immunol. 1981 Jul;127(1):184–191. [PubMed] [Google Scholar]
- Spellman J. M., Reed N. D. Immune and mitogenic responses by BALB/c, C3H/HeJ, and nude mice to Brucella abortus bacterin and lipopolysaccharide. Infect Immun. 1979 May;24(2):371–378. doi: 10.1128/iai.24.2.371-378.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W., Galanos C. Characterisation of protein co-extracted together with LPS in Escherichia coli, Salmonella minnesota and Yersinia enterocolitica. Microb Pathog. 1987 Jan;2(1):29–36. doi: 10.1016/0882-4010(87)90112-4. [DOI] [PubMed] [Google Scholar]
- Sveen K., Hofstad T., Milner K. C. Lethality for mice and chick embryos, pyrogenicity in rabbits and ability to gelate lysate from amoebocytes of Limulus polyphemus by lipopolysaccharides from Bacteroides, Fusobacterium and Veillonella. Acta Pathol Microbiol Scand B. 1977 Dec;85B(6):388–396. doi: 10.1111/j.1699-0463.1977.tb01994.x. [DOI] [PubMed] [Google Scholar]
- Takayama K., Rothenberg R. J., Barbour A. G. Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1987 Sep;55(9):2311–2313. doi: 10.1128/iai.55.9.2311-2313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vinh T., Adler B., Faine S. Ultrastructure and chemical composition of lipopolysaccharide extracted from Leptospira interrogans serovar copenhageni. J Gen Microbiol. 1986 Jan;132(1):103–109. doi: 10.1099/00221287-132-1-103. [DOI] [PubMed] [Google Scholar]
- Vordermeier H. M., Bessler W. G. Polyclonal activation of murine B lymphocytes in vitro by Salmonella typhimurium porins. Immunobiology. 1987 Sep;175(3):245–251. doi: 10.1016/S0171-2985(87)80033-5. [DOI] [PubMed] [Google Scholar]
- Vordermeier M., Stäb K., Bessler W. G. A defined fragment of bacterial protein I (OmpF) is a polyclonal B-cell activator. Infect Immun. 1986 Jan;51(1):233–239. doi: 10.1128/iai.51.1.233-239.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannemuehler M. J., Michalek S. M., Jirillo E., Williamson S. I., Hirasawa M., McGhee J. R. LPS regulation of the immune response: Bacteroides endotoxin induces mitogenic, polyclonal, and antibody responses in classical LPS responsive but not C3H/HeJ mice. J Immunol. 1984 Jul;133(1):299–305. [PubMed] [Google Scholar]
- Wollenweber H. W., Rietschel E. T., Hofstad T., Weintraub A., Lindberg A. A. Nature, type of linkage, quantity, and absolute configuration of (3-hydroxy) fatty acids in lipopolysaccharides from Bacteroides fragilis NCTC 9343 and related strains. J Bacteriol. 1980 Dec;144(3):898–903. doi: 10.1128/jb.144.3.898-903.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]