Abstract
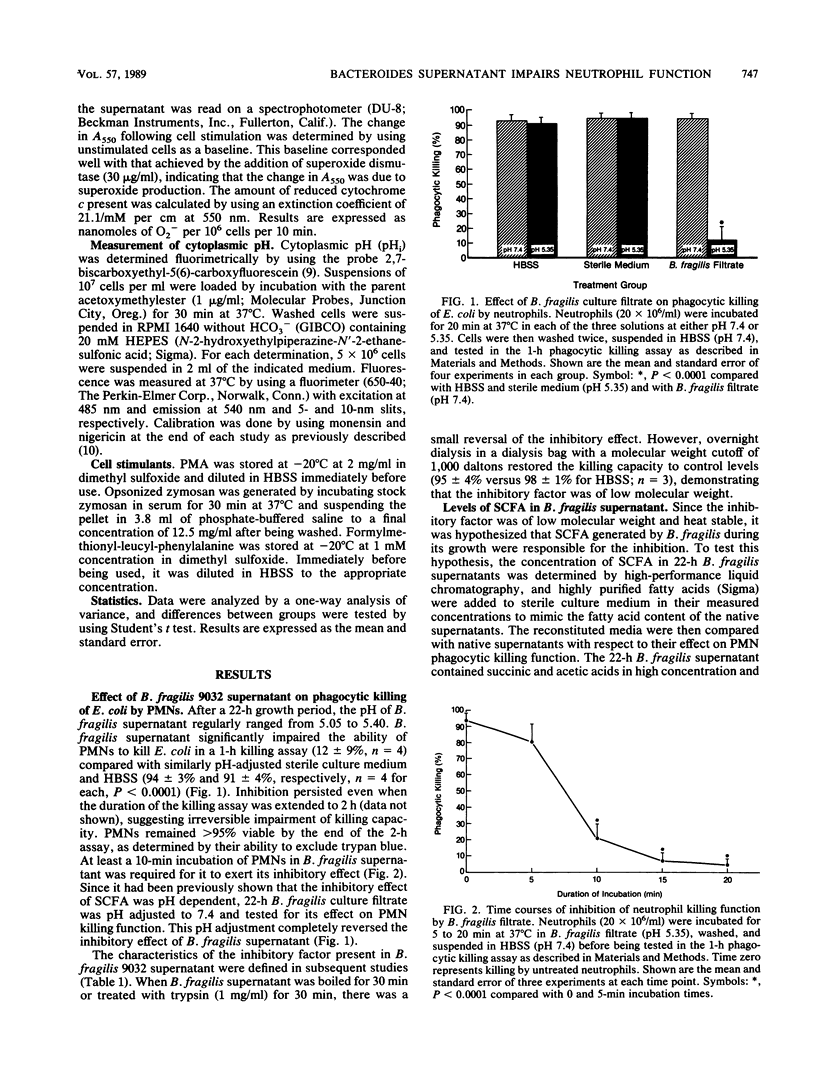
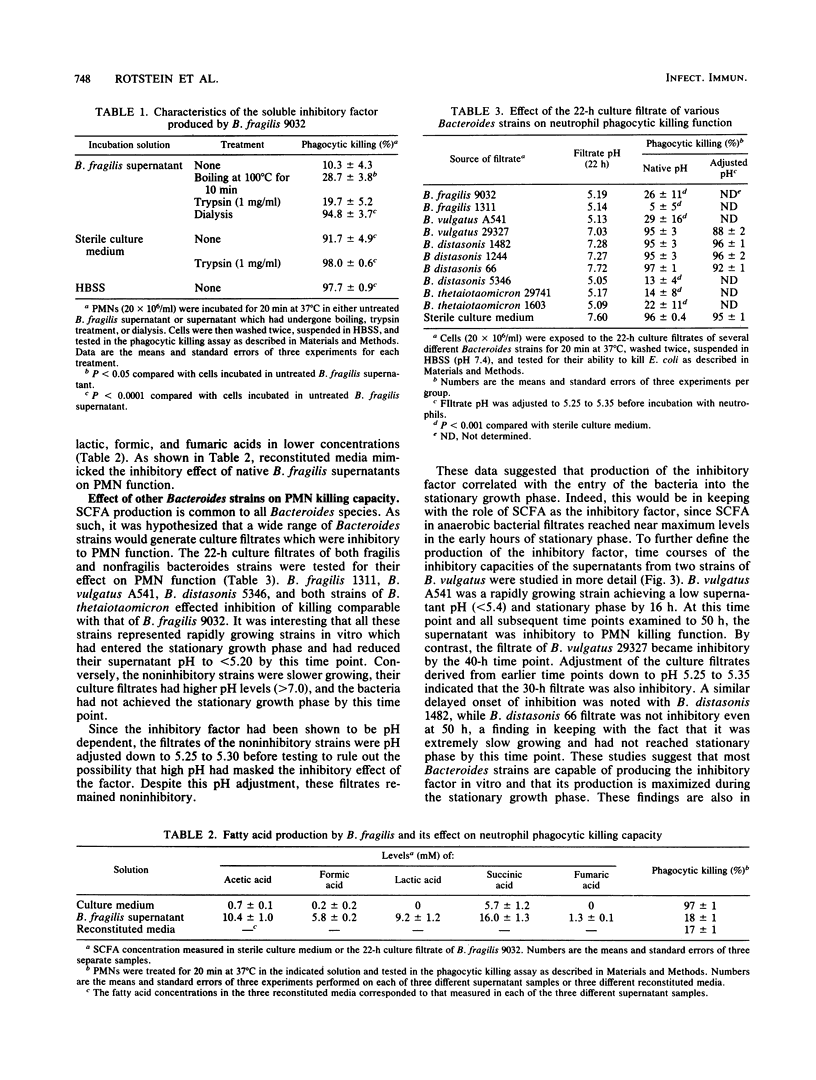
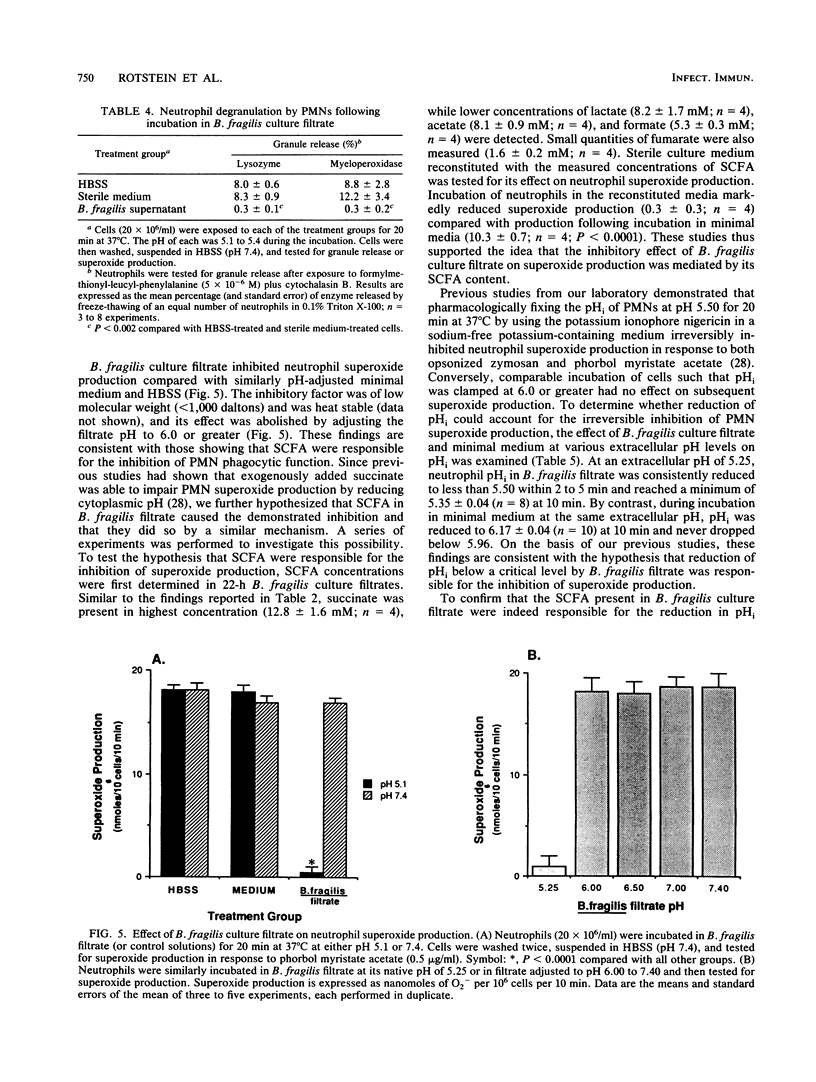
The effect of Bacteroides culture filtrate on killing of Escherichia coli by neutrophils was examined as a potential mechanism for E. coli-Bacteroides microbial synergy. A low-molecular-weight heat-stable factor present in the 22-h culture filtrate of Bacteroides fragilis 9032 impaired neutrophil killing function. To determine whether short-chain fatty acids present in the filtrate could account for the inhibition, the fatty acid content of the culture filtrate was determined and sterile medium supplemented with measured concentrations of fatty acids was tested for its effect on neutrophil function. Succinic and acetic acids were measured in high concentrations, while lactic, formic, and fumaric acids were present in lower concentrations. Reconstituted media mimicked the inhibitory effect of B. fragilis filtrate on neutrophil killing capacity. In further support of the hypothesis that short-chain fatty acids were responsible for the inhibition, the filtrates of other Bacteroides strains were found to be inhibitory only after bacterial growth had entered the stationary phase, a period during which fatty acid production is maximized. Further studies investigating the mechanism of impaired neutrophil killing showed that B. fragilis 9032 culture filtrate inhibited both phagocytosis of [3H]thymidine-labeled E. coli by neutrophils and the intrinsic microbicidal functions of the neutrophil. Impairment of neutrophil superoxide production was mediated via the ability of short-chain fatty acids present in B. fragilis filtrate to reduce neutrophil cytoplasmic pH. These studies suggest that Bacteroides strains capable of reaching stationary phase in vivo may contribute to the pathogenesis of mixed infections by direct inhibition of neutrophil function.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I. Encapsulated anaerobic bacteria in synergistic infections. Microbiol Rev. 1986 Dec;50(4):452–457. doi: 10.1128/mr.50.4.452-457.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I., Hunter V., Walker R. I. Synergistic effect of bacteroides, Clostridium, Fusobacterium, anaerobic cocci, and aerobic bacteria on mortality and induction of subcutaneous abscesses in mice. J Infect Dis. 1984 Jun;149(6):924–928. doi: 10.1093/infdis/149.6.924. [DOI] [PubMed] [Google Scholar]
- Bryant R. E., Rashad A. L., Mazza J. A., Hammond D. beta-Lactamase activity in human pus. J Infect Dis. 1980 Oct;142(4):594–601. doi: 10.1093/infdis/142.4.594. [DOI] [PubMed] [Google Scholar]
- Deacon A. G., Duerden B. I., Holbrook W. P. Gas-lipuid chromatographic analysis of metabolic products in the identification of bacteroidaceae of clinical interest. J Med Microbiol. 1978 May;11(2):81–99. doi: 10.1099/00222615-11-2-81. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. A rapid one-step procedure for purification of mononuclear and polymorphonuclear leukocytes from human blood using a modification of the Hypaque-Ficoll technique. J Immunol Methods. 1978;24(3-4):389–393. doi: 10.1016/0022-1759(78)90143-6. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L., Mayhew J. W., Bartlett J. G., Thadepalli H., Onderdonk A. B. Rapid diagnosis of anaerobic infections by direct gas-liquid chromatography of clinical speciments. J Clin Invest. 1976 Feb;57(2):478–484. doi: 10.1172/JCI108300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Assessment of Na+-H+ exchange activity in phagosomal membranes of human neutrophils. Am J Physiol. 1988 Feb;254(2 Pt 1):C272–C285. doi: 10.1152/ajpcell.1988.254.2.C272. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Cytoplasmic pH regulation in phorbol ester-activated human neutrophils. Am J Physiol. 1986 Jul;251(1 Pt 1):C55–C65. doi: 10.1152/ajpcell.1986.251.1.C55. [DOI] [PubMed] [Google Scholar]
- Guerrant G. O., Lambert M. A., Moss C. W. Analysis of short-chain acids from anaerobic bacteria by high-performance liquid chromatography. J Clin Microbiol. 1982 Aug;16(2):355–360. doi: 10.1128/jcm.16.2.355-360.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. H., Spencer L. K., Nulsen M. F., McDonald P. J., Finlay-Jones J. J. Neutrophil activity in abscess-bearing mice: comparative studies with neutrophils isolated from peripheral blood, elicited peritoneal exudates, and abscesses. Infect Immun. 1986 Mar;51(3):936–941. doi: 10.1128/iai.51.3.936-941.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham H. R., Sisson P. R., Tharagonnet D., Selkon J. B., Codd A. A. Inhibition of phagocytosis in vitro by obligate anaerobes. Lancet. 1977 Dec 17;2(8051):1252–1254. doi: 10.1016/s0140-6736(77)92662-9. [DOI] [PubMed] [Google Scholar]
- Jones G. R., Gemmell C. G. Impairment by Bacteroides species of opsonisation and phagocytosis of enterobacteria. J Med Microbiol. 1982 Aug;15(3):351–361. doi: 10.1099/00222615-15-3-351. [DOI] [PubMed] [Google Scholar]
- Kelly M. J. The quantitative and histological demonstration of pathogenic synergy between Escherichia coli and Bacteroides fragilis in guinea-pig wounds. J Med Microbiol. 1978 Nov;11(4):513–523. doi: 10.1099/00222615-11-4-513. [DOI] [PubMed] [Google Scholar]
- LITWACK G. Photometric determination of lysozyme activity. Proc Soc Exp Biol Med. 1955 Jul;89(3):401–403. doi: 10.3181/00379727-89-21824. [DOI] [PubMed] [Google Scholar]
- Lindner J. G., Marcelis J. H. Quantitative gas chromatography of Bacteroides species under different growth conditions. Antonie Van Leeuwenhoek. 1978;44(1):1–14. doi: 10.1007/BF00400072. [DOI] [PubMed] [Google Scholar]
- Lorber B., Swenson R. M. The bacteriology of intra-abdominal infections. Surg Clin North Am. 1975 Dec;55(6):1349–1354. doi: 10.1016/s0039-6109(16)40792-9. [DOI] [PubMed] [Google Scholar]
- Mackowiak P. A. Microbial synergism in human infections (second of two parts). N Engl J Med. 1978 Jan 12;298(2):83–87. doi: 10.1056/NEJM197801122980206. [DOI] [PubMed] [Google Scholar]
- Mayhew J. W., Onderdonk A. B., Gorbach S. L. Effects of time and growth media on short-chain fatty acid production by Bacteroides fragilis. Appl Microbiol. 1975 Apr;29(4):472–475. doi: 10.1128/am.29.4.472-475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand D., McBride B. C. Exological relationships of bacteria involved in a simple, mixed anaerobic infection. Infect Immun. 1980 Jan;27(1):44–50. doi: 10.1128/iai.27.1.44-50.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney M. I., Thompson L. U., Cuff D. J., Jenkins D. J. Comparison of ileal effluents, dietary fibers, and whole foods in predicting the physiological importance of colonic fermentation. Am J Gastroenterol. 1988 May;83(5):536–540. [PubMed] [Google Scholar]
- Namavar F., Verweij A. M., Bal M., van Steenbergen T. J., de Graaff J., MacLaren D. M. Effect of anaerobic bacteria on killing of Proteus mirabilis by human polymorphonuclear leukocytes. Infect Immun. 1983 Jun;40(3):930–935. doi: 10.1128/iai.40.3.930-935.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Bartlett J. G., Louie T., Sullivan-Seigler N., Gorbach S. L. Microbial synergy in experimental intra-abdominal abscess. Infect Immun. 1976 Jan;13(1):22–26. doi: 10.1128/iai.13.1.22-26.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett T. L., Rotstein O. D., Fiegel V. D., Sorenson J. J., Nelson R. D., Simmons R. L. Mechanism of the adjuvant effect of hemoglobin in experimental peritonitis: VIII. A leukotoxin is produced by Escherichia coli metabolism in hemoglobin. Surgery. 1984 Aug;96(2):375–383. [PubMed] [Google Scholar]
- Quie P. G. Perturbation of the normal mechanisms of intraleukocytic killing of bacteria. J Infect Dis. 1983 Aug;148(2):189–193. doi: 10.1093/infdis/148.2.189. [DOI] [PubMed] [Google Scholar]
- Rotstein O. D., Nasmith P. E., Grinstein S. The Bacteroides by-product succinic acid inhibits neutrophil respiratory burst by reducing intracellular pH. Infect Immun. 1987 Apr;55(4):864–870. doi: 10.1128/iai.55.4.864-870.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotstein O. D., Pruett T. L., Fiegel V. D., Nelson R. D., Simmons R. L. Succinic acid, a metabolic by-product of Bacteroides species, inhibits polymorphonuclear leukocyte function. Infect Immun. 1985 May;48(2):402–408. doi: 10.1128/iai.48.2.402-408.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotstein O. D., Pruett T. L., Simmons R. L. Lethal microbial synergism in intra-abdominal infections. Escherichia coli and Bacteroides fragilis. Arch Surg. 1985 Feb;120(2):146–151. doi: 10.1001/archsurg.1985.01390260016003. [DOI] [PubMed] [Google Scholar]
- Salt W. G., Samara M. A., Stretton R. J. The influence of incubation time and medium composition on fatty acid production by some Bacteroides species. Microbios. 1985;42(171S):273–285. [PubMed] [Google Scholar]
- Silver I. A., Murrills R. J., Etherington D. J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988 Apr;175(2):266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- Simon G. L., Klempner M. S., Kasper D. L., Gorbach S. L. Alterations in opsonophagocytic killing by neutrophils of Bacteroides fragilis associated with animal and laboratory passage: effect of capsular polysaccharide. J Infect Dis. 1982 Jan;145(1):72–77. doi: 10.1093/infdis/145.1.72. [DOI] [PubMed] [Google Scholar]
- Tally F. P., Goldin B. R., Jacobus N. V., Gorbach S. L. Superoxide dismutase in anaerobic bacteria of clinical significance. Infect Immun. 1977 Apr;16(1):20–25. doi: 10.1128/iai.16.1.20-25.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofte R. W., Peterson P. K., Schmeling D., Bracke J., Kim Y., Quie P. G. Opsonization of four Bacteroides species: role of the classical complement pathway and immunoglobulin. Infect Immun. 1980 Mar;27(3):784–792. doi: 10.1128/iai.27.3.784-792.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- Wade B. H., Kasper D. L., Mandell G. L. Interactions of Bacteroides fragilis and phagocytes: studies with whole organisms, purified capsular polysaccharide and clindamycin-treated bacteria. J Antimicrob Chemother. 1983 Oct;12 (Suppl 100):51–62. doi: 10.1093/jac/12.suppl_c.51. [DOI] [PubMed] [Google Scholar]
- West B. C., Rosenthal A. S., Gelb N. A., Kimball H. R. Separation and characterization of human neutrophil granules. Am J Pathol. 1974 Oct;77(1):41–66. [PMC free article] [PubMed] [Google Scholar]


