Abstract
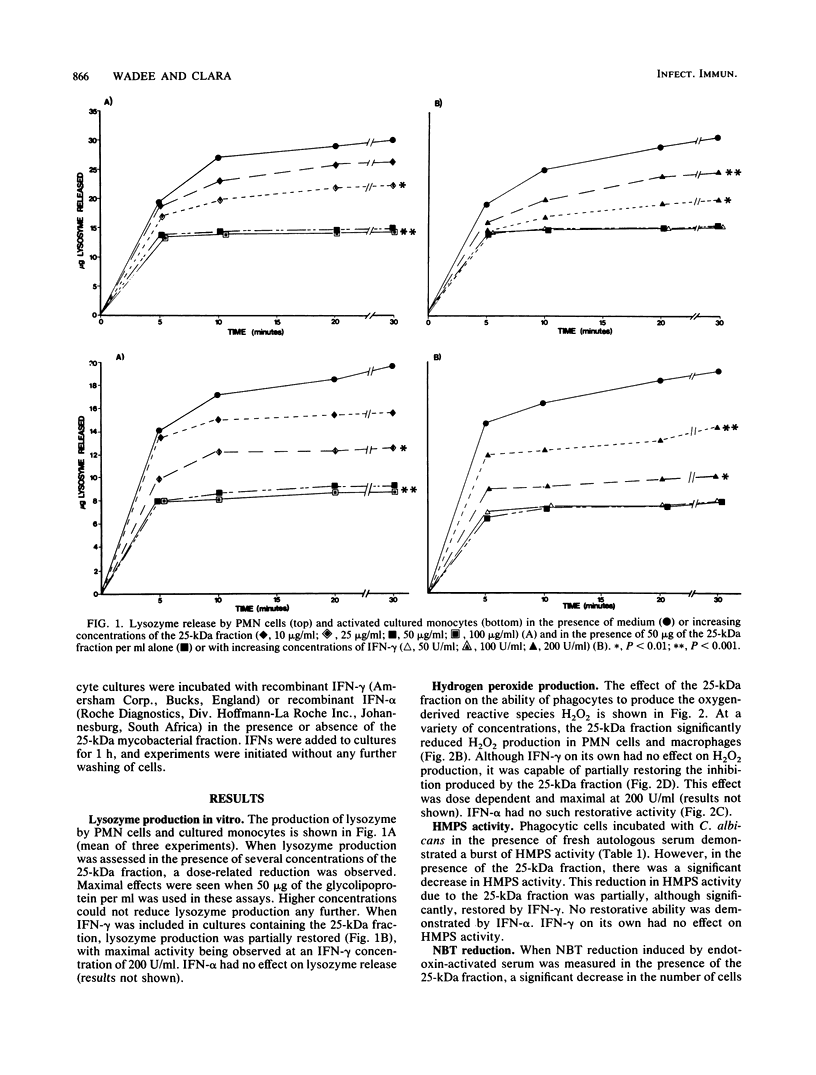
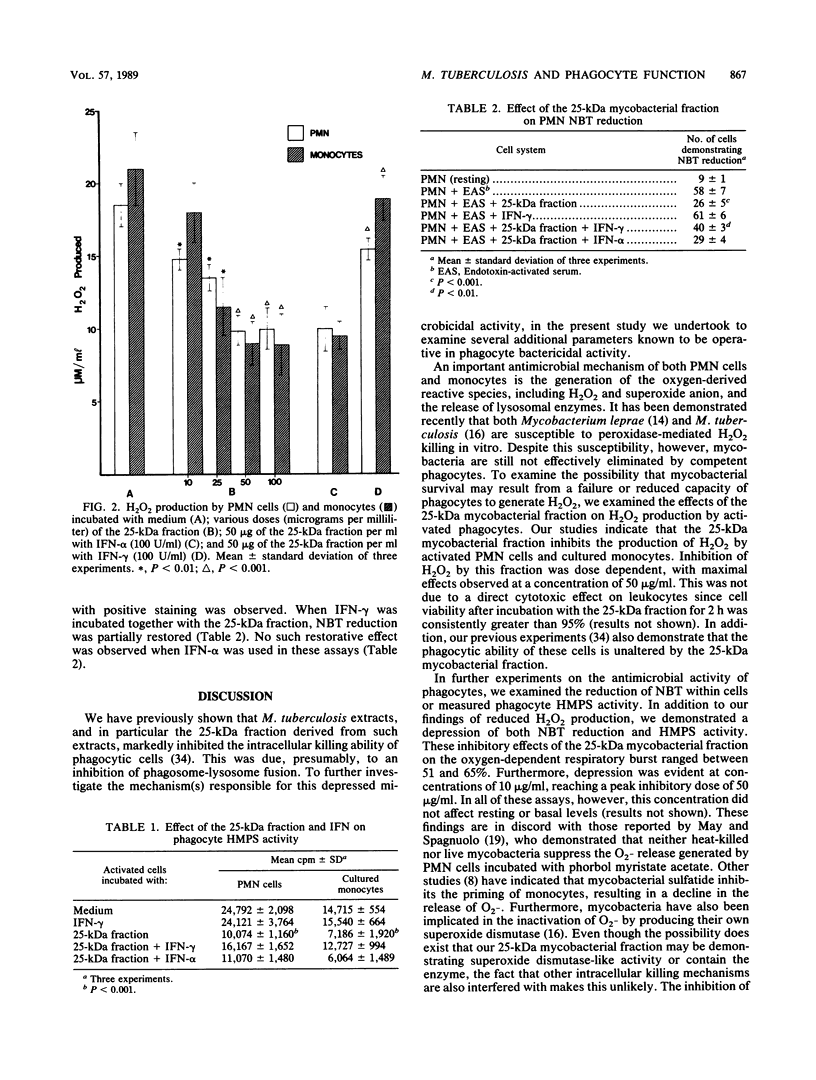
This study examined the effects of a 25-kilodalton (kDa) glycolipoprotein derived from Mycobacterium tuberculosis on phagocyte functions associated with antimicrobial activity. The 25-kDa fraction inhibited the ability of both polymorphonuclear cells and cultured monocytes to release lysozyme and produce hydrogen peroxide. In addition, the glycolipoprotein was capable of reducing hexose monophosphate shunt activity and interfered with the ability of polymorphonuclear cells to reduce Nitro Blue Tetrazolium. Inhibition of these antimicrobial systems was optimal at a 50-micrograms/ml concentration of the 25-kDa fraction. Gamma interferon, but not alpha interferon, partially reversed the inhibitory effect of the mycobacterial component in all of the systems assessed. These studies indicate important mechanisms in the understanding of the pathogenesis of tuberculosis and suggest that gamma interferon may have a therapeutic role in mycobacterial diseases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj N., Nash T. W., Horwitz M. A. Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1986 Oct 15;137(8):2662–2669. [PubMed] [Google Scholar]
- Bortolussi R., Vandenbroucke-Grauls C. M., van Asbeck B. S., Verhoef J. Relationship of bacterial growth phase to killing of Listeria monocytogenes by oxidative agents generated by neutrophils and enzyme systems. Infect Immun. 1987 Dec;55(12):3197–3203. doi: 10.1128/iai.55.12.3197-3203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Chaparas S. D. The immunology of mycobacterial infections. Crit Rev Microbiol. 1982;9(2):139–197. doi: 10.3109/10408418209104488. [DOI] [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Phagocyte strategy vs. microbial tactics. Rev Infect Dis. 1980 Sep-Oct;2(5):817–838. doi: 10.1093/clinids/2.5.817. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Hedegaard H. B., Zlotnik A., Gangadharam P. R., Johnston R. B., Jr, Pabst M. J. Chronic infection due to Mycobacterium intracellulare in mice: association with macrophage release of prostaglandin E2 and reversal by injection of indomethacin, muramyl dipeptide, or interferon-gamma. J Immunol. 1986 Mar 1;136(5):1820–1827. [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover D. L., Nacy C. A., Meltzer M. S. Human monocyte activation for cytotoxicity against intracellular Leishmania donovani amastigotes: induction of microbicidal activity by interferon-gamma. Cell Immunol. 1985 Sep;94(2):500–511. doi: 10.1016/0008-8749(85)90274-6. [DOI] [PubMed] [Google Scholar]
- Jackett P. S., Aber V. R., Lowrie D. B. Virulence and resistance to superoxide, low pH and hydrogen peroxide among strains of Mycobacterium tuberculosis. J Gen Microbiol. 1978 Jan;104(1):37–45. doi: 10.1099/00221287-104-1-37. [DOI] [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M. Studies of lysosomal enzymes in macrophages. III. Lysosomal enzyme activities in cultured macrophages infected with some species of mycobacteria. Hiroshima J Med Sci. 1981 Jun;30(2):99–107. [PubMed] [Google Scholar]
- Klebanoff S. J., Shepard C. C. Toxic effect of the peroxidase-hydrogen peroxide-halide antimicrobial system on Mycobacterium leprae. Infect Immun. 1984 May;44(2):534–536. doi: 10.1128/iai.44.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossack R. E., Guerrant R. L., Densen P., Schadelin J., Mandell G. L. Diminished neutrophil oxidative metabolism after phagocytosis of virulent Salmonella typhi. Infect Immun. 1981 Feb;31(2):674–678. doi: 10.1128/iai.31.2.674-678.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusunose E., Ichihara K., Noda Y., Kusunose M. Superoxide dismutase from Mycobacterium tuberculosis. J Biochem. 1976 Dec;80(6):1343–1352. doi: 10.1093/oxfordjournals.jbchem.a131407. [DOI] [PubMed] [Google Scholar]
- Lowrie D. B. The macrophage and mycobacterial infections. Trans R Soc Trop Med Hyg. 1983;77(5):646–655. doi: 10.1016/0035-9203(83)90193-1. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M. E., Spagnuolo P. J. Evidence for activation of a respiratory burst in the interaction of human neutrophils with Mycobacterium tuberculosis. Infect Immun. 1987 Sep;55(9):2304–2307. doi: 10.1128/iai.55.9.2304-2307.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokoena T., Gordon S. Human macrophage activation. Modulation of mannosyl, fucosyl receptor activity in vitro by lymphokines, gamma and alpha interferons, and dexamethasone. J Clin Invest. 1985 Feb;75(2):624–631. doi: 10.1172/JCI111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrvik Q. N., Leake E. S., Wright M. J. Disruption of phagosomal membranes of normal alveolar macrophages by the H37Rv strain of Mycobacterium tuberculosis. A correlate of virulence. Am Rev Respir Dis. 1984 Feb;129(2):322–328. [PubMed] [Google Scholar]
- Nandoskar M., Ferrante A., Bates E. J., Hurst N., Paton J. C. Inhibition of human monocyte respiratory burst, degranulation, phospholipid methylation and bactericidal activity by pneumolysin. Immunology. 1986 Dec;59(4):515–520. [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Gross J. M., Brozna J. P., Goren M. B. Inhibition of macrophage priming by sulfatide from Mycobacterium tuberculosis. J Immunol. 1988 Jan 15;140(2):634–640. [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., LeBlanc P. A., Murasko D. M. Comparative effects of various classes of mouse interferons on macrophage activation for tumor cell killing. J Immunol. 1985 Feb;134(2):977–981. [PubMed] [Google Scholar]
- Paton J. C., Ferrante A. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect Immun. 1983 Sep;41(3):1212–1216. doi: 10.1128/iai.41.3.1212-1216.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Harcus J. L., Symes P. H., Romito R., Donowitz G. R. Failure of the phagocytic oxidative response to protect human monocyte-derived macrophages from infection by Leishmania donovani. J Immunol. 1982 Sep;129(3):1282–1286. [PubMed] [Google Scholar]
- Ridley M. J., Oates C., Waters M. F., Ridley D. S. Lysozyme as a measure of cellular dynamics in the lesions of leprosy. Br J Exp Pathol. 1985 Feb;66(1):109–122. [PMC free article] [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Murray H. W. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J Immunol. 1983 Nov;131(5):2542–2544. [PubMed] [Google Scholar]
- Sharp A. K., Banerjee D. K. Effect of gamma interferon on hydrogen peroxide production by cultured mouse peritoneal macrophages. Infect Immun. 1986 Nov;54(2):597–599. doi: 10.1128/iai.54.2.597-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD H. G., KATZ J., LANDAU B. R. ESTIMATION OF PATHWAYS OF CARBOHYDRATE METABOLISM. Biochem Z. 1963;338:809–847. [PubMed] [Google Scholar]
- Wadee A. A., Cohen J. D., Rabson A. R. Gamma interferon reverses inhibition of leukocyte bactericidal activity by a 25-kilodalton fraction from Mycobacterium tuberculosis. Infect Immun. 1987 Nov;55(11):2777–2782. doi: 10.1128/iai.55.11.2777-2782.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadee A. A., Sher R., Rabson A. R. Production of a suppressor factor by human adherent cells treated with mycobacteria. J Immunol. 1980 Sep;125(3):1380–1386. [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]


