Abstract
Amyotrophic lateral sclerosis (ALS) is an incurable neurodegenerative disease that primarily affects motoneurons in the brain and spinal cord. Dominant mutations in superoxide dismutase-1 (SOD1) cause a familial form of ALS. Mutant SOD1-damaged glial cells contribute to ALS pathogenesis by releasing neurotoxic factors, but the mechanistic basis of the motoneuron-specific elimination is poorly understood. Here, we describe a motoneuron-selective death pathway triggered by activation of lymphotoxin-β receptor (LT-βR) by LIGHT, and operating by a novel signaling scheme. We show that astrocytes expressing mutant SOD1 mediate the selective death of motoneurons through the proinflammatory cytokine interferon-γ (IFNγ), which activates the LIGHT-LT-βR death pathway. The expression of LIGHT and LT-βR by motoneurons in vivo correlates with the preferential expression of IFNγ by motoneurons and astrocytes at disease onset and symptomatic stage in ALS mice. Importantly, the genetic ablation of Light in an ALS mouse model retards progression, but not onset, of the disease and increases lifespan. We propose that IFNγ contributes to a cross-talk between motoneurons and astrocytes causing the selective loss of some motoneurons following activation of the LIGHT-induced death pathway.
Keywords: amyotrophic lateral sclerosis, interferon-γ, LIGHT, astrocytes, motoneurons
Amyotrophic lateral sclerosis (ALS) is a devastating motoneuron disease, characterized by the selective and gradual degeneration of both upper and lower motoneurons. Approximately, 10% of ALS cases are inherited and among these, 20% are caused by dominant mutations in the superoxide dismutase-1 (SOD1) gene. Mice expressing human SOD1 mutations develop a motor syndrome with features of the human disease.1 Both cell-autonomous and non-cell-autonomous processes contribute to motoneuron degeneration: a toxic action of mutant SOD1 within motoneurons has been documented as crucial for the onset and the early phase of disease progression,2 whereas a non-cell-autonomous component, involving damage to astrocytes and microglia is determinant for disease progression.3 Astrocytes have a pivotal role in the pathogenic process by determining the extent of the inflammatory response from microglia,3 but also by releasing soluble factors selectively toxic for motoneurons.4, 5, 6, 7, 8 The specificity of this toxicity toward motoneurons might be explained by the activation of a motoneuron-specific death pathway; a hypothesis that has been tested in several studies.
Active killing of neurons by death receptors of the tumor necrosis factor (TNF) receptor superfamily, including TNFR1, p75NTR or Fas has been documented.9, 10 TNFα can efficiently trigger the death of cultured motoneurons,11 but may not directly participate to motoneuron degeneration in disease.12 Nerve growth factor in combination with nitric oxide (NO), produced by reactive astrocytes, has been proposed to induce a p75NTR-dependent motoneuron death in vitro,13 but conflicting results have not yet demonstrated a functional relevance of p75NTR in the direct killing of motoneurons in ALS models.9 We previously demonstrated that Fas triggers a motoneuron-restricted death pathway, which is exacerbated in a cell-autonomous manner by mutant SOD1.14, 15 Interestingly, a functional involvement of the Fas death pathway in motoneuron degeneration in mutant SOD1 mice has been shown.15, 16, 17 Regarding the pathogenic processes, the mutant astrocyte-mediated toxicity to motoneurons would occur independently of the Fas death pathway,8 suggesting that other sources, such as microglia or serum, activate Fas.14, 18 Our understanding of the selective degenerative process integrating external death triggers remains, however, incomplete.
LIGHT (TNFSF14) is a type II transmembrane protein of the TNF superfamily that can engage the lymphotoxin-β receptor (LT-βR), the herpes virus entry mediator (HVEM) and the decoy receptor 3. LIGHT, which is expressed by immature dendrocytes, activated lymphocytes, monocytes and natural killer cells, and is important for both innate and adaptive immune processes.19 Remarkably, LIGHT can function with the immunomodulatory cytokine interferon-γ (IFNγ) to induce a singular slow apoptotic death in tumor cells,20 reminiscent of the progressive nature of motoneuron degeneration in the disease.
Here, we report that the activation of LT-βR by LIGHT triggers a novel motoneuron-selective death pathway, which shows an additive killing potency with the activation of Fas. We demonstrate that IFNγ selectively induces death of motoneurons through the LIGHT-LT-βR pathway and mediates the neurotoxic effect of astrocytes expressing mutant SOD1. LIGHT and LT-βR are expressed by motoneurons both in control and mutant SOD1 mice, whereas expression of IFNγ is observed in motoneurons and astrocytes at the onset and symptomatic stage in ALS mice. Finally, deficiency of Light in ALS mice delays the progression, but not the onset of disease and extends life expectancy. We propose that besides its proinflammatory activity, IFNγ induces a motoneuron-specific LIGHT-dependent death pathway that contributes to the loss of motoneuron in ALS.
Results
LIGHT triggers a motoneuron-selective death pathway
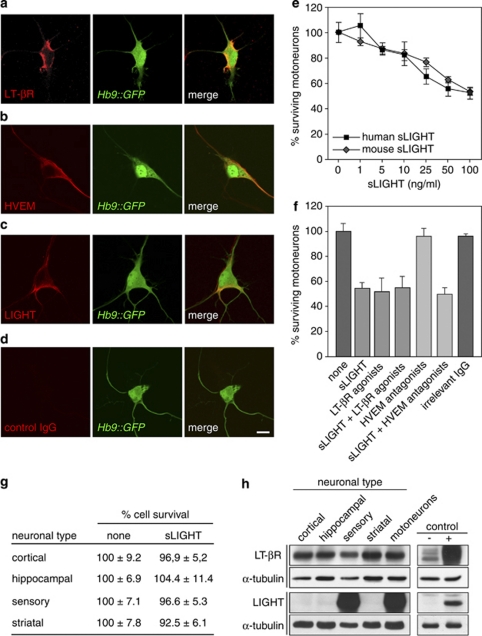
To investigate the potential role of LIGHT in triggering death of motoneurons, we first asked whether cultured motoneurons express LIGHT, LT-βR and HVEM. We isolated embryonic motoneurons from mice expressing the green fluorescent protein (GFP) under the control of the motoneuron-selective Hb9 promoter (Hb9∷GFP) to facilitate motoneuron tracing.8 We found that all motoneurons cultured for 24 h express LIGHT, LT-βR and HVEM (Figure 1a–d). We next exposed motoneurons for 48 h to increasing concentrations of mouse or human soluble LIGHT (sLIGHT) and assessed survival by counting phase-bright neurons using morphological criteria,14 or GFP-positive neurons isolated from Hb9∷GFP embryos. In both cases, we observed that mouse and human sLIGHT induce death of about 50% of motoneurons in a dose-dependent manner (Figure 1e and not shown). We next investigated motoneuron survival with respect to sLIGHT in a time-dependent manner. Cell survival was not significantly altered 24 h following sLIGHT addition, was diminished by about half after 48 h and was unchanged after 72 or 96 h of LIGHT treatment (Supplementary Figure 1a).
Figure 1.
sLIGHT selectively induces death of motoneurons. (a–d) Hb9∷GFP motoneurons were cultured for 24 h and immunostained with anti-LT-βR (a), anti-HVEM (b) and anti-LIGHT (c) antibodies. Goat (d) or rabbit (not shown) irrelevant IgGs were used as control. Scale bar, 10 μm. (e) Mouse motoneurons were cultured for 24 h and then incubated with increasing concentrations of human or mouse sLIGHT. Motoneuron survival was determined 48 h later and expressed relative to non-treated cells. Henceforth, sLIGHT will refer to the human form of the recombinant protein. The distance between the x-axis values is arbitrary. (f) After 24 h in culture, motoneurons were treated (or not) with agonistic anti-LT-βR antibodies (100 ng/ml), antagonistic anti-HVEM antibodies (100 ng/ml) or irrelevant goat IgG (100 ng/ml) in combination (or not) with sLIGHT (100 ng/ml). Cell survival was determined 48 h later. (g) Twenty four hours after plating, cortical, hippocampal, sensory and striatal neurons were treated (or not) with sLIGHT (100 ng/ml). Neuron survival was determined 48 h later. (h) Protein extracts from cortical, hippocampal, sensory, striatal and motoneurons cultured for 24 h were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis, followed by Western blotting with specific antibodies to LT-βR and LIGHT. The loading control was α-tubulin. Lysates from Cos-7 cells transfected (+) or not (−) with LT-βR or LIGHT expression vectors were used as controls. Graphs in (e), (f) and table in (g) show means values of three independent experiments, each done in triplicate
To ensure that the LIGHT killing effect functions specifically through LT-βR and/or HVEM, sLIGHT was challenged with soluble decoy receptors, including LT-βR-, HVEM-, Fas- or TNFR1-Fc chimeras. Although Fas- or TNFR1-Fc inhibited motoneuron death induced by their respective ligands, they did not show any inhibitory activity on sLIGHT. LT-βR-Fc and HVEM-Fc, both of which compete with sLIGHT, however, efficiently rescued motoneurons from LIGHT-induced death (Supplementary Figure 1b and c), supporting the specificity of the death signal triggered by LIGHT.
As LIGHT can engage both LT-βR and HVEM, it raised the question whether these receptors function cooperatively, or if not, which of both triggers motoneuron death. We show that agonistic anti-LT-βR antibodies show the same killing activity as sLIGHT, without unspecific additive toxicity when combined with sLIGHT (Figure 1f). On the contrary, antagonistic anti-HVEM antibodies21 did not inhibit LIGHT-induced death (Figure 1f). These results suggest that LT-βR activation is sufficient to induce death of motoneurons and that HVEM does not cooperate with LT-βR to mediate LIGHT killing effect.
The identification of LIGHT as a novel death-inducing ligand in motoneurons prompted us to investigate its target-cell specificity. When we examined whether cortical, hippocampal, sensory or striatal neurons were sensitive to LIGHT, we observed that none of these neuronal types was killed by sLIGHT (Figure 1g). This motoneuron-specificity to LIGHT cannot be attributed to the absence of expression of the death receptor, as all these neuronal types express substantial levels of LT-βR (Figure 1h), but is likely due to an intrinsic property of motoneurons.
Fas and LT-βR-triggered death are additive and differ in their signaling
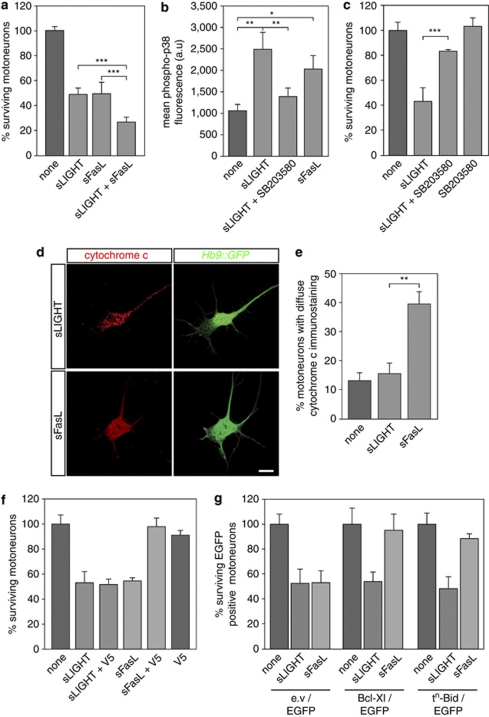
The activation of Fas that triggers death of about 50% of motoneurons,14 raised the question about its functional complementarity with LIGHT. Interestingly, LIGHT and FasL added together induced death of about 70% of motoneurons (Figure 2a). We next investigated whether LT-βR and Fas use the same signaling network. The p38 kinase controls crucial steps in Fas death signaling, including NO production through upregulation of the neuronal NO synthase (NOS) and cytochrome c release from mitochondria.14 We first determined the phosphorylation levels of nuclear p38 by quantitative confocal miscroscopy.15, 22 Treatment of motoneurons with sLIGHT, as well as sFasL, resulted in a significant increase in levels of p38 phosphorylation (Figure 2b and Supplementary Figure 2a). SB203580, a pyridinyl imidazole inhibitor of p38, prevented phosphorylation of p38, consistent with its inhibitory effect on the autophosphorylation-dependent activation of p38.23 In addition, we found that SB203580 blocked degeneration of motoneurons induced by sLIGHT (Figure 2c), supporting the role of p38 in the LIGHT death pathway. Interestingly, inhibition of NO production by the broad-spectrum NOS inhibitor L-NAME did not provide protection against LIGHT-induced death (Supplementary Figure 2b), although it saved motoneurons from death induced by trophic factor deprivation (Supplementary Figure 2c).
Figure 2.
sLIGHT and sFasL show additive killing effect but LIGHT in contrary to FasL functions independently of mitochondrial cytochrome c release. (a) Mouse motoneurons were cultured for 24 h and treated (or not) with sLIGHT (100 ng/ml), recombinant human soluble FasL (sFasL, 100 ng/ml in the presence of 1 μg/ml enhancer antibody), or a combination of both. Motoneuron survival was assessed 48 h after treatment. Note that the enhancer antibody used to cross-link sFasL had no effect on motoneuron survival when added alone or with sLIGHT (not shown)(n=4, ***P<0.001). (b) Quantification of phospho-p38 kinase fluorescence in Hb9∷GFP motoneurons treated (or not) with sLIGHT in the presence or the absence of SB203580 (10 μM) for 1 h. Treatment with sFasL served as a control.15 Confocal fluorescence imaging and ImageJ image analysis were used to determine the nuclear mean fluorescence intensity of p38 in Hb9∷GFP neurons (a.u, arbitrary unit, *P<0.05, **P<0.01). (c) Motoneurons were maintained in culture for 24 h and treated or not with sLIGHT (100 ng/ml) and SB203580 (10 μM). Survival was determined 48 h later and expressed relative to survival in the absence of any treatment. (d and e) Hb9∷GFP motoneurons were incubated or not with 100 ng/ml of sLIGHT or sFasL, fixed and immunostained with anti-cytochrome c antibody 30 h later. sLIGHT-treated Hb9∷GFP motoneuron shows the same punctuate labeling of mitochondria as non-treated motoneurons (not shown), whereas following Fas activation a proportion of motoneurons show diffuse labeling of cytochrome c. Scale bar, 10 μm. (e) The percentage of Hb9∷GFP motoneurons showing diffuse versus punctuated cytochrome c labeling was determined by direct counting under fluorescence microscope (n=4). (f) Survival assay was done as in (c). The Bax inhibitory peptide V5 was used at the concentration of 50 μM. (g) Mouse motoneurons were co-electroporated with an equimolar ratio of a vector coding for EGFP and a vector coding for either Bcl-Xl, a dominant negative form of Bid (tn-Bid) or an empty vector (e.v) and cultured for 24 h before being treated or not with sLIGHT or sFasL. The survival of EGFP-positive motoneurons for each combination of vectors was expressed as a percentage of surviving motoneurons in the absence of sLIGHT or sFasL. Histograms show mean values±S.D. of at least three independent experiments, each done in triplicate
We then examined the contribution of the mitochondrial pathway in the death program. Fas activation leads to a redistribution of cytochrome c from a punctuated mitochondrial to a diffuse cytoplasmic immunoreactivity in motoneurons.14 Interestingly, we did not observe a cytoplasmic redistribution of cytochrome c following sLIGHT treatment at 24, 30 or 36 h (Figure 2d and e and not shown). We next inhibited Bax, a major effector of cytochrome c release, using the inhibitory pentapeptide VPMLK (V5). Although V5 efficiently rescued motoneurons from Fas-induced death, V5 did not protect motoneurons against LIGHT-killing effect (Figure 2f). We also investigated the functional impact of the overexpression of two other factors acting at the mitochondrial checkpoint: Bcl-Xl and tn-Bid, a dominant negative form of Bid.24 Consistently, LIGHT-induced death signaling was not affected by the overexpression of either Bcl-Xl or tn-Bid in motoneurons in contrast to the Fas death pathway (Figure 2g). Thus, the LIGHT-LT-βR death pathway appears to proceed independently of cytochrome c release, suggesting that a previously unknown cascade of events is involved.
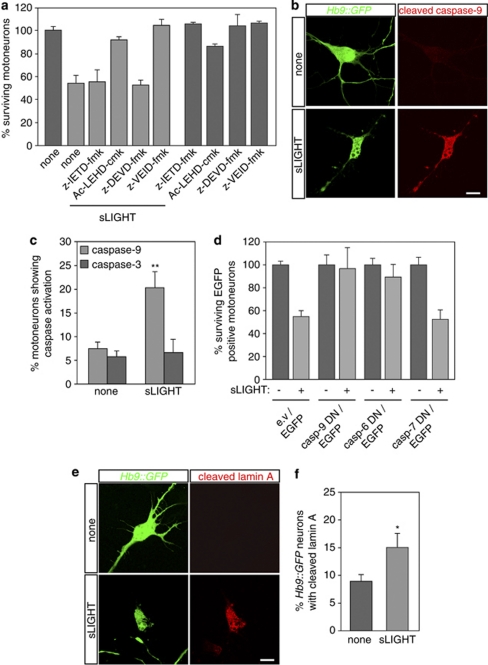
LIGHT triggers an unconventional caspase-dependent death process
Caspases are pivotal components in the apoptotic death program. We, therefore, explored the protective effect of inhibiting prototypic initiator (caspase-8 and -9) and effector (caspase-3, -7 and -6) caspases. We observed that motoneuron death induced by sLIGHT was not blocked by the caspase-8 inhibitor, z-IETD-fmk, which effectively blocked death induced by trophic factor deprivation or Fas activation (Figure 3a, Supplementary Figure 3a and not shown). However, Ac-LEHD-cmk, that inhibits caspase-9, rescued motoneurons from LIGHT-induced death (Figure 3a). Surprisingly, when we examined executioner caspases, we found that z-DEVD-fmk, which inhibits the caspase-3/-7 axis, did not save motoneurons from the sLIGHT killing effect (Figure 3a), whereas it efficiently rescued motoneurons from death induced by trophic factor deprivation or Fas activation (Supplementary Figure 3a and not shown). We also found that the caspase-6 inhibitor, z-VEID-fmk blocked death of motoneurons triggered by sLIGHT (Figure 3a). We further confirmed the requirement of caspase-9 independently of caspase-3 by examining their respective activation using specific antibodies against cleaved forms (Figure 3b and Supplementary Figure 3b). We detected a significant increase in caspase-9 activation, but not of caspase-3, in motoneurons following sLIGHT treatment (Figure 3c). We further examined whether the LIGHT-LT-βR pathway was functionally dependent on caspase-9 and -6 but independent of caspase-7 by overexpressing their catalytically inactive mutants that function as dominant-negative forms.25 Consistently, we showed that the caspase-9 and -6, but not caspase-7 mutants saved motoneurons from LIGHT-induced death (Figure 3d), whereas caspase-7 mutant saved motoneurons from Fas-induced death (Supplementary Figure 3c). Implication of caspase-6, but not of caspase-3/-7, was further confirmed using agonistic anti-LT-βR (Supplementary Figure 3d). Finally, we confirmed the functional implication of caspase-6 by probing its processed specific substrate, lamin A.26 Results showed that, although sLIGHT increased lamin A cleavage in motoneurons (Figure 3e and f), it was prevented by caspase-6, but not by caspase-3, inhibitor (Supplementary Figure 3e). Thus, LIGHT triggers a novel caspase-dependent signaling pathway in motoneurons.
Figure 3.
sLIGHT triggers motoneuron death through an unconventional caspase cascade. (a) Motoneurons were incubated with z-IETD-fmk (10 μM), Ac-LEHD-cmk (1 μM), z-DEVD-fmk (10 μM) or z-VEID-fmk (10 μM) added together with sLIGHT (100 ng/ml). Motoneuron survival was determined 48 h later and expressed relative to non-treated condition. (b) Activation of caspase-9 was visualized in sLIGHT-treated Hb9∷GFP motoneurons with antibodies specific to the cleaved form of caspase-9. (c) The percentage of Hb9∷GFP motoneurons immunopositive for active caspase-9 (b) or -3 (see Supplementary Figure 3b) was determined 30 h later following addition of sLIGHT by direct counting using fluorescence microscopy. Statistical attribute is shown for none versus sLIGHT-treated cells (n=3, **P<0.01). (d) Motoneurons were co-electroporated with a combination of expression vectors encoding EGFP and catalytically inactive mutants of caspase-9 (casp-9 DN), -6 (casp-6 DN) or -7 (casp-7 DN), and incubated in the presence or absence of sLIGHT. The effect of the dominant negative caspases on motoneuron survival was determined by counting the EGFP-positive motoneurons 48 h after treatment. e.v, empty vector. (e) The experimental procedure was performed as in (b) only that the percentage of motoneurons positive for cleaved lamin A was determined 38 h after treatment using an anti-cleaved lamin A antibody. Scale bars, 10 μm. (f) The percentage of cleaved lamin-A-positive Hb9∷GFP motoneurons for LIGHT-treated or untreated conditions was determined by counting under fluorescent microscope (n=3, *P<0.05). All values are expressed as the means±S.D. of three independent experiments
IFNγ induces motoneuron death in a LIGHT-dependent manner
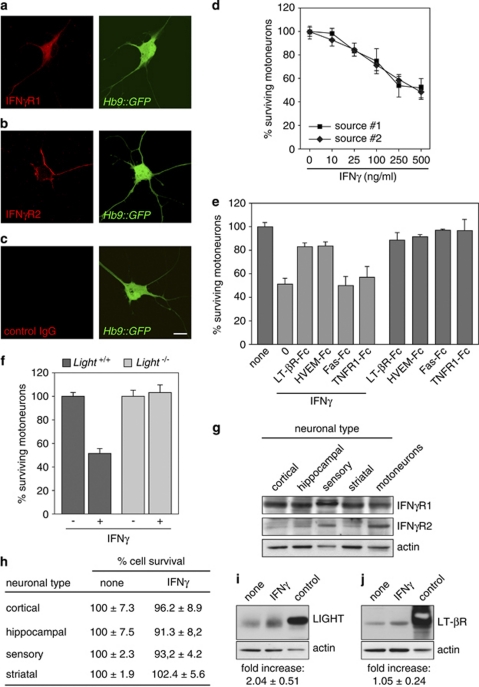
IFNγ is a cytokine known to function synergistically with LIGHT to activate caspases in tumor cells. Therefore, we investigated the influence of IFNγ on the modulation of the LIGHT-LT-βR system in motoneurons. We first demonstrated the expression of IFNγ receptor (IFNγR) chain 1 and 2 in nearly all motoneurons in vitro (Figure 4a–c). We next tested whether IFNγ was functionally relevant to LIGHT-induced death. By exposing motoneurons to a suboptimal dose of sLIGHT and increasing doses of IFNγ, we found that IFNγ substantially potentiates the LIGHT-killing effect (Supplementary Figure 4a), while having no effect on sFasL-induced death (Supplementary Figure 4b).
Figure 4.
IFNγ selectively kills motoneurons in a LIGHT-dependent manner. (a–c) Isolated Hb9∷GFP motoneurons were immunostained 24 h after seeding with antibodies directed against IFNγR1 (a), IFNγR2 (b) or with hamster (c) or mouse (not shown) irrelevant IgG as control. Scale bar, 10 μm. (d) Motoneurons were cultured for 24 h and incubated with increasing concentrations of soluble mouse recombinant IFNγ from two different sources. The percentage of surviving motoneurons was determined 48 h later. The distance between the x-axis values is arbitrary. (e) Motoneuron survival was determined 48 h following treatment or not with LT-βR-Fc (100 ng/ml), HVEM-Fc (10 ng/ml), Fas-Fc (1 μg/ml) or TNFR1-Fc (100 ng/ml) in combination or not with 250 ng/ml of IFNγ. The number of surviving motoneurons is expressed as a percentage of the number of motoneurons in the control condition (none). (f) Motoneurons were isolated from E12.5 embryos of indicated genotype and cultured for 24 h before being treated with 250 ng/ml of IFNγ. Motoneuron survival was determined 48 h later and expressed relative to the non-treated condition for each genotype. (g) Immunoblot analysis of cortical, hippocampal, sensory, striatal and motoneurons proteins separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Actin was used as a loading control. (h) Cell survival assay was performed as in (d), with neuronal cells being treated with 250 ng/ml of IFNγ. (i and j) Motoneurons were cultured for 24 h and incubated with 250 ng/ml of IFNγ. Eight hours later, cells were lysed and expression levels of LIGHT (i) and LT-βR (j) were determined by western blotting with indicated antibodies. Fold-increase of LIGHT and LT-βR over non-treated conditions was determined by densitometric analysis of immunoreactive bands, normalized to their respective actin signals (n=3, means±S.D.). Results shown in (d, e, f and h) are the mean values±S.D. of three independent experiments performed in triplicate
We then found that IFNγ induced death of about 50% of motoneurons in a dose-dependent manner (Figure 4d). The similarity with the loss of half of the motoneurons and the slow death kinetics of LIGHT-induced death prompted us to examine whether IFNγ-triggered death involved the LIGHT-LT-βR pathway. We thus competed endogenous LIGHT with LT-βR or HVEM-Fc and showed that both decoys saved motoneurons from the killing effect of IFNγ, whereas neither Fas- nor TNFR1-Fc conferred any protective effect (Figure 4e). To further argue for a LIGHT-dependent mechanism, we showed that inhibition of caspase-6, but not caspase-3, saved motoneurons from IFNγ-induced death (Supplementary Figure 4c). Finally, we investigated whether the IFNγ responsiveness was altered in Light-deficient motoneurons.27 We found that motoneurons isolated from Light−/− embryos are resistant to IFNγ-killing effect (Figure 4f), confirming that IFNγ triggers motoneuron death in a LIGHT-dependent manner.
To assess whether IFNγ could trigger death of other neuronal types through a LIGHT-independent pathway, we examined the responsiveness of cortical, hippocampal, sensory and striatal neurons to IFNγ. After ensuring that all these neurons express both IFNγ receptors (Figure 4g), we exposed them to an efficient dose of IFNγ or to the potent combination of IFNγ with sLIGHT, and observed that none of them were sensitive to IFNγ (Figure 4h and Supplementary Figure 4d).
The ability of IFNγ to activate the LIGHT-LT-βR pathway raised the possibility that IFNγ might enhance expression levels of LIGHT and/or LT-βR. Densitometric analysis of LIGHT and LT-βR immunoblots revealed that IFNγ significantly increased levels of LIGHT, but not of LT-βR, in motoneurons (Figure 4i and j). Altogether, these findings implicate a mechanism in which IFNγ specifically triggers a death program in motoneurons by eliciting the LIGHT-LT-βR pathway.
ALS mutant astrocytes selectively kill motoneurons through the IFNγ/LT-βR pathway
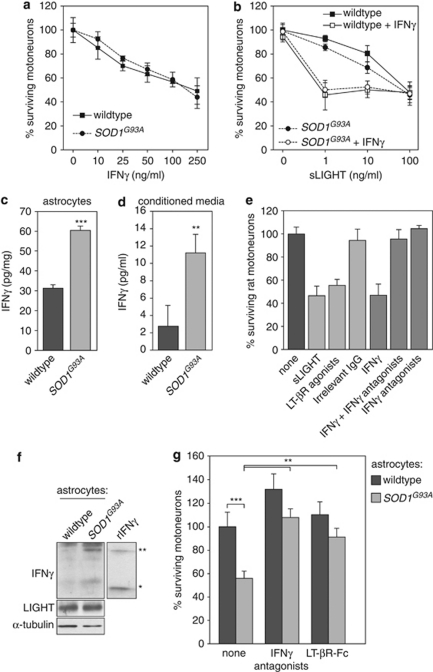
The motoneuron selectivity of IFNγ-triggered LIGHT-mediated death prompted us to investigate whether it has a function in the selective loss of motoneurons in a pathological context. We cultured motoneurons from mice overexpressing the G93A SOD1 mutation and asked whether mutant motoneurons show a differential susceptibility to IFNγ and LIGHT compared with wild-type motoneurons. Dose–response curves of IFNγ or its synergistic combination with sLIGHT on mutant motoneurons did not differ from those of wild-type motoneurons (Figure 5a and b), showing that the SOD1G93A mutant does not exacerbate the IFNγ and LIGHT effect.
Figure 5.
SOD1 mutant astrocytes kill motoneurons in an IFNγ/LIGHT-dependent pathway. (a) Twenty four hours after plating, indicated concentrations of mouse recombinant IFNγ were added to motoneurons isolated from SOD1G93A or wild-type embryos of the same littermate. Motoneuron survival was determined 48 h later and expressed relative to non-treated condition of corresponding genotype. (b) Mutant SOD1G93A and wild-type motoneurons were treated after 24 h in culture with increasing concentrations of sLIGHT in combination or not with IFNγ (10 ng/ml). Motoneuron survival was determined 48 h after treatment. (c and d) IFNγ levels in extracts (c) and conditioned media (d) of astrocytes of indicated genotype were quantified by ELISA (n=4, means±S.D.). (e) Immunopurified E14 rat motoneurons were treated or not with sLIGHT (100 ng/ml), agonistic anti-LT-βR antibodies (100 ng/ml), irrelevant IgG (100 ng/ml), recombinant soluble rat IFNγ (250 ng/ml) in combination or not with antagonistic anti-IFNγ (500 ng/ml) or anti-IFNγ antibodies alone. Survival of motoneurons was determined 48 h after treatment by direct counting. (f) Total protein extract of wild-type and SOD1G93A rat astrocytes were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by immunoblotting with anti-IFNγ specific antibodies. Recombinant rat IFNγ was used as a control. Asterisks indicate the monomeric (*) form of IFNγ and the apparent stable dimeric (**) biologically active form of IFNγ. (g) Wild-type motoneurons were plated on astrocyte monolayer of indicated genotype (wild type, SOD1G93A) and incubated or not with function-blocking anti-IFNγ antibodies (500 ng/ml) or LT-βR-Fc (100 ng/ml) for 48 h. Survival of motoneurons is expressed as the percentage of the number of motoneurons surviving on wild-type astrocyte monolayer in the absence of any treatment. The graphs show the mean values±S.D. of at least three independent experiments performed in triplicate
Coculture systems have demonstrated that mutant astrocytes release factors that selectively trigger death of motoneurons, independently of the expression of mutant SOD1 in neurons.5, 7, 8 Interestingly, we found that levels of IFNγ, as determined by ELISA, were significantly augmented in SOD1G93A astrocytes and in their corresponding conditioned media compared with wild-type cells (Figure 5c and d). We, therefore, investigated the involvement of the IFNγ-induced LIGHT-mediated death pathway in mutant astrocyte-conferred neurotoxicity. We used a coculture system of highly purified wild-type rat motoneurons and rat astrocyte monolayers of different genotypes.4 Firstly, we demonstrated that rat motoneurons are as responsive as mouse motoneurons to sLIGHT, agonistic anti-LT-βR antibodies and IFNγ (Figure 5e). We next found that, in contrast to the wild type, SOD1G93A astrocytes express substantial levels of IFNγ, whereas we did not observe any difference in LIGHT levels (Figure 5f). We then investigated the effect of mutant astrocyte-derived IFNγ on motoneuron survival. After 48 h of coculture, about 50% of wild-type motoneurons plated on SOD1G93A astrocytes died compared with motoneurons cultured on wild-type astrocytes (Figure 5g). Motoneurons cocultured with wild-type and SOD1G93A astrocytes were then treated with neutralizing anti-IFNγ antibodies that we had first validated as functional by saving motoneurons from IFNγ-induced death (Figure 5e). We found that inhibition of IFNγ activity substantially prevented the death of motoneurons induced by mutant astrocytes (Figure 5g). Consistently, inhibition of the LIGHT-LT-βR death pathway significantly increased the percentage of surviving motoneurons cultured with SOD1G93A astrocytes (Figure 5g). Collectively, these results suggest that an IFNγ-induced motoneuron-selective death is involved in the astrocytic neurotoxicity conferred by mutant SOD1.
Evidence of a role for IFNγ in the cross-talk between motoneurons and astrocytes in ALS
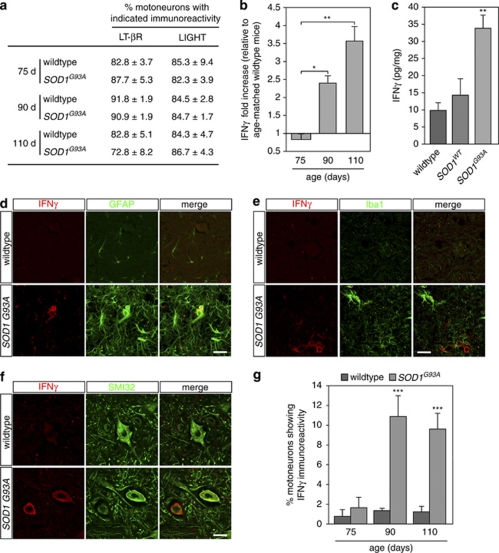
We next sought evidence for the involvement of the IFNγ-LIGHT-LT-βR pathway in SOD1G93A transgenic mice. We examined the expression of LT-βR and LIGHT by lumbar spinal cord motoneuron of SOD1G93A at presymptomatic (75 days), early onset (90 days) and symptomatic (110 days) stages and of age-matched wild-type mice. We observed that in wild-type and SOD1G93A mice the vast majority of motoneurons, identified by their selective expression of vesicular acetylcholine transferase (VAChT), constitutively express both LT-βR and LIGHT (Figure 6a and Supplementary Figure 5a and b).
Figure 6.
IFNγ is upregulated in spinal cords of ALS mice. (a) The percentage of motoneurons, as identified with VAChT immunostaining on adjacent sections (Supplementary Figure 5a and b), immunoreactive for LIGHT and LT-βR was determined in lumbar spinal cord of wild-type and SOD1G93A mice at 75, 90 and 110 days (d) of age. (b) Total protein extracts from lumbar spinal cords of wild-type and SOD1G93A mice at indicated age were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and probed with antibodies to IFNγ and actin (Supplementary Figure 5c). IFNγ signals were quantified, normalized to actin signals and expressed as the ratio of SOD1G93A to wild-type values. (c) ELISA quantification of IFNγ levels in dissociated spinal cord of 110-day-old wild-type and SOD1G93A mice and 365-day-old SOD1WT mice. Values in (a–c) are means±S.D., n=3. (d and f) Immunostainings of wild-type and SOD1G93A mice lumbar spinal cord sections at 90 days of age using antibody against IFNγ in combination with either GFAP (d), Iba1 (e) or SMI32 (f). Scale bar, 30 μm. (g) Lumbar spinal cord sections of wild-type and SOD1G93A mice were immunostained as in (f) at 75, 90 and 110 days of age and the percentage of IFNγ immunoreactive motoneurons was determined by counting under fluorescent microscope (75 days, n=3; 90 days, n=4; 110 days, n=5, values are means±S.D.)
We next monitored the expression of IFNγ during the course of the disease. We observed by western blot analysis that, although barely detectable at presymptomatic stage and indistinguishable from those seen in non-transgenic mice, levels of IFNγ in the spinal cord of SOD1G93A significantly increased at early disease onset and were further enhanced at symptomatic stages compared with wild-type mice (Figure 6b and Supplementary Figure 5c). As measured by ELISA, IFNγ was found at higher levels in dissociated spinal cords of symptomatic SOD1G93A mice compared with wild type or with mice overexpressing non-pathogenic SOD1WT (Figure 6c). We then questioned the identity of cells expressing IFNγ in SOD1G93A mice. Consistent with previous results (Figure 6b), we were not able to detect IFNγ in the spinal cord of both wild-type and presymptomatic mutant SOD1 mice. Interestingly, at early onset and symptomatic stages, IFNγ was readily detected in astrocytes (GFAP+), but not in microglial cells (Iba1+) (Figure 6d and e). It is to be noted that, IFNγ was also detected in spheroid GFAP+ cells reminiscent of degenerative astrocytes in spinal cords of SOD1G93A mice at 90 and 110 days of age28 (Supplementary Figure 5d). The only other cell type positive for IFNγ at early onset and symptomatic stages were motoneurons (Figure 6f). Consistently, we found that the percentage of motoneurons immunoreactive for IFNγ increased significantly at 90 and 110 days compared with 75-day-old SOD1G93A and age-matched wild-type mice (Figure 6g). A similar increase in IFNγ was observed in symptomatic SOD1G85R mice, whereas SOD1WT showed no increase in IFNγ either in motoneurons or glial cells (Supplementary Figure 5e). Expression of IFNγ by mutant astrocytes and motoneurons, at onset and symptomatic stages, suggests that IFNγ potentially contributes to the progression rather than the onset of motoneuron disease.
Genetic ablation of Light in SOD1G93A mice retards disease progression
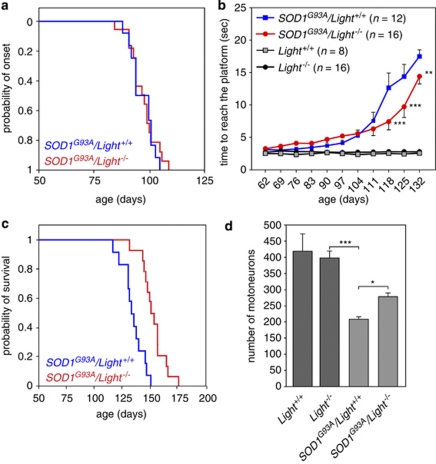
To evaluate the functional involvement of the IFNγ-LIGHT-LT-βR pathway in ALS pathogenesis, we genetically deleted Light in mice overexpressing SOD1G93A by cross-breeding. Light-deficient mice are viable and fertile with no behavioral abnormalities.27 We ensured that the expression levels of SOD1 mutant in the spinal cords of SOD1G93A/Light+/+ and SOD1G93A/Light−/− mice were similar (not shown). The time of disease onset in SOD1G93A/Light+/+ and SOD1G93A/Light−/− was determined by the time of peak weight (Supplementary Figure 6).2 We did not observe any significant difference between SOD1G93A/Light+/+ and SOD1G93A/Light−/− mice (97±1.7 days and 97.2±1.6 days, respectively, mean±S.E.M., log-rank test P=0.7765) (Figure 7a). To investigate the impact of Light deletion on disease progression, the swimming performance was evaluated weekly in the cohorts of Light+/+, Light−/−, SOD1G93A/Light+/+ and SOD1G93A/Light−/− mice.29 We found that Light deficiency significantly retarded the decline of motor function in ALS mice (Figure 7b). Furthermore, the loss of body weight was significantly slowed in SOD1G93A/Light−/− compared with SOD1G93A/Light+/+ mice (Supplementary Figure 6). We also observed that deletion of Light increases the lifespan of SOD1 mutant mice by 17.9 days (SOD1G93A/Light+/+, 135.5±2.8 days and SOD1G93A/Light−/−, 153.4±2.9 days, mean±S.E.M., log-rank test ***P=0.0002) (Figure 7c). We next investigated whether the amelioration in motor performance of Light-deficient SOD1 mice was associated with an increase in motoneuron survival. We quantified the number of surviving motoneurons on VAChT-immunostained sections, taken from the lumbar region of 120-day-old mice spinal cords of different genotypes. We observed that Light ablation in SOD1G93A mice leads to a significant increase in the number of surviving motoneurons compared with the marked loss seen in SOD1 mutant mice (Figure 7d). These results indicate that LIGHT contributes to disease progression, but not disease onset, in ALS mice.
Figure 7.
Targeted deletion of Light in ALS mice delays progression but not onset of the disease. (a) The cumulative probability of onset of SOD1G93A/Light+/+ (n=12) and SOD1G93A; Light−/− (n=16) was determined by the peak of weight curve. (b) The progressive motor deficit of SOD1G93A/Light+/+, SOD1G93A/Light−/−, Light+/+ and Light−/− was determined by evaluating weekly the swimming performance of mice (values are means±S.E.M.). Statistical attributes are shown only for SOD1G93A/Light+/+ versus SOD1G93A/Light−/− (Supplementary Table). (c) Kaplan–Meier survival curves for SOD1G93A/Light+/+ (n=12) and SOD1G93A/Light−/− (n=14) mice. (d) The mean motoneuron survival was determined by counting the number of VAChT-immunostained motoneurons in 28 sections of lumbar spinal cord from 120-day-old Light+/+ (n=3), Light−/− (n=3), SOD1G93A/Light+/+ (n=4) and SOD1G93A/Light−/− (n=4) (values are means±S.D.)
Discussion
Our study reveals a novel death signaling pathway triggered by the activation of LT-βR by LIGHT, which appears restricted to motoneurons and can be triggered by IFNγ delivered by astrocytes expressing mutant SOD1. We provide evidence that strongly support the potential role of this death pathway in the progression of motoneuron disease.
The selective vulnerability of motoneurons to mutant astrocyte-derived toxicity has been recapitulated in cocultures of rat, mouse or human embryonic stem-cell-derived motoneurons and astrocytes expressing different SOD1 mutations.4, 5, 7, 8 Among the potential actors of the neurotoxicity of mutant astrocytes, proinflammatory molecules have been proposed. In particular, it has been suggested that prostaglandin D2 (PGD2) contributes to astrocyte-derived toxicity.5 Interestingly, it has been demonstrated that the genetic or pharmacological inhibition of the PGD2/PGD2 receptor signaling markedly reduced astrogliosis,30 raising the question whether PGD2 function directly on motoneurons as a death trigger and/or indirectly by consolidating the inflammatory phenotype of mutant astrocytes. In the present study, we show that inhibition of IFNγ activity by antagonist antibodies greatly improves the survival of motoneurons cocultured with mutant astrocytes, whereas blocking the interaction between LIGHT and LT-βR by LT-βR-Fc produced a more moderate improvement in motoneuron survival. This difference in efficacy could be attributed to the proinflammatory activity of IFNγ in astrocytes. Accumulating evidence suggests that IFNγ induces and/or functions in combination with reactive oxygen species (ROS) to potently activate the astrocytic reaction. It has been demonstrated that IFNγ induces NO production and increases levels of GFAP in primary astrocytes.31 Expression of SOD1G93A or SOD1G37R in astrocytes is accompanied by increased levels of ROS, including NO,4, 7 and expression of SOD1G93A in glioblastoma leads to the production of IFNγ.32 How IFNγ and ROS cooperate in the acquisition or the consolidation of the reactive status of astrocytes expressing SOD1 mutations remains to be elucidated.
In this study, we show that astrocytes derived from the ALS rat or mouse models also produce IFNγ, which can directly trigger death of motoneurons. In contrast, it has been shown that exposure of freshly isolated motoneurons to IFNγ fails to alter neuron survival.33 In our survival experiments, motoneurons were cultured for at least 24 h before treatment and indeed we observed that freshly isolated motoneurons were resistant to IFNγ or LIGHT (not shown), suggesting that motoneurons need at least 24 h to acquire competence to die through IFNγ and LIGHT. In the light of these studies, IFNγ can be proposed as a key player of toxicity of mutant astrocytes by directly promoting death of motoneurons through LIGHT and by promoting release of other glial-derived neurotoxic factors.
Toxic mechanisms elicited by mutant astrocytes to promote death of motoneurons might involve additional effectors, such as those acting through Bax activation.10 Indeed, Nagai et al.,8 showed that mutant astrocytes kill embryonic stem-cell-derived motoneurons in a Bax-dependent manner. The discrepancies observed between our coculture system and that of those authors could be related to the different species origin (mouse or rat) and motoneuron sources (primary or ES), as well as the use of different culture conditions (timeframe, cell density and so on). The implication of a Bax-dependent pathway is consistent with some evidence demonstrating that the classical mitochondrial pathway has a function in the pathogenic process of motoneuron disease.1, 18 However, several arguments indicate that alternative cell death mechanisms may participate in the neurodegenerative process: overexpression of Bcl-2 in mutant SOD1 mice delays the onset but has no effect on the duration of the disease;34 the targeted deletion of caspase-11 in SOD1G93A mice, which results in a marked reduction of caspase-3 activity, failed to prevent neurodegeneration;35 Bax deletion in mutant SOD1 mice does not prevent neuromuscular denervation and the fatal outcome of the disease.36 Here, we show that ablating LIGHT, which triggers death of motoneurons following a differential mitochondrial execution phase, delays progression of the disease and increases motoneuron survival in SOD1G93A mice. We, therefore, propose that the diversity of motoneurons regarding their functional identity or their selective vulnerability to disease may be related to the way in which they execute a death sentence.
Around 20 years ago, IFNγ immunoreactivity was observed in motoneurons after axotomy and was proposed to induce glial reaction.37 In our experimental conditions, motoneurons and astrocytes were the only identifiable intraparenchymal sources of IFNγ, which strikingly attests to the cross-talk between motoneurons and astrocytes in motoneuron disease. Our results are also corroborated by a study showing an increase in IFNγ levels in SOD1G93A spinal cords at end-stage.38 It is to be noted that, increased levels of IFNγ have been detected in the serum of ALS patients as the disease progresses,39 and we found increased serum levels of IFNγ in SOD1G93A mice at symptomatic stage of the disease compared with age-matched wild-type or SOD1WT mice (Supplementary Figure 7). Recently, IFNγ levels were also found significantly increased in the cerebrospinal fluid (CSF) of sporadic ALS patients compared with patients with non-inflammatory neurological diseases.40 This evidence supports a potential role of IFNγ, provided by motoneurons, serum, CSF and/or astrocytes, in the pathogenic process (Figure 8). IFNγ could participate in an inflammatory cascade that would also include the activation of microglial cells, as IFNγ has been shown to enhance microglial cell activity in vivo.41 Whether IFNγ directly activates microglial cells or mediates astrocytic-dependent microglia activation remains to be determined. Moreover, activated microglia could participate with reactive astrocytes in the chronic production of neurotoxic molecules that could function through different mechanisms in different motoneuron populations (Figure 8). Recently, interleukin (IL)-1β has been proposed to promote a microglial-mediated neuroinflammation process contributing to disease progression in SOD1G93A mice.42 In our study, the timing of IFNγ expression in SOD1G93A mice suggests that IFNγ participates in the progression rather than the onset of disease, and Light deletion in SOD1G93A mice delayed progression, but not onset, of motoneuron disease. Cooperation between IFNγ and IL-1β provides a potential pathological mechanism for initiating and/or expanding the neuroinflammatory response in ALS.
Figure 8.
Model for the non-cell-autonomous effect of mutant SOD1 on the activation of motoneuron selective death pathways. IFNγ produced by mutant astrocytes can selectively trigger death of some motoneurons through activation of the LIGHT-LT-βR pathway. IFNγ from circulating blood (serum) and the cerebrospinal fluid (CSF)40 may also contribute to this neurodegenerative process. Motoneurons might represent an additional source of IFNγ that could participate to the inflammatory process. In grey, neurotoxic factors, including NO, or members of the TNF family, such as FasL, produced by mutant microglial cells and/or astrocytes, could also participate in the elimination of motoneurons in the disease.10, 14 Vulnerable motoneurons, having distinct intrinsic features, might be differentially susceptible to these non-cell-autonomous death triggers
Collectively, the motoneuron-selective IFNγ-induced LIGHT-mediated death pathway we describe would represent a non-cell-autonomous pathogenic mechanism that contributes to the progression phase of the disease. To complete our understanding of the role of the IFNγ-LIGHT pathway in motoneuron disease and the development of pertinent therapeutic strategies, it will be important to conduct a detailed analysis of the cell-type-specific genetic ablation of IFNγ in mutant SOD1 mice, as well as a correlative study between expression pattern/levels of IFNγ and clinical parameters in ALS patients.
Materials and Methods
Animals
All animal experiments were approved by the National Ethics Committee on Animal Experimentation, and were done in compliance with the European Community and National Directives for the Care and Use of Laboratory Animals. Hb9∷GFP mice (TM Jessell's laboratory, Columbia University, NY, USA) were maintained on a CD1 background (Charles River laboratories, Wilmington, MA, USA).43 SOD1G93A mice were maintained on a B6SJL background (Jackson laboratories, Bar Harbor, ME, USA).44 SOD1G85R (DW Cleveland's laboratory, University of California, San Diego, CA, USA), SOD1WT (S Przedborski's laboratory, Columbia University, NY, USA) and Light−/− mice (K Pfeffer's laboratory, Heinrich-Heine University, Düsseldorf, Germany) mice were maintained on a C57BL/6 background.27, 45, 46 Sprague-Dawley SOD1G93A L26H rats (DS Howland, Wyeth Research, Princeton, NJ, USA) were maintained as described.4
Cell cultures
Motoneurons from E12.5 spinal cord of CD1, Hb9∷GFP, Light−/− or SOD1G93A embryos were isolated as described47 modified by,14 using iodixanol density gradient centrifugation. Motoneurons were plated on poly-ornithine/laminin-treated wells in the presence (or not when mentioned) of a cocktail of neurotrophic factors (0.1 ng/ml glial-derived neurotrophic factor (GDNF), 1 ng/ml brain-derived neurotrophic factor (BDNF) and 10 ng/ml ciliary neurotrophic factor (CNTF)) in supplemented neurobasal medium. When needed, motoneurons were electroporated before plating with the indicated expression constructs as we previously described.14 For survival assays, between 60 and 130 motoneurons in 2-cm diameters of each well were counted as described previously.14, 47 In our conditions, the number of living motoneurons remained relatively constant between 24 and 96 h of culture: if 24 h is set at 100% surviving motoneurons, 48 h, 96.1±6% 72 h, 91.2±4.5% and 96 h, 92.9±6.6% (means±S.D., n=4). To allow for comparison of values from different experiments, survival values were expressed relative to the value in the presence of neurotrophic factors only (taken as 100%). Motoneurons from E14 rat embryos were immunopurified using Ig192 mouse monoclonal anti-p75 antibody as previously described4 and cultured in supplemented neurobasal medium in the presence of GDNF (0.1 ng/ml). Motoneuron cultures were free of GFAP1+ or Iba1+ glial cells. Cortical, hippocampal, dorsal root ganglion neurons and striatal neurons were isolated from E17.5 embryos as described.14, 48 Cortical, hippocampal, sensory and striatal neurons were plated on poly-ornithine/laminin-treated wells and cultured in neurobasal medium complemented with 1 mM sodium pyruvate, 2% B27 supplement (Invitrogen, Carlsbad, CA, USA) at the exception of sensory neurons that were maintained in the same supplemented neurobasal medium used for motoneurons but in the presence of 100 ng/ml nerve growth factor instead of GDNF, BDNF and CNTF. Unless otherwise indicated, cell survival experiments were done on neurons isolated from CD1 mice. All neuronal types were seeded at the density of 1500 cells/cm2 and surviving neurons were directly counted under light or fluorescence microscopy. Cos-7 cells were maintained in Dulbecco Modified Eagle Medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS). For expression analysis of FLAG-tagged LT-βR and HA-tagged LIGHT (D Olive's laboratory, Inserm U891, Marseille, France), cells were transfected using Fugene 6 following manufacturer's instruction (Roche diagnostics, Indianapolis, IN, USA).
Proteins and chemicals for survival assay
Soluble human recombinant LIGHT and FasL, enhancer antibodies used for aggregating tagged sFasL, human HVEM-Fc, LT-βR-Fc, Fas-Fc, TNFR1-Fc, LG-nitro--arginine-methyl ester.HCI and LG-nitro--arginine-methyl ester.HCI were purchased from Alexis Biochemicals (San Diego, CA, USA). Soluble mouse recombinant LIGHT, functional goat polyclonal anti-LT-βR anti-HVEM antibodies were purchased from R&D systems (Minneapolis, MN, USA). Soluble mouse recombinant IFNγ (source no. 1), the pyridinyl imidazole p38 inhibitor SB203580, and the caspase inhibitors z-IETD-fmk, Ac-LEHD-cmk, Z-DEVD-fmk were purchased from Calbiochem (San Diego, CA, USA). Recombinant rat TNFα was from BD Biosciences (Franklin Lakes, NJ, USA). Soluble mouse recombinant IFNγ (source no. 2) and soluble recombinant rat IFNγ were from PBL Biomedical laboratories (Piscataway, NJ, USA). z-VEID-fmk, Bax inhibitor peptide V5, neutralizing goat polyclonal anti-IFNγ antibodies (I5027) were purchased from Sigma-Aldrich (St Louis, MO, USA).
Expression constructs
Expression of enhanced GFP (EGFP), Bcl-Xl, tn-Bid, the catalytically inactive caspase-9C287A and caspase-7C186A, were placed under the control of the cytomegalovirus promoter. The active site mutant caspase-6C163S was introduced by PCR-based site-directed mutagenesis with the following primer (sense): 5′-TTATCATTCAGGCATCTCGGGGAAAC-3′ using the pcDNA3.1-caspase-6 as template (Quickchange site-directed mutagenesis kit, Stratagen, La Jolla, CA, USA). The construct was checked by sequencing.
Immunocytochemistry
Hb9∷GFP motoneurons were seeded on poly-ornithine/laminin-treated glass coverslips at the density of 5000 cells/cm2 and cultured in the supplemented neurobasal medium as above. At indicated time, neurons were processed for immunocytochemistry as previously described.14, 15 Primary antibodies we used were: anti-LT-βR (sc-8376, Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1 : 50), anti-HVEM (AF2516, R&D systems, 1 : 50), anti-LIGHT (sc-28880, Santa Cruz Biotechnology, 1 : 50), anti-phospho-p38 kinase (V1211, Promega, Madison, WI, USA, 1 : 500), anti-cytochrome c (6H2.B4, BD Biosciences, 1 : 500), anti-cleaved caspase-9 (9509, Cell Signaling Technology, Beverly, MA, USA, 1 : 200), anti-cleaved caspase-3 (9661, Cell Signaling Technology 1 : 200), anti-cleaved lamin A (30H5, Cell Signaling Technology, 1 : 200), anti-IFNγR1 (559911, BD Biosciences, 1 : 250) and anti-IFNγR2 (ab31606, Abcam, Cambridge, MA, USA, 1 : 2000). Alexa Fluor 555-conjugated donkey anti-goat, anti-rabbit or anti-mouse antibody was used as secondary antibodies (Invitrogen). Images were taken using a Zeiss LSM510 laser scanning confocal microscope. Images of phospho-p38 immunostaining were collected with an Olympus BX50WI confocal laser-scanning microscope. Fluorescence analysis was performed using the NIH ImageJ software on Hb9∷GFP motoneurons that were selected on GFP native fluorescence. Analysis of nuclear mean fluorescence intensity was done on 100–200 motoneurons per culture condition.22
Western blot
Neurons were plated at the density of 20 000 cells/cm2 in 6-cm diameter dishes containing corresponding complemented neurobasal medium (see above). Sodium dodecyl sulfate polyacrylamide gel electrophoresis and western blotting were carried out on neurons, astrocytes and mouse lumbar spinal cords using the protocol previously described.29 Primary antibodies were anti-LT-βR (sc-8377, 1 : 500), anti-LIGHT (sc-28880, 1 : 500), anti-mouse IFNγ (sc-52557, 1:500) from Santa Cruz Biotechnology, anti-rat IFNγ (I5027, Sigma-Aldrich, 1 : 500), anti-IFNγR1 (559911, BD Biosciences, 1 : 500), anti-IFNγR2 (ab31606, Abcam, 1 : 2000), anti-α-tubulin (B-5-1-2, Sigma-Aldrich, 1 : 20 000) and anti-actin (AC-40, Sigma-Aldrich, 1 : 20 000). Proteins were detected using horseradish peroxidase (HRP)-conjugated secondary antibodies and visualized with the chemiluminescent HRP substrate (Millipore, Bedford, MA, USA). Where indicated, immunoblot images were quantified and normalized relative to the α-tubulin or actin levels using ImageJ software (National Institutes of Health, USA).
IFNγ ELISA
We prepared primary spinal cord astrocytes from mouse P1-P2 pups as previously described.8 At the first passage, astrocytes were cultured in supplemented neurobasal medium until confluency. Media was then changed and 8 h later, conditioned media were collected and cleared by centrifugation at 1500 × g for 5 min. Astrocytes were then washed and homogenized into in 100 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.1% Triton-X100. Lumbar spinal cords of wild-type, SOD1WT and SOD1G93A mice were dissociated in 100 mM Tris-Hcl pH 7.4, 150 mM NaCl and cleared by centrifugation at 1500 × g for 5 min. Levels of IFNγ were determined using OptEIA mouse IFNγ ELISA Kit II according to manufacturer's instructions (BD Biosciences).
Motoneuron–astrocyte cocultures
Primary astrocyte cultures were prepared from spinal cord of P1-P2 wild-type and SOD1G93A rats as previously described.4 Dissociated cells were plated into tissue culture flasks and maintained in 5% CO2 at 37 °C in DMEM containing 10% FBS until they reach confluency. After 7–9 days, the flasks were shaken at 37 °C for 48 h to eliminate weakly adherent microglial cells. Adherent cells were then removed by Trypsin and plated at a density of 2 × 104 cells/cm2 and maintained in DMEM supplemented with 10% FBS, HEPES (3.6 g/l), penicillin (100 IU/ml) and streptomycin (100 μg/ml) until confluency. Astrocyte monolayers were 98% pure as determined by GFAP immunoreactivity and devoid of OX42-positive microglial cells. Wild-type motoneurons, purified as above, were plated on rat astrocyte monolayer of different genotypes at the density of 300 cells/cm2 and maintained for 48 h in L15 medium (Invitrogen) supplemented with 2% horse serum, 0.63 mg/ml bicarbonate, 5 μg/ml insulin, 0.1 mg/ml conalbumin, 0.1 mM putrescine, 30 nM sodium selenite, 20 nM progesterone, 20 mM glucose, 100 U/ml penicillin, 100 μg/ml streptomycin.
Immunohistochemistry
Immunostaining of lumbar spinal cord sections was performed as we described previously.29 The following antibodies were used: anti-mouse IFNγ (I5027, Sigma-Aldrich, 1 : 100), anti-non-phosphorylated neurofilament (SMI32, Sternberger Monoclonals, Covance, Princeton, NJ, USA, 1 : 500), anti-GFAP (MAB360, Millipore, 1 : 500), anti-Iba1 (Wako Chemical Industries, Osaka, Japan, 1 : 100), anti-LT-βR (sc-8376, 1 : 50), anti-LIGHT (sc-28880, 1 : 50) from Santa Cruz Biotechnology and anti-VAChT (V5387, Sigma-Aldrich, 1 : 2500). Proteins were detected using either fluorochrome-conjugated secondary antibodies (Alexa Fluor 488 or 555) or the peroxidase/DAB detection system following the manufacturer's instruction (Dako, Glostrup, Denmark).
Analysis of Light and SOD1G93A mutant mice
Light−/− and SOD1G93A mice were genotyped as previously described.27, 49 Light−/− males were crossed with SOD1G93A females to obtain SOD1G93A/Light+/− mice. SOD1G93A/Light+/− male mice were then backcrossed with Light+/− female mice. Following the double cross-breeding, only Light+/+, Light−/−, SOD1G93A/Light+/+ and SOD1G93A/Light−/− mice were chosen for the behavioral assays. To define the onset of disease, we measured the body weight twice a week and determined the time mice reached their peak body weight before muscle atrophy and weight loss.3 To assess progression of motor decline, we weekly performed a swimming tank test starting at the age of 50 days and measured swimming speed as we previously described.29 For statistical purposes, we set the maximum swimming latency at 20 s. The mortality was defined as the point in time when the mice are unable to right themselves within 30 s after being placed upon their back. All behavioral studies were done in a blinded manner.
Statistical analysis
Statistical significance was determined by unpaired two-tailed t-test or by a one-way analysis of variance (ANOVA) followed by a Tukey's post hoc tests. Statistical analysis of swimming performance was done using a two-way (group × time) repeated measures ANOVA followed by a Newman-Keuls's post hoc test. A log-rank test was used to calculate the statistical differences in the onset and survival of the different mouse cohorts. Kaplan–Meier survival curves were plotted using GraphPad Prism Software. GraphPad Prism (GraphPad Software, La Jolla, CA, USA) and StatSoft Statistica software (Statsoft, Tulsa, OK, USA) were used for calculations. Significance was accepted at the level of P<0.05. Please see Supplementary Table for statistical details.
Acknowledgments
We thank K Dudley and P Bomont for their critical reading of the manuscript, and all members of the Avenir team for their helpful comments throughout the work. We are grateful to D Olive who provided the LIGHT and LT-βR constructs, DE Bredesen who provided the caspase-9 and -7 constructs, H Esumi who provided the caspase-6 construct, AO Hueber for Bcl-Xl construct and J-C Martinou for Bid construct. We thank K Pfeffer and S Scheu for the gift of Light-deficient mice. We thank J-C Bensadoun for help with statistical analysis and I Medina for help with confocal image analysis. This work was supported by a PACA conseil regional – Trophos SA Ph.D fellowship to JA and grants from the Institut National de la Santé et de la Recherche Médicale (Inserm), Association Française contre les Myopathies (AFM), Association Française pour la Recherche sur la SLA (ARS) and the Thierry Latran foundation.
Glossary
- ALS
amyotrophic lateral sclerosis
- SOD1
superoxide dismutase-1
- LT-βR
lymphotoxin β receptor
- IFNγ
interferon-γ
- TNF
tumor necrosis factor
- NO
nitric oxide
- HVEM
herpes virus entry mediator
- GFP
green fluorescent protein
- NOS
nitric oxide synthase
- IFNγR
IFNγ receptor
- VAChT
vesicular acetylcholine transferase
- GFAP
glial fibrillary acidic protein
- PGD2
prostaglandin D2
- ROS
reactive oxygen species
- CSF
cerebrospinal fluid
- IL
interleukin
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by L Greene
Supplementary Material
References
- Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annu Rev Neurosci. 2010;33:409–440. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MR, Pehar M, Cassina P, Beckman JS, Barbeito L. Increased glutathione biosynthesis by Nrf2 activation in astrocytes prevents p75NTR-dependent motor neuron apoptosis. J Neurochem. 2006;97:687–696. doi: 10.1111/j.1471-4159.2006.03742.x. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase G, Pettmann B, Raoul C, Henderson CE. Signaling by death receptors in the nervous system. Curr Opin Neurobiol. 2008;18:284–291. doi: 10.1016/j.conb.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugolini G, Raoul C, Ferri A, Haenggeli C, Yamamoto Y, Salaun D, et al. Fas/tumor necrosis factor receptor death signaling is required for axotomy-induced death of motoneurons in vivo. J Neurosci. 2003;23:8526–8531. doi: 10.1523/JNEUROSCI.23-24-08526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing G, Dequen F, Soucy G, Julien JP. Absence of tumor necrosis factor-alpha does not affect motor neuron disease caused by superoxide dismutase 1 mutations. J Neurosci. 2006;26:11397–11402. doi: 10.1523/JNEUROSCI.0602-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehar M, Cassina P, Vargas MR, Castellanos R, Viera L, Beckman JS, et al. Astrocytic production of nerve growth factor in motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J Neurochem. 2004;89:464–473. doi: 10.1111/j.1471-4159.2004.02357.x. [DOI] [PubMed] [Google Scholar]
- Raoul C, Estevez AG, Nishimune H, Cleveland DW, deLapeyriere O, Henderson CE, et al. Motoneuron death triggered by a specific pathway downstream of Fas. Potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35:1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- Raoul C, Buhler E, Sadeghi C, Jacquier A, Aebischer P, Pettmann B, et al. Chronic activation in presymptomatic amyotrophic lateral sclerosis (ALS) mice of a feedback loop involving Fas, Daxx, and FasL. Proc Natl Acad Sci USA. 2006;103:6007–6012. doi: 10.1073/pnas.0508774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri S, Kiaei M, Wille E, Calingasan NY, Flint Beal M. Loss of Fas ligand-function improves survival in G93A-transgenic ALS mice. J Neurol Sci. 2006;251:44–49. doi: 10.1016/j.jns.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Locatelli F, Corti S, Papadimitriou D, Fortunato F, Del Bo R, Donadoni C, et al. Fas small interfering RNA reduces motoneuron death in amyotrophic lateral sclerosis mice. Ann Neurol. 2007;62:81–92. doi: 10.1002/ana.21152. [DOI] [PubMed] [Google Scholar]
- Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- Rooney IA, Butrovich KD, Glass AA, Borboroglu S, Benedict CA, Whitbeck JC, et al. The lymphotoxin-beta receptor is necessary and sufficient for LIGHT-mediated apoptosis of tumor cells. J Biol Chem. 2000;275:14307–14315. doi: 10.1074/jbc.275.19.14307. [DOI] [PubMed] [Google Scholar]
- Gavalda N, Gutierrez H, Davies AM. Developmental regulation of sensory neurite growth by the tumor necrosis factor superfamily member LIGHT. J Neurosci. 2009;29:1599–1607. doi: 10.1523/JNEUROSCI.3566-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langou K, Moumen A, Pellegrino C, Aebischer J, Medina I, Aebischer P, et al. AAV-mediated expression of wild-type and ALS-linked mutant VAPB selectively triggers death of motoneurons through a Ca2+-dependent ER-associated pathway. J Neurochem. 2010;114:795–809. doi: 10.1111/j.1471-4159.2010.06806.x. [DOI] [PubMed] [Google Scholar]
- Ge B, Gram H, Di Padova F, Huang B, New L, Ulevitch RJ, et al. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291–1294. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- Kudla G, Montessuit S, Eskes R, Berrier C, Martinou JC, Ghazi A, et al. The destabilization of lipid membranes induced by the C-terminal fragment of caspase 8-cleaved bid is inhibited by the N-terminal fragment. J Biol Chem. 2000;275:22713–22718. doi: 10.1074/jbc.M003807200. [DOI] [PubMed] [Google Scholar]
- Sperandio S, de Belle I, Bredesen DE. An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci USA. 2000;97:14376–14381. doi: 10.1073/pnas.97.26.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchaud S, Korfali N, Villa P, Kottke TJ, Dingwall C, Kaufmann SH, et al. Caspase-6 gene disruption reveals a requirement for lamin A cleavage in apoptotic chromatin condensation. EMBO J. 2002;21:1967–1977. doi: 10.1093/emboj/21.8.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U, Pfeffer K. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med. 2002;195:1613–1624. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D, Brambilla L, Valori CF, Roncoroni C, Crugnola A, Yokota T, et al. Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ. 2008;15:1691–1700. doi: 10.1038/cdd.2008.99. [DOI] [PubMed] [Google Scholar]
- Raoul C, Abbas-Terki T, Bensadoun JC, Guillot S, Haase G, Szulc J, et al. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11:423–428. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- Mohri I, Taniike M, Taniguchi H, Kanekiyo T, Aritake K, Inui T, et al. Prostaglandin D2-mediated microglia/astrocyte interaction enhances astrogliosis and demyelination in twitcher. J Neurosci. 2006;26:4383–4393. doi: 10.1523/JNEUROSCI.4531-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari S, Fung YK, Pahan K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci. 2006;26:4930–4939. doi: 10.1523/JNEUROSCI.5480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri A, Nencini M, Casciati A, Cozzolino M, Angelini DF, Longone P, et al. Cell death in amyotrophic lateral sclerosis: interplay between neuronal and glial cells. FASEB J. 2004;18:1261–1263. doi: 10.1096/fj.03-1199fje. [DOI] [PubMed] [Google Scholar]
- Mir M, Asensio VJ, Tolosa L, Gou-Fabregas M, Soler RM, Llado J, et al. Tumor necrosis factor alpha and interferon gamma cooperatively induce oxidative stress and motoneuron death in rat spinal cord embryonic explants. Neuroscience. 2009;162:959–971. doi: 10.1016/j.neuroscience.2009.05.049. [DOI] [PubMed] [Google Scholar]
- Kostic V, Jackson-Lewis V, de Bilbao F, Dubois-Dauphin M, Przedborski S. Bcl-2: prolonging life in a transgenic mouse model of familial amyotrophic lateral sclerosis. Science. 1997;277:559–562. doi: 10.1126/science.277.5325.559. [DOI] [PubMed] [Google Scholar]
- Kang SJ, Sanchez I, Jing N, Yuan J. Dissociation between neurodegeneration and caspase-11-mediated activation of caspase-1 and caspase-3 in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2003;23:5455–5460. doi: 10.1523/JNEUROSCI.23-13-05455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TW, Buss RR, Vinsant S, Prevette D, Sun W, Knudson CM, et al. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J Neurosci. 2006;26:8774–8786. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson T, Kristensson K, Ljungdahl A, Maehlen J, Holmdahl R, Klareskog L. Gamma-interferon-like immunoreactivity in axotomized rat motor neurons. J Neurosci. 1989;9:3870–3875. doi: 10.1523/JNEUROSCI.09-11-03870.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K, Fedynyshyn J, Ferrell S, Floyd RA, Gordon B, Grammas P, et al. Message and protein-level elevation of tumor necrosis factor alpha (TNF alpha) and TNF alpha-modulating cytokines in spinal cords of the G93A-SOD1 mouse model for amyotrophic lateral sclerosis. Neurobiol Dis. 2003;14:74–80. doi: 10.1016/s0969-9961(03)00087-1. [DOI] [PubMed] [Google Scholar]
- Babu GN, Kumar A, Chandra R, Puri SK, Kalita J, Misra UK. Elevated inflammatory markers in a group of amyotrophic lateral sclerosis patients from Northern India. Neurochemical Research. 2008;33:1145–1149. doi: 10.1007/s11064-007-9564-x. [DOI] [PubMed] [Google Scholar]
- Tateishi T, Yamasaki R, Tanaka M, Matsushita T, Kikuchi H, Isobe N, et al. CSF chemokine alterations related to the clinical course of amyotrophic lateral sclerosis. J Neuroimmunol. 2010;222:76–81. doi: 10.1016/j.jneuroim.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Jensen MB, Hegelund IV, Lomholt ND, Finsen B, Owens T. IFNgamma enhances microglial reactions to hippocampal axonal degeneration. J Neurosci. 2000;20:3612–3621. doi: 10.1523/JNEUROSCI.20-10-03612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner F, Molawi K, Zychlinsky A. From the Cover: Mutant superoxide dismutase 1-induced IL-1{beta} accelerates ALS pathogenesis. Proc Natl Acad Sci USA. 2010;107:13046–13050. doi: 10.1073/pnas.1002396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- Arce V, Garces A, de Bovis B, Filippi P, Henderson C, Pettmann B, et al. Cardiotrophin-1 requires LIFRbeta to promote survival of mouse motoneurons purified by a novel technique. J Neurosci Res. 1999;55:119–126. doi: 10.1002/(SICI)1097-4547(19990101)55:1<119::AID-JNR13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Zala D, Benchoua A, Brouillet E, Perrin V, Gaillard MC, Zurn AD, et al. Progressive and selective striatal degeneration in primary neuronal cultures using lentiviral vector coding for a mutant huntingtin fragment. Neurobiol Dis. 2005;20:785–798. doi: 10.1016/j.nbd.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Duplan L, Bernard N, Casseron W, Dudley K, Thouvenot E, Honnorat J, et al. Collapsin response mediator protein 4a (CRMP4a) is upregulated in motoneurons of mutant SOD1 mice and can trigger motoneuron axonal degeneration and cell death. J Neurosci. 2010;30:785–796. doi: 10.1523/JNEUROSCI.5411-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.