Abstract
Anoikis resistance is a hallmark of transformed epithelial cells. Here, we show that treatment of anoikis-resistant carcinoma cell lines with the endogenous lectin galectin-1 (Gal-1) promoted apoptosis via interaction with the unligated fibronectin receptor α5β1-integrin. Gal-1 efficiency correlated with expression of α5β1-integrin, and transfection of the α5-subunit into deficient cell lines conferred Gal-1 binding and anoikis stimulation. Furthermore, Gal-1 and the α5- and β1-integrin subunits co-precipitated in Gal-1-stimulated cells undergoing anoikis. Other members of the galectin family failed to be active. The functional interaction between Gal-1 and α5β1-integrin was glycan dependent with α2,6-sialylation representing a switch-off signal. Desialylation of cell surface glycans resulted in increased electrophoretic mobility of α5β1-integrin and facilitated Gal-1 binding and anoikis stimulation. On the level of signaling, Gal-1-stimulated anoikis was prevented by filipin, which impaired the internalization of α5β1-integrin via cholesterol-enriched microdomains, and by pretreatment with a caspase-8 inhibitor. We propose that Gal-1/α5β1-integrin interaction participates in the control of epithelial integrity and integrin sialylation may enable carcinoma cells to evade this Gal-1-dependent control mechanism.
Keywords: anoikis, caspase-8, fibronectin receptor, galectin, integrin, sialylation
Epithelial cells require attachment to an appropriate extracellular matrix (ECM) in order to survive.1, 2 This anchorage is provided by integrins, heterodimeric transmembrane receptors that recognize and bind ECM ligands, and thereby induce reorganization of the actin-based adhesive structures of the cytoskeleton. In addition to this physical linkage, integrins act as sensors of the ECM environment.3 They initiate intracellular signal transduction and/or create signaling platforms that modify signals generated by other classes of cell surface receptors.
When bound to appropriate ECM components, integrins cooperate with growth factors to activate mitogenic and anti-apoptotic signaling pathways.1 However, failure to engage with proper ECM components will not only deprive epithelial cells of these mitogenic and protective signals, but generate pro-apoptotic signals reminiscent of death-receptor activation.4, 5 In consequence, epithelial cells that detach from the ECM or lack appropriately composed ECM undergo apoptosis in a process named ‘anoikis' (Greek for homelessness)2 or integrin-mediated cell death,6 respectively. Integrins thereby provide a central safeguard mechanism to preclude survival of epithelial cells in inappropriate locations. Conversely, escape from anchorage dependence is a hallmark of carcinoma cells.
Nonetheless, cancer cells frequently retain or increase integrin expression, and overexpression of individual integrins correlated to invasive and metastatic phenotypes in clinical cancer specimens.7 In experimental settings, ligated integrins promoted malignant cell behavior of carcinoma cells, but frequently fail to initiate cell death, when unligated. This failure may result from alterations either in downstream signaling cascades or integrin properties. In this context, changes in integrin glycosylation are receiving increasing attention.8 In fact, altered glycosylation has long been associated with the malignant phenotype and inflammation.9, 10
We previously identified galectin-1 (Gal-1) as a novel ligand that functionally interacts with the fibronectin receptor α5β1-integrin via binding to glycans.11 Gal-1 belongs to the galectin family of adhesion/growth-regulatory endogenous lectins, which share specificity for β-galactosides and derivatives thereof, sequence similarity in the carbohydrate recognition domain and the jelly-roll folding.12 Structurally, Gal-1 is a proto-type homodimeric protein with one binding site per subunit.13 Cellular binding partners are suitable carbohydrate moieties of distinct glycoproteins and glycolipids.12, 14 The ability for receptor-type-specific crosslinking renders it suited to modulate cell adhesion, migration and growth.9, 12
Gal-1 interaction with the fibronectin receptor on adherent carcinoma cells attenuated cell cycle progression via induction of p21 and p27.11 Upon cell detachment biological responses to Gal-1 might also be modified, prompting us to analyze Gal-1/fibronectin receptor interaction in carcinoma cells subjected to anoikis conditions. Our results identify Gal-1 as a potent activator of pro-apoptotic α5β1-integrin signaling, which imposed anchorage dependence by activating integrin-associated caspase-8.
Results
Gal-1 sensitizes HepG2 cells to anoikis
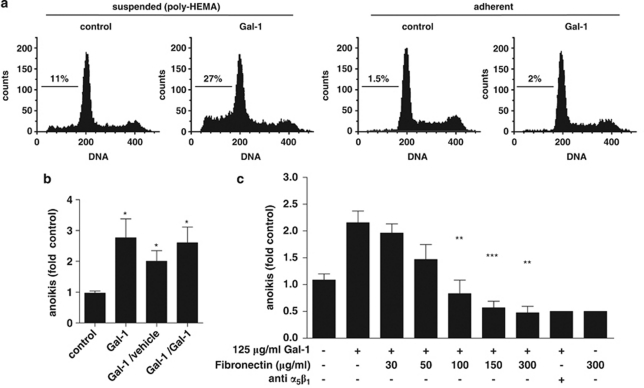
In initial experiments, we examined the effects of Gal-1 on growth and survival, when cells were deliberately kept from attaching to the substratum. Extending previous work with adherent cells,11 hepatocellular HepG2 carcinoma cells were chosen as a representative Gal-1-responsive cell line with endogenous α5-integrin expression. These cells, when cultured on poly-HEMA, responded with a significant induction of apoptosis when treated with Gal-1, but not with vehicle or when kept under adherent conditions (Figure 1a). Importantly, anoikis was also induced, when adherent cells first received Gal-1 treatment and were subsequently transferred to suspension cultures without further addition of Gal-1 (Figure 1b). Thus, Gal-1 treatment sensitized HepG2 cells to anoikis, prompting us to identify the functional binding partner.
Figure 1.
Gal-1 stimulates anoikis in HepG2 cells. (a) HepG2 cells were cultured overnight either adherent or suspended on poly-HEMA-coated plates and received Gal-1 (125 μg/ml) as indicated. The pre-G1 fractions were quantitated by flow cytometry to determine apoptosis (adherent) and anoikis (suspended). (b) Adherent HepG2 cells were pretreated with either vehicle or Gal-1 (125 μg/ml) and subsequently transferred to poly-HEMA-coated plates. Anoikis was then determined following overnight incubation in the presence or absence of Gal-1. Control cells received vehicle throughout the experiment, for the other samples conditions were as indicated (pretreatment/treatment). Pretreatment with Gal-1 was sufficient to sensitize HepG2 cells to anoikis (*P<0.05). (c) Functional modulation of Gal-1-stimulated cell death by addition of the indicated concentrations of soluble fibronectin or 2 μg/ml of an α5β1-integrin-blocking antibody. Cells received the treatment immediately after detachment and were then kept in suspension for 24 h before cell death rates were determined. Data represent mean±S.E.M. of at least three independent experiments (**P<0.01, ***P<0.001 versus. Gal-1-treated cells)
The fibronectin receptor is required for anoikis induction by Gal-1
As Gal-1 effects on growth of adherent cells required α5β1-integrin,11 we assumed a similar interaction in Gal-1-stimulated anoikis of HepG2 cells and tested, whether anoikis could be prevented by coincubation with soluble fibronectin. Indeed, fibronectin prevented Gal-1-stimulated anoikis in a dose-dependent manner (Figure 1c). Similarly, Gal-1-stimulated anoikis was prevented by a neutralizing α5β1-integrin-specific antibody (Figure 1c). A functional interaction with α5β1-integrin was apparently required to elicit the pro-apoptotic effect, as was independently confirmed in a second cell line (Supplementary Figure 1).
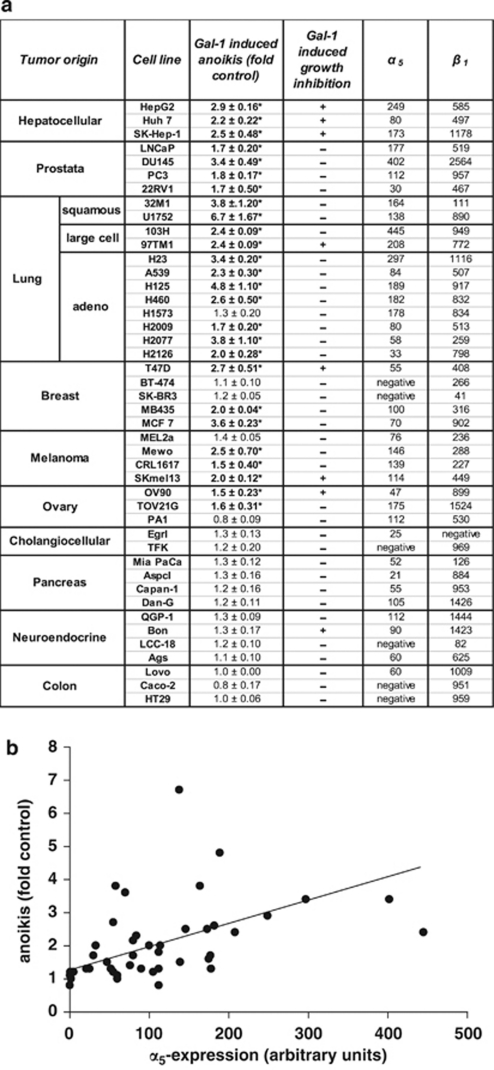
Encouraged by this evidence for α5β1-integrin involvement in Gal-1-mediated anoikis, we characterized a panel of 44 tumor cell lines of different histogenetic origin with respect to its expression and anoikis stimulation by Gal-1 (Figure 2). The data collected provided the following insights: (i) Gal-1 significantly increased anoikis in 27 of the 44 cell lines (Figure 2a); (ii) the six cell lines that were α5-subunit deficient were entirely insensitive to Gal-1 treatment; (iii) α5-integrin presence and susceptibility to Gal-1-mediated anoikis were significantly correlated (Pearson's r=0.6028, P=0.006; Figure 2b); and (iv) Gal-1 responsiveness differed between cell lines derived from tumors of different histogenetic origin.
Figure 2.
Gal-1 stimulates anoikis in tumour cells that express the fibronectin receptor. (a) The α5-expression level and anoikis stimulation by Gal-1 (125 μg/ml) were determined in 44 cell lines of different histogenetic origin. Gal-1-stimulated anoikis is given relative to control values of anoikis determined in untreated controls (fold control) and ratios >1.5 are highlighted in bold (*). For comparison, growth inhibition by Gal-1 in adherent cultures is indicated. Antiproliferative effects were determined on the basis of cell numbers. Gal-1-treated cultures with a >30% reduction of cell numbers after 96 h were considered as responsive (+). Cellular α5β1-integrin surface expression was determined in parallel by flow cytometry. Data are derived from three independent experimental series. (b) The linear regression analysis (Pearson's r=0.6028, P=0.006) between anoikis and α5-integrin expression is shown
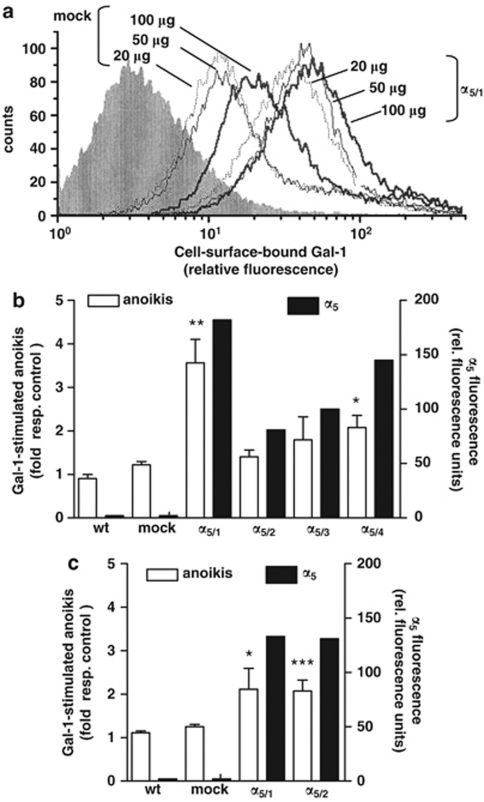
To provide further evidence for a functional role of α5β1-integrin in Gal-1-mediated anoikis, we used the Caco-2 cell system described previously.11 Caco-2 wild-type (wt) cells are α5 deficient and entirely resistant to both Gal-1-dependent cell cycle regulation and stimulation of anoikis. Following transfection with cDNA for the α5-integrin subunit, Caco-2 cells had become responsive to Gal-1-induced cell cycle inhibition.11 Using this cell model in suspension culture, enhanced Gal-1 surface reactivity (Figure 3a) and Gal-1-stimulated anoikis were seen in α5-transfected Caco-2 cells, but not in their mock-transfected controls (Figure 3b). Maximal anoikis rates were reached in clones that displayed the highest level of α5-integrin expression (Figure 3b). Furthermore, anoikis induction in clones with minute α5-integrin subunit expression did not reach statistical significance, suggesting that a certain density of α5β1-integrin was required either for efficient Gal-1 binding or pro-apoptotic downstream signaling. Similar corroborating evidence was obtained from α5-integrin-transfected HT29 cell populations (Figure 3c). Together, these experiments suggested that the fibronectin receptor conferred susceptibility to Gal-1-stimulated anoikis.
Figure 3.
The fibronectin receptor confers capacity for Gal-1-stimulated anoikis to α5-deficient colon cancer cells. (a) Cell surface binding of Gal-1 in mock- and α5-transfected (clone α5/1) Caco-2 cells. Number inserts indicate the amount of biotinylated Gal-1 added (μg/ ml). (b and c) Determination of Gal-1-stimulated anoikis after incubation with 125 μg/ml Gal-1 for 24 h (open bars) and α5-expression levels (closed bars) in different clones of α5-transfected Caco-2 cells (α5/1 to α5/4), wild-type (wt) cells and mock control (panel b) and in two α5-transfected HT29 clones (α5/1 and α5/2), wt cells and mock control (panel c). Data represent at least three independent experiments (anoikis as fold stimulation without treatment, *P<0.05, **P<0.01, ***P<0.001) and a representative determination of α5 expression
Anoikis stimulation by Gal-1 is galectin-type specific and inhibited by cognate sugar
We next explored the galectin-type specificity of anoikis stimulation using galectins-3 and -7 and the N-terminal domain of galectin-4, which represent another proto-type galectin very similar to Gal-1 (Gal-7), the chimera type (Gal-3) and the N-terminal part of a tandem-repeat-type galectin (Gal-4), respectively. We found that these other galectin family members were unable to stimulate anoikis (Supplementary Figure 2). This observation was confirmed in two additional α5-integrin-positive, Gal-1-responsive cell lines, that is, DU145 and 97TM1 (Supplementary Figure 3).
Gal-1 interacts with cellular effectors via lectin-carbohydrate/protein interactions.14 To document involvement of the carbohydrate recognition domain, the cognate sugar lactose, which inhibits binding of Gal-1 to glycans on the cell surface, was utilized. Addition of lactose, but not sucrose abrogated Gal-1-stimulated anoikis, whereas the haptenic inhibitor had no effect by itself (Supplementary Figure 2).
Sialylation modulates Gal-1 binding and Gal-1-stimulated anoikis
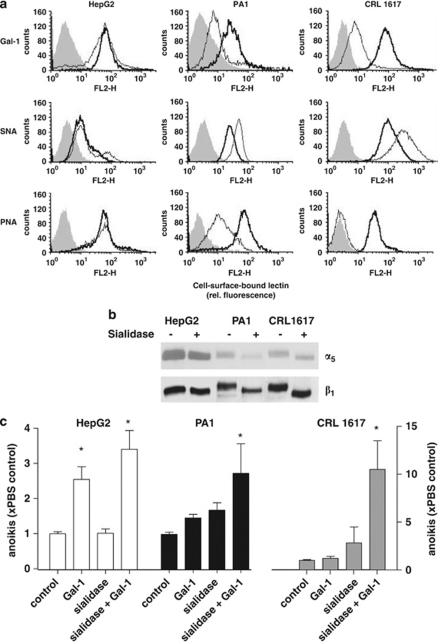
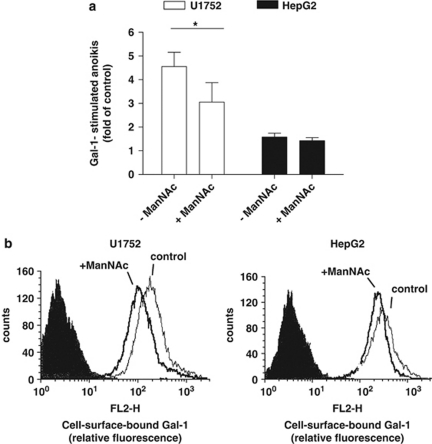
Overall, the correlation between α5-integrin expression and Gal-1-stimulated anoikis supported the concept that glycans of the α5β1-integrin represent the functional interaction partner. However, several cell lines did not follow the assumed reactivity pattern. They may have a saturating level of Gal-1, prompting us to explore Gal-1 expression in our panel of cell lines by immunoblotting (Supplementary Figure 4). However, Gal-1 (and also Gal-3) presence was variable, and high endogenous levels could occur in cell lines, which responded to addition of exogenous Gal-1, precluding a clear-cut correlation. These results turned attention to monitoring Gal-1 reactivity, testing PA1 and CRL1617 cells, which revealed less apoptosis induction than expected from their α5β1-integrin level, as well as U1752 cells, which were exceptionally responsive, although α5β1-integrin presentation was moderate (please see Figure 2). HepG2 cells were also included, as they responded to Gal-1 exposure as predicted from their α5-integrin content. Using biotinylated Gal-1 (Gal-1-bio) as probe, we barely detected probe binding to PA1 or CRL1617 cells when compared with HepG2 or U1752 cells (Figure 4a).
Figure 4.
Sialidase treatment enhances Gal-1 binding and Gal-1-stimulated anoikis. (a) Flow cytometry analysis of binding of SNA, PNA or biotinylated Gal-1 to control (light) and (bold) sialidase-treated cells. Background fluorescence (shaded) was similar in the PBS and sialidase-treated cells. (b) Western blot for detection of α5- and β1-integrin subunits in cells with or without sialidase treatment. (c) Anoikis rates in HepG2, PA1 and CRL1617 cells with or without previous sialidase treatment measured after 24 h on suspension culture either in the presence or absence of Gal-1 are expressed as relative to the corresponding PBS-treated controls. Data represent mean±S.E.M. of at least three independent experiments (*P<0.05)
As terminal α2,6-sialylation of glycans blocks Gal-1 binding,14 we hypothesized that low-level probe binding might result from sialylation of α5β5-integrin. Cells were thus cautiously treated with sialidase. The removal of α2 → 6-linked sialyl moieties from PA1 and CRL1617 cells were confirmed by reduced binding of Sambucus nigra agglutinin (SNA; a probe for α2,6-sialylated Gal/GalNAc residues). As a consequence of desialylation, the reactivities for Gal-1 and peanut agglutinin (PNA; a probe for sialic acid-free type-1 O-glycans) were increased in sialidase-treated PA1 and CRL1617 cells, but not in HepG2 cells (Figure 4a). The rather small changes for HepG2 cells indicate a small degree of sialidase sensitivity under the given conditions. Overall, removal of masking sialylation increased Gal-1-binding sites. Parallel immunoblot analyses of α5- and β1-integrin subunits revealed a sialidase-dependent increase in electrophoretic mobility primarily for the β1-integrin subunit from PA1 and CRL1617 cells (Figure 4b). In summary, Gal-1-stimulated anoikis in PA1 and CRL1617 cells was enhanced by sialidase treatment (Figure 4c). Conversely, increased sialylation may reduce the responsiveness to Gal-1. As experimental test, we pretreated highly responsive U1752 cells for 48 h with 5 mM N-acetylmannosamine (ManNAc), a biosynthetic precursor of sialic acid, to enhance cell surface sialylation. Addition of ManNAc to U1752 cells cultures reduced the ability of Gal-1 to stimulate anoikis (Figure 5a), as it did with the extent of lectin binding to the cell surface (Figure 5b). Gal-1 reactivity and Gal-1-stimulated anoikis of HepG2 cells remained nearly unaffected, although we could detect sufficient amounts of free sialic acid in cell supernatants (not shown). Detailed monitoring of α2,3/6-sialylation on the cell surface with a panel of plant/human lectins after cell treatment with 5 and 20 mM ManNAc for 48 h fittingly revealed only slight increases (up to 12%), in full accord with the given data for Gal-1 (Figure 5b). SNA binding, for example, increased from about 40 to 42–45% (percentage of positive cells) and from 15.8 to about 16.7 (mean fluorescence intensity). Evidently, cell surface sialylation did not critically depend on synthesis of free sugar in these cells.
Figure 5.
ManNAc supplementation reduces Gal-1 stimulated cell death and Gal-1 binding. (a) Gal-1-mediated cell death in U1752 and HepG2 cells previously cultured for 48 h with 5 mM or without ManNAc supplementation. Cells were then subjected to suspension culture with continued ManNAc supplementation and Gal-1 was added as indicated. Anoikis rates were determined after 24 h on the basis of the pre-G1 fraction from DNA-histograms. Anoikis is expressed as fold of the respective controls in the absence of Gal-1. Data represent mean±S.E.M. of at least three independent experiments (*P<0.05, one tailed t-test). (b) Binding of biotinylated Gal-1 to U1752 (left panel) or to HepG2 (right panel) cells that had been cultured for 48 h in the presence of ManNAc (+ManNAc) or vehicle (control). Flow cytometry analysis shows control (light), ManNAc-treated (bold) cells and background fluorescence via binding of indocarbocyanine-streptavidin conjugate in the absence of biotinylated Gal-1 (shaded)
In summary, our data indicate that accessibility and/or functionality of a critical-binding partner of Gal-1 were modulated by sialylation.
Gal-1 directly associates and functionally interacts with α5β1-integrin
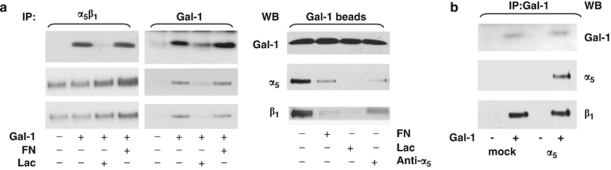
On the basis of (i) the requirement for α5β1-integrin expression for Gal-1-stimulated anoikis, (ii) increased Gal-1 binding in α5-integrin-transfected cells, and (iii) anoikis protection by fibronectin and the α5β1-integrin-blocking antibody, Gal-1 likely associated with α5β1-integrin. The predicted complexes containing Gal-1 were found with a fibronectin-receptor-specific antibody in Gal-1-treated HepG2 cells (Figure 6a, left). Addition of lactose, but not fibronectin abolished this co-immunoprecipitation. Using an antibody to Gal-1, the α5- and β1-integrin subunits were pulled down in the absence, but not in the presence of lactose (Figure 6a, middle). Again, fibronectin did not prevent this complex formation, although it abrogated anoikis induction by Gal-1. Using Gal-1-coated magnetic beads α5β1-integrin was detected (Figure 6a, right). Different from immunoprecipitations, fibronectin reduced the recovery of α5β1-integrin from Gal-1-coated beads, likely due to the altered experimental conditions with disparate Gal-1 presentation and a 30 min fibronectin pre-incubation before the application of Gal-1.
Figure 6.
Gal-1 binds to the α5β1-fibronectin receptor. Coimmunoprecipitation of Gal-1 with α5β1-integrin. (a) Whole-cell lysates from HepG2 cells that had been cultured on PolyHEMA-coated dishes and treated for 1 h with (+) or without (−) 125 μg/ml Gal-1. For co-incubation conditions, 300 μg/ml fibronectin (FN), 20 μM lactose (Lac), or 100 μg/ml of α5-blocking antibody were added as indicated. Cells were lysed and immunoprecipitations were conducted with antibodies to α5β1-integrin (left panel) or Gal-1 (middle panel). In an alternative approach, HepG2 cells were incubated for 5 min with Gal-1-coated magnetic beads after pretreatment with 300 μg/ml fibronectin (FN), 20 μM lactose (Lac), or 100 μg/ml of α5-blocking antibody, respectively (right panel). (b) Co-immunoprecipitation of Gal-1 and α5β1-integrin in α5-transfected Caco-2 cells and mock controls, using an antibody to Gal-1 after treatment with (+) or without (−) 125 μg/ml Gal-1for 1 h. Immunoblots using antibodies to either α5- or β1-integrin subunits or to Gal-1 are shown
Taken together, Gal-1 appeared to physically interact with the fibronectin receptor outside the fibronectin-binding domain, fully in line with contact to integrin's N-glycans.
In the Caco-2 cell system, we co-precipitated the β1-subunit not only from Caco-2/α5-expressing cells, but also from parental α5-deficient cells with the Gal-1-specific antibody (Figure 6b). Thus, Gal-1 can interact with the β-integrin subunit independent of α5-integrin expression. Having revealed evidence for direct interaction, we addressed Gal-1-initiated signaling next.
Gal-1 engagement of α5β1-integrin results in caspase-8 activation
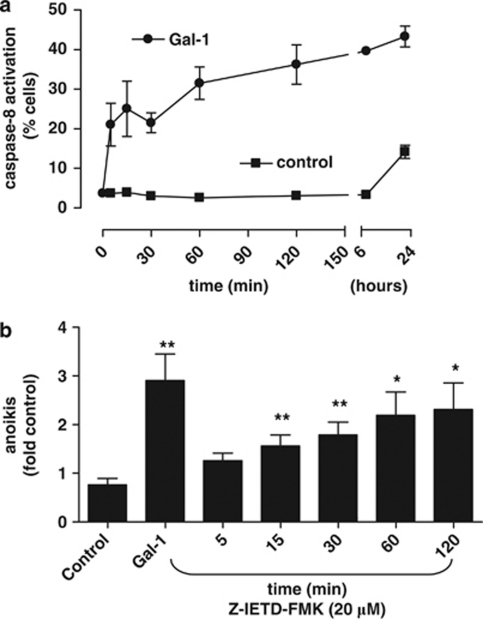
As integrins may modulate survival via caspase-8 activation, we determined caspase-8 activity in Gal-1- treated HepG2 suspension cultures (Figure 7). Treatment with Gal-1 resulted in rapid and progressive activation of caspase-8, when compared with untreated controls. To define the significance of this caspase-8 activation for Gal-1-stimulated anoikis, a specific caspase-8 inhibitor (Z-IETD-FMK) was added at various time points following Gal-1 stimulation. The inhibitor prevented Gal-1-dependent anoikis completely when added within 5 min of starting Gal-1 treatment (Figure 7b). When added at later time points, Gal-1-mediated stimulation of anoikis could only be partially inhibited. Hence, Gal-1 stimulated anoikis required the activation of caspase-8. This observation prompted us to further explore the mechanism, whereby Gal-1/α5β1-integrin complexes might activate caspase-8.
Figure 7.
Caspase-8 is activated in Gal-1-stimulated cells undergoing anoikis. (a) HepG2 cells were subjected to suspension culture in the presence or absence of Gal-1, and caspase-8 activity was determined at the indicated time points. (b) Cells were cultured and Gal-1 stimulation (125 μg/ml) was conducted as above. At the time points indicated, the caspase-8 inhibitor Z-IEDT-FMK (20 μM) was added to cells in suspension culture in the presence of Gal-1. After 24 h, anoikis rates were determined by flow cytometry. Data represent mean±S.E.M. of four independent experiments. (*P<0.05, **P<0.01)
Gal-1-stimulated anoikis is associated with internalization of α5β1-integrin via cholesterol-enriched microdomains
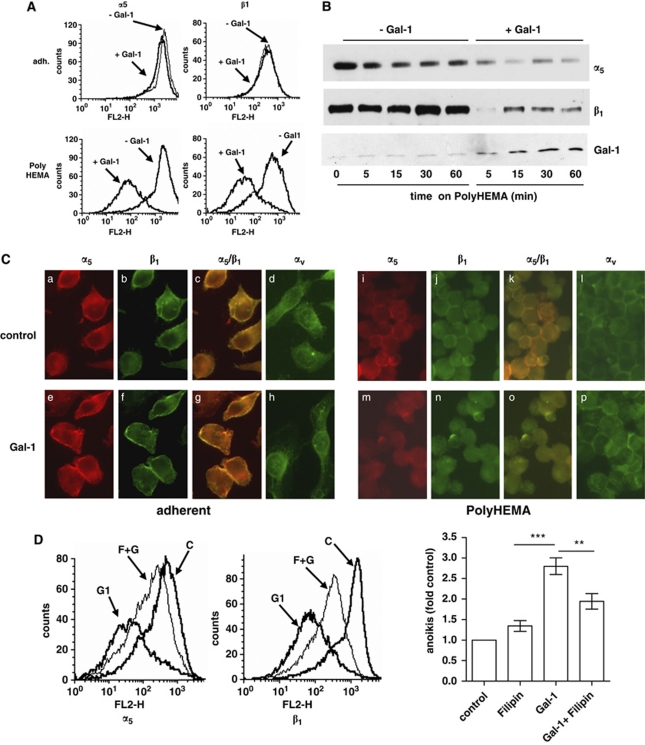
As cell detachment is followed by a rapid internalization of cholesterol-enriched microdomains that can result in cell death, we examined the internalization of Gal-1 and α5β1-integrin in our anoikis model. Although detachment per se did not alter α5β1-integrin surface immunoreactivity, reactivity rapidly declined following Gal-1 treatment in detached, but not adherent cells (Figure 8A). Immunoblotting of membrane fractions corroborated the loss of α5β1-integrin (Figure 8B), whereas the overall integrin content of whole-cell lysates remained unchanged (Supplementary Figure 5). Immunofluorescence microscopy similarly illustrated a redistribution of α5β1-integrin membrane reactivity into the cytosol of Gal-1-treated detached HepG2 cells (Figure 8C). Examination of other integrins (Figure 8C and Supplementary Figure 6a) or stimulation with Gal-3 suggested that internalization of α5β1-integrin occurred specifically on addition of Gal-1. We then compared Gal-1-stimulated internalization of α5β1-integrin in cell lines that differed with respect to extent of Gal-1-stimulated anoikis and observed internalization exclusively in cell lines, which underwent anoikis (Supplementary Figure 7). To directly test the functional relevance of the internalization process for Gal-1-stimulated anoikis, we used the macrolide filipin to perturb microdomain integrity (Figure 8D). Filipin treatment did not alter level of spontaneous anoikis, but potently reduced Gal-1-stimulated internalization of α5β1-integrin (Figure 8D left and Supplementary Figure 8a) and anoikis (Figure 8D right and Supplementary Figure 8b). Thus, microdomain integrity and Gal-1-induced internalization of its cognate integrin have a role for anoikis induction.
Figure 8.
Gal-1-stimulated anoikis is associated with microdomain-mediated internalization of α5β1-integrin and prevented by filipin. (A) HepG2 cells were cultured either under adherent conditions (adh.) or subjected to suspension culture and treated with 125 μg/ml Gal-1 as indicated. After 30 min incubation time, an aliquot was withdrawn and native cells were stained with anti-α5 or anti-β1 integrin antibodies, respectively, to determine surface abundance of these integrins. The remaining cells were further incubated on poly-HEMA for confirmation of maintained anoikis induction (not shown). (B) Immunoblotting of membrane-enriched fractions (separated at 100 000 × g, 20 μg protein/lane) obtained from HepG2 cells that were cultured on poly-HEMA and treated after with Gal-1 or vehicle for the indicated time periods. Both α5- and β1-integrin subunits were reduced in cells that had received Gal-1. (C) Immunofluorescence of α5- and β1-integrin subunits. Cells were cultured as described above and treated with Gal-1 for 1 h, and fixed before α5- and β1-integrin subunits were examined by immunofluorescence microscopy. Left part shows integrin localization in adherent cells incubated without (a–d) or with Gal-1 (e–h) for 1 h. Right part shows results from cells cultured on poly-HEMA treated without (i–l) or with Gal-1 (m–p) for 1 h. Shown are immunoreactive fluorescence signals for α5- (a, e, i and m), β1- (b, f, j and n) subunits, overlay of α5- and β1- (c, g, k and o) and αv- (d, h, l and p) integrin subunits. (D) Effect of filipin on Gal-1-stimulated α5β1-integrin internalization and anoikis. Cells were detached and incubated without or with Gal-1 in the presence or absence of filipin. Subsequently, α5β1-integrin immunoreactivity was determined in native cells (left). Labels are as follows: control cells (C), Gal-1 treated cells (G1) and cells pretreated with filipin (4 μg/ml) for 60 min before Gal-1 incubation (F). To determine effects of filipin on anoikis, cells were cultured on Poly-HEMA and treated with Gal-1 (125 μg/ml) or filipin (4 μg/ml) or a combination thereof. For filipin conditions, cells had also been pretreated for 60 min before they were transferred to suspension culture. Anoikis rates were determined after 24 h. Results are expressed as fold of control and represent the mean±S.E.M. of at least three independent experiments. ANOVA was performed using Prism software (Prism, San Diego, CA, USA). **P<0.01, ***P<0.001
Discussion
The current study identifies human Gal-1 as a potent modulator of anchorage-independent survival in epithelial cancer cells. We provide evidence that (i) Gal-1 is capable of promoting anoikis in a series of cell lines of different histogenetic origin, (ii) Gal-1 binding depends on the presence of the fibronectin receptor and is modulated by sialylation of cell surface glycans, (iii) anoikis stimulation by Gal-1 requires a functional interaction with the fibronectin receptor and (iv) complex formation with this integrin promotes internalization of the integrin via filipin-sensitive cholesterol-enriched microdomains and activation of caspase-8, a process cascade triggering anoikis.
Previously, we had found that Gal-1 interacted with the fibronectin receptor in adherent cells to induce cell cycle inhibition.11 Here, we focused on Gal-1 interactions with the unligated integrin in suspension culture, which intriguingly promoted anoikis. When screening a panel of 44 carcinoma cells lines, we found the majority anoikis resistant despite expression of α5β1-integrin, suggesting the integrin had lost the capacity to impose anchorage dependence. Addition of Gal-1 restored anoikis susceptibility in most of these cells. Several lines of evidence indicated that the unligated fibronectin receptor represented the relevant functional target of Gal-1: (i) α5β1-integrin expression was mandatory for anoikis stimulation via Gal-1, (ii) overall, the extent of Gal-1-mediated anoikis correlated to the amount of α5-integrin surface expression, (iii) transfection with cDNA specific for the α5-integrin subunit conferred Gal-1 binding and responsiveness and (iv) fibronectin engagement and a neutralizing antibody against α5β1-integrin, respectively, prevented Gal-1-stimulated anoikis. The latter findings are in full accord with the functional antagonism between Gal-1 and fibronectin noted under adherent conditions.11 In addition, Gal-1 also stimulated anoikis in cells, which failed to respond under anchorage-dependent conditions (Figure 2a), suggesting differential downstream signaling. Glycans of integrins have previously been shown to interact with galectins, resulting in either impaired or enhanced integrin-mediated adhesion.15, 16, 17 Close association of the β1-integrin subunit and Gal-1 was evident from cross-linking and co-precipitation experiments, and Gal-1 has been implicated in β1-subunit surface presentation and trafficking.18, 19 Also, Gal-1 was recovered from α5β1-integrin complexes in transfected K562 chronic myelogenous leukemia cells in a recent proteomic analysis.20 The requirement for α5β1-integrin in Gal-1-stimulated anoikis furthermore fits the profile of this integrin as a relevant determinant of anchorage dependence.21, 22, 23
Although expression of the fibronectin receptor was a prerequisite for Gal-1-stimulated anoikis, the capacity of the cell to produce Gal-1 turned out to be less predictive. Evidently, the signal intensity of western blots was not correlated to cell reactivity, possibly due to insufficient routing to the integrin and/or presence of other binding partners. A coordinated upregulation of Gal-1 production and its surface presentation is orchestrated via reexpression of the tumor suppressor p16INK4a in a pancreatic cancer cell line.24 Induction of Gal-1 was accompanied by reprogramming of glycosylation, leading to increased Gal-1 reactivity and potent induction of anoikis involving α5β1-integrin.24
With respect to the cellular reactivity, accessibility of Gal-1-binding sites emerged as a crucial modifier. Specifically, enzymatic reduction of cell surface sialylation increased binding and anoikis response of cell lines that appeared resistant to Gal-1 despite abundant α5-integrin expression. Thus, consequences of integrin sialylation may extend beyond altered binding of protein ligands 8, 25, 26 due to an effective masking of lectin-reactive sites. Indeed, the presence of α2,6-linked sialic acid precluded cell binding of Gal-1,27 even in N-acetyllactosamine repeats.28 The current findings suggest α2,6-sialylation of α5β1-integrin constitutes a relevant (patho-)physiologic response modifier for Gal-1-stimulated cancer cell anoikis. Interestingly, this type of N-glycan branch-end structure is increased in various malignant cells.10
In line with this concept, our immunoprecipitation analyses confirm a physical, lactose-inhibitable interaction of Gal-1 with the fibronectin receptor. Presence of both integrin subunits appears mandatory, as Gal-1 binding and pro-apoptotic signaling are negligible in Caco-2 or HT29 cell lines with abundant β1-integrin expression, unless they are transfected to produce the α5-integrin subunit. Although fibronectin counteracts Gal-1-stimulated anoikis, it did not consistently displace Gal-1 from α5β1-integrin complexes, suggesting physically separate interaction sites. Information on the precise localization of the glycans acting as docking sites will be required to answer topological aspects of this question.
Which signals trigger anoikis following the binding of Gal-1 to α5β1-integrin? Initiation and execution of the anoikis process rely on the activation of caspases via integrins,1 though variability exists with respect to the sequence of caspase activation. Studies on α5β1- and αvβ3-integrins describe an early and functionally relevant activation of procaspase-8.5, 29 In fact, procaspase-8 was shown to promote migration and invasion of cancer cells, unless turned into an apoptotic signal by activation.30 Here, we report a rapid procaspase-8 activation upon Gal-1 engagement of α5β1-integrin and impairment of Gal-1-stimulated anoikis with a selective caspase-8 inhibitor. Involvement of caspase-8 has also recently been demonstrated for anoikis induction in p16 INK4a-reconstituted cells, which required autocrine Gal-1 availability.31
Different mechanisms are proposed, whereby integrins activate or suppress caspase-8 activity in a ligand-dependent manner, including proximity-induced activation of integrin-associated procaspase-8 due to integrin clustering,6 prevention of autocatalytic cleavage by post-translational modifications,30, 32 redistribution of endogenous caspase inhibitors,33 or increased availability of pro-apoptotic bcl-2 family members.34 Here, we observed rapid internalization of α5β1-integrin via cholesterol-enriched microdomains, a process required for full anoikis induction. Thus, Gal-1 may provoke clustering and/or repartition of the unligated α5β1-integrin into endocytotic microdomains and thereby enable or amplify its capacity to activate caspase-8. Enhanced caspase-8 activation by death receptors, such as CD95, following recruitment to lipid rafts and endocytosis provides an example for modulation of apoptotic signaling by endocytotic trafficking.35
Association of ganglioside GM1, which by itself is a potent Gal-1 counterreceptor, with this integrin, as noted in T effector cells,36 may favor clustering in cholesterol-enriched microdomains, leading to enhanced Gal-1 reactivity37 thereby supporting recruitment to microdomains and endocytosis.
In summary, we report Gal-1 as a counter receptor of N-glycans of the α5β1-integrin. Binding to the unligated integrin initiates apoptosis. Thus, Gal-1 is capable of implementing anchorage dependence in epithelial cancer cells. Cancer cells may circumvent this control mechanism by reducing access of Gal-1 to its target(s) on the cell surface via α2,6-sialylation. Thus, an acquired anoikis resistance of carcinoma cells may be encoded in glycans at the cell surface, besides being exerted by oncogenic alterations in downstream survival signaling pathways. Consequently, shifting the glycophenotype to a Gal-1-responsive profile offers an attractive alternate avenue for therapeutic intervention.
Materials and Methods
Cell culture
Human tumor cell lines were obtained from ATCC, the German Cancer Research Center (Heidelberg, Germany) (DanG cells), or the Japan Health Sciences Foundation (Health Science Research Resources Bank, Osaka, Japan) (QGP-1), and were grown exactly as recommended. BON (a generous gift from CM Townsend, Galveston, TX, USA) and LCC-18 neuroendocrine cancer cell lines were maintained in DMEM, supplemented with 10% (v/v) FCS, 100 units/ml penicillin and 100 μg/ml streptomycin.
Induction and detection of anoikis
For determination of anoikis, 2 × 105 cells were cultured as suspension cultures on plates coated with poly(2-hydroxyethyl methacrylate) (poly-HEMA) (Sigma-Aldrich, Munich, Germany) for the indicated periods of time. Apoptotic cells were then quantitated from the pre-G1 fraction in cell cycle analyses.23
Production and purification of galectins
Human galectins -1, -3 and -7 and the N-terminal domain of rat tandem-repeat-type galectin-4 were purified with affinity chromatography as crucial step, rigorously checked for purity by one- and two-dimensional gel electrophoresis, mass spectrometry and gel filtration, and bioactivity was ascertained by solid-phase and cell assays as previously described.38, 39
Antibodies
Antibodies for immunoblotting of integrins α5 (clone 1), β1 (clone 18), α4 (clone 7) and αv (clone 21) were from BD Transduction Laboratories (Heidelberg, Germany); the antibody utilized to immunoprecipitate α5β1-integrin and as blocking antibody (AB1950) was from Chemicon (Hofheim, Germany). The antibody to PCNA from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies used for flow cytometry were as follows: α5 (CD49e R-PE CBL497P) and β1 (CD29PE CBL481P) from Cymbus Biotechnology (Hampshire, UK). Secondary antibodies were from Dianova (Hamburg, Germany). Polyclonal antibodies against Gal-1 free of cross-reactivity for other galectins have been described previously.24
Protein extraction and western blotting
Cells were lysed in 1 × RIPA buffer (50 mM Tris-HCl pH 7.5, 0.15M NaCl, 0.25% SDS, 0.05% sodium deoxycholate, 1% NP-40, 1 mM dithiothreitol, 1 μg/ml aprotinin, 2 mM leupeptin, 1 mM Na3VO4, 1 mM NaF and 1 mM PMSF), and sonicated on ice. Aliquots (5–10 μg) were subjected to SDS-PAGE and were electroblotted onto PVDF membranes (NEN, Cologne, Germany). Blots were incubated overnight at 4 °C with the respective antibodies (diluted 1 : 1000 in 5% non-fat dried milk in PBS with 0.5% Tween 20). Immunoreactive bands were visualized by enhanced chemoluminescence (NEN, Cologne, Germany).
Sialidase treatment
4 × 105 cells were washed with PBS, resuspended in 50 μl PBS and incubated with 10 mUnits of neuraminidase from Clostridium perfringens (specific for α2,3/6-sialylation; Roche Diagnostic GmbH, Mannheim, Germany) 40 min at 37 °C. For western blot analysis 30–40 μg protein extract were processed instead of whole cells.
Analysis of integrin content by flow cytometry
Approximately 105 cells were resuspended in 300 μl PBS with 10 μl of subunit-specific phycoerythrin (PE)-labeled integrin antibodies (α5, β1). After incubation for 15 min at 4 °C, cells were washed with PBS and fluorescence was recorded on a FACSCalibur (Becton Dickinson, Heidelberg, Germany) and analyzed with CellQUEST software (Becton Dickinson).
Stable transfection of α5-integrin constructs
Full-length α5-integrin-subunit-specific cDNA was originally obtained from E. D. Kreuser (Department of Hematology and Oncology, University Medical Center Benjamin Franklin, Free University of Berlin, Berlin, Germany) and was subcloned in pRC-CMV (pRC-α5).40 To generate stably transfected cells Effectene Transfection Reagent (Qiagen, Hilden, Germany) was used following the manufacturer's protocol. Subsequent selection of stably transfected cells was carried out with 0.8 mg/ml G418.11
Determination of Gal-1 binding
Cells were incubated with 125 μg/ml of biotinylated Gal-1, prepared and checked for activity and label incorporation as described,24 washed twice with PBS and bound Gal-1 (and also labeled plant lectins) was then detected by flow cytometry using a fluorescent-streptavidin derivative.39 Carbohydrate-dependent binding was ascertained by controls with sugars.
Immunoprecipitation of integrin/galectin complexes
Immunoprecipitations were carried out as previously described.23 Briefly, cells were lysed in 50 mM Hepes, pH 7.4, 150 mM sodium chloride, 1 mM EDTA, 2.5 mM EGTA, 10% glycerol, 0.1% Tween 20, 1 mM DTT, 1 mM sodium fluoride, 10 mM β-glycerolphosphate, 0.1 mM sodium orthovanadate, 0.1 mM PMSF, 3 mg/ml aprotinin, and 2 mM leupeptin. Cell lysates were immunoprecipitated with antibodies as indicated, and immune complexes were recovered with protein A-Sepharose or protein G-Sepharose (α5β1-integrin) beads (Sigma-Aldrich) overnight at 4 °C. Precipitated proteins were separated on SDS-PAGE gels and electroblotted to PVDF membranes.
Determination of caspase-8 activity
Cells with activated caspase-8 were detected using the carboxyfluorescein-labelled derivative of the caspase-8 inhibitor Z-LETD-FMK (FAM-LETD-FMK) (Biocarta, Hamburg, Germany), which irreversibly binds to activated caspase-8. Fluorescence intensity was evaluated by flow cytometry.
Magnetic separation of galectin-containing complexes
For separation of Gal-1-associated complexes, 2 × 108 tosyl-activated Dynabeads M-280 (Dynal) were coated overnight with either 250 μg Gal-1 or BSA at 37 °C. Dynabeads were recovered on a magnetic separation stand, washed twice with PBS, deactivated with 0.2M Tris-HCl pH 8.5 for 4 h at 37 °C and washed again with PBS. Beads were then added to 106 cells for 5 min at RT. Cells and beads were rinsed twice with PBS and homogenization buffer (20 mM Tris-HCl pH 7.6, 10 mM MgCl2, 1 μg/ml aprotinin, 2 mM leupeptin and 1 mM PMSF) was added. Proteins attached to the beads were washed twice with homogenization buffer and resuspended in SDS-DTT protein loading buffer.
Subcellular fractionation
All steps were carried out at 4 °C. Cells were rinsed with PBS, resuspended in ice-cold homogenization buffer (20 mM Tris-HCl pH 7.6, 10 mM MgCl2) containing protease inhibitors and lysed by pipeting. Following centrifugation (2 min at 40 × g) the pellet (cell debris) was discarded and the supernatant centrifuged again (10 min at 750 × g). The pellet (nuclear fraction) was stored in homogenization buffer and the supernatant centrifuged 60 min at 100 000 × g. The supernatant (cytosolic fraction) was stored and the pellet (membrane enriched fraction) resuspended in homogenization buffer. Aliquots (20 μg) of the membrane enriched fraction were separated by SDS-PAGE and further processed as described for western blotting above.
Immunofluorescence microscopy
For immunofluorescence microscopy, adherent cells were grown on Lab-Tek chamber slides (Nalge Nunc Int., Rochester, NY, USA) and suspended cells were centrifuged onto microscope slides before analysis. Cells were fixed with cold methanol/acetone (2 : 1) for 10 min at −20 °C.
The following monoclonal antibodies were used: α5 (CD49e R-PE CBL497P), β1 (CD29PE CBL481P; CD29F:P5.2 CBL481F) from Cymbus Biotechnology LTD (Hampshire, UK), α2, αí, β5 from Dianova (Hamburg, Germany), β4 from Telios Pharmaceuticals (San Diego, CA, USA).
Statistical analysis
Unless indicated unpaired Student's t-test analyses were performed using Prism software (Prism, San Diego, CA, USA). Data were considered significant at P<0.05. Spearman's correlation and Deming's regression, respectively, were applied to describe the relation between α5 integrin expression and extent of Gal-1-mediated anoikis.
Acknowledgments
We wish to express our gratitude to the expert review providing us with valuable suggestions. This work was supported by grants from Deutsche Krebshilfe, DFG, Wilhelm–Sander Stiftung, Else Kröner Fresenius Stiftung, Berliner Krebsgesellschaft and Sonnenfeld–Stiftung to SR, and by a grant from Wilhelm-Sander Stiftung to KD. HJG was supported by an EC Marie Curie Research Training Network grant (contract no. MRTN-CT-2005-019561) and the Verein zur Förderung des biologisch-technologischen Fortschritts in der Medizin.
Glossary
- ECM
extracellular matrix
- Gal-1
galectin-1
- ManNAc
N-acetylmannosamine
- SNA
Sambucus nigra agglutinin
- PNA
peanut agglutinin
- Erk
extracellular signal-regulated protein kinase
- PKB/AKT
protein kinase B
- PCNA
proliferating cell nuclear antigen
- poly-HEMA
poly(2-hydroxyethyl methacrylate)
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by RA Knight
Supplementary Material
References
- Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24:425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- Rytomaa M, Martins LM, Downward J. Involvement of FADD and caspase-8 signalling in detachment-induced apoptosis. Curr Biol. 1999;9:1043–1046. doi: 10.1016/s0960-9822(99)80454-0. [DOI] [PubMed] [Google Scholar]
- Frisch SM. Evidence for a function of death-receptor-related, death-domain-containing proteins in anoikis. Curr Biol. 1999;9:1047–1049. doi: 10.1016/s0960-9822(99)80455-2. [DOI] [PubMed] [Google Scholar]
- Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol. 2001;155:459–470. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellis SL. Variant glycosylation: an underappreciated regulatory mechanism for β1 integrins. Biochim Biophys Acta. 2004;1663:52–60. doi: 10.1016/j.bbamem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Gabius H-J.(ed).The Sugar Code Fundamentals of Glycosciences 2009Wiley-VCH: Weinheim, Germany [Google Scholar]
- Kobata A, Amano J. Altered glycosylation of proteins produced by malignant cells, and application for the diagnosis and immunotherapy of tumours. Immunol Cell Biol. 2005;83:429–439. doi: 10.1111/j.1440-1711.2005.01351.x. [DOI] [PubMed] [Google Scholar]
- Fischer C, Sanchez-Ruderisch H, Welzel M, Wiedenmann B, Sakai T, André S, et al. Galectin-1 interacts with the α5β1 fibronectin receptor to restrict carcinoma cell growth via induction of p21 and p27. J Biol Chem. 2005;280:37266–37277. doi: 10.1074/jbc.M411580200. [DOI] [PubMed] [Google Scholar]
- Gabius HJ. Glycans: bioactive signals decoded by lectins. Biochem Soc Trans. 2008;36 (Part 6:1491–1496. doi: 10.1042/BST0361491. [DOI] [PubMed] [Google Scholar]
- López-Lucendo MF, Solis D, André S, Hirabayashi J, Kasai K, Kaltner H, et al. Growth-regulatory human galectin-1: crystallographic characterisation of the structural changes induced by single-site mutations and their impact on the thermodynamics of ligand binding. J Mol Biol. 2004;343:957–970. doi: 10.1016/j.jmb.2004.08.078. [DOI] [PubMed] [Google Scholar]
- Gabius H-J. Cell surface glycans: the why and how of their functionality as biochemical signals in lectin-mediated information transfer. Crit Rev Immunol. 2006;26:43–79. doi: 10.1615/critrevimmunol.v26.i1.30. [DOI] [PubMed] [Google Scholar]
- Hughes RC. Galectins as modulators of cell adhesion. Biochimie. 2001;83:667–676. doi: 10.1016/s0300-9084(01)01289-5. [DOI] [PubMed] [Google Scholar]
- Zhuo Y, Chammas R, Bellis SL. Sialylation of β1 integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J Biol Chem. 2008;283:22177–22185. doi: 10.1074/jbc.M8000015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadari YR, Arbel-Goren R, Levy Y, Amsterdam A, Alon R, Zakut R, et al. Galectin-8 binding to integrins inhibits cell adhesion and induces apoptosis. J Cell Sci. 2000;113 (Part 13:2385–2397. doi: 10.1242/jcs.113.13.2385. [DOI] [PubMed] [Google Scholar]
- Moiseeva EP, Williams B, Goodall AH, Samani NJ. Galectin-1 interacts with beta-1 subunit of integrin. Biochem Biophys Res Commun. 2003;310:1010–1016. doi: 10.1016/j.bbrc.2003.09.112. [DOI] [PubMed] [Google Scholar]
- Fortin S, Le Mercier M, Camby I, Spiegl-Kreinecker S, Berger W, Lefranc F, et al. Galectin-1 Is implicated in the protein kinase C ɛ/vimentin-controlled trafficking of integrin-β1 in glioblastoma cells. Brain Pathol. 2010;20:39–49. doi: 10.1111/j.1750-3639.2008.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Bass MD, Craig SE, Pinney JW, Knight D, et al. Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6. Sci Signal. 2009;2:ra51. doi: 10.1126/scisignal.2000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Reed JC, Ruoslahti E. The α5β1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath T, Detjen K, Welzel M, von Marschall Z, Murphy D, Schirner M, et al. A novel function for the tumor suppressor p16INK4a: induction of anoikis via upregulation of the α5β1 fibronectin receptor. J Cell Biol. 2000;150:1467–1478. doi: 10.1083/jcb.150.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André S, Sanchez-Ruderisch H, Nakagawa H, Buchholz M, Kopitz J, Forberich P, et al. Tumor suppressor p16INK4a - modulator of glycomic profile and galectin-1 expression to increase susceptibility to carbohydrate-dependent induction of anoikis in pancreatic carcinoma cells. FEBS J. 2007;274:3233–3256. doi: 10.1111/j.1742-4658.2007.05851.x. [DOI] [PubMed] [Google Scholar]
- Semel AC, Seales EC, Singhal A, Eklund EA, Colley KJ, Bellis SL. Hyposialylation of integrins stimulates the activity of myeloid fibronectin receptors. J Biol Chem. 2002;277:32830–32836. doi: 10.1074/jbc.M202493200. [DOI] [PubMed] [Google Scholar]
- Bartik P, Maglott A, Entlicher G, Vestweber D, Takeda K, Martin S, et al. Detection of a hypersialylated β1 integrin endogenously expressed in the human astrocytoma cell line A172. Int J Oncol. 2008;32:1021–1031. [PubMed] [Google Scholar]
- Amano M, Galvan M, He J, Baum LG. The ST6Gal I sialyltransferase selectively modifies N-glycans on CD45 to negatively regulate galectin-1-induced CD45 clustering, phosphatase modulation, and T cell death. J Biol Chem. 2003;278:7469–7475. doi: 10.1074/jbc.M209595200. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Gabius H-J, Kaltner H, André S, Kuwabara I, Lin F-T, et al. Thermodynamic binding studies of cell surface carbohydrate epitopes to galectins-1, -3, and -7: Evidence for differential binding specificities. Can J Chem. 2002;80:1096–1104. [Google Scholar]
- Aoudjit F, Vuori K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J Cell Biol. 2001;152:633–643. doi: 10.1083/jcb.152.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM. Caspase-8: fly or die. Cancer Res. 2008;68:4491–4493. doi: 10.1158/0008-5472.CAN-08-0952. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ruderisch H, Fischer C, Detjen KM, Welzel M, Wimmel A, Manning JC, et al. Tumor suppressor p16INK4a: downregulation of galectin-3, an endogenous competitor of the pro-anoikis effector galectin-1, in a pancreatic carcinoma model. FEBS J. 2010;277:3552–3563. doi: 10.1111/j.1742-4658.2010.07764.x. [DOI] [PubMed] [Google Scholar]
- Barbero S, Mielgo A, Torres V, Teitz T, Shields DJ, Mikolon D, et al. Caspase-8 association with the focal adhesion complex promotes tumor cell migration and metastasis. Cancer Res. 2009;69:3755–3763. doi: 10.1158/0008-5472.CAN-08-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain KH, Landowski TH, Dalton WS. Adhesion-mediated intracellular redistribution of c-Fas-associated death domain-like IL-1-converting enzyme-like inhibitory protein-long confers resistance to CD95-induced apoptosis in hematopoietic cancer cell lines. J Immunol. 2002;168:2544–2553. doi: 10.4049/jimmunol.168.5.2544. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Villunger A, O'Reilly LA, Beaumont JG, Coultas L, Cheney RE, et al. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 2001;293:1829–1832. doi: 10.1126/science.1062257. [DOI] [PubMed] [Google Scholar]
- Schutze S, Tchikov V, Schneider-Brachert W. Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat Rev Mol Cell Biol. 2008;9:655–662. doi: 10.1038/nrm2430. [DOI] [PubMed] [Google Scholar]
- Wang J, Lu ZH, Gabius HJ, Rohowsky-Kochan C, Ledeen RW, Wu G. Cross-linking of GM1 ganglioside by galectin-1 mediates regulatory T cell activity involving TRPC5 channel activation: possible role in suppressing experimental autoimmune encephalomyelitis. J Immunol. 2009;182:4036–4045. doi: 10.4049/jimmunol.0802981. [DOI] [PubMed] [Google Scholar]
- Kopitz J, Bergmann M, Gabius HJ. How adhesion/growth-regulatory galectins-1 and -3 attain cell specificity: case study defining their target on neuroblastoma cells (SK-N-MC) and marked affinity regulation by affecting microdomain organization of the membrane. IUBMB Life. 2010;62:624–628. doi: 10.1002/iub.358. [DOI] [PubMed] [Google Scholar]
- André S, Pei Z, Siebert HC, Ramström O, Gabius HJ. Glycosyldisulfides from dynamic combinatorial libraries as O-glycoside mimetics for plant and endogenous lectins: their reactivities in solid-phase and cell assays and conformational analysis by molecular dynamics simulations. Bioorg Med Chem. 2006;14:6314–6326. doi: 10.1016/j.bmc.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Patsos G, André S, Roeckel N, Gromes R, Gebert J, Kopitz J, et al. Compensation of loss of protein function in microsatellite-unstable colon cancer cells (HCT116): a gene-dependent effect on the cell surface glycan profile. Glycobiology. 2009;19:726–734. doi: 10.1093/glycob/cwp040. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Streit M, Kaiser R, Herzberg F, Schirner M, Schramm K, et al. De novo expression of the α5β1-fibronectin receptor in HT29 colon-cancer cells reduces activity of C-SRC Increase of C-SRC activity by attachment on fibronectin. Int J Cancer. 1998;76:91–98. doi: 10.1002/(sici)1097-0215(19980330)76:1<91::aid-ijc15>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.