Abstract
The receptor structure recognized by Escherichia coli possessing K99 fimbriae and by isolated K99 fimbrial fractions was examined by using an equine erythrocyte hemagglutination inhibition test. Both K99-positive organisms (strain B41) and fimbrial preparations reacted with N-glycolylneuraminyl-lactosyl-ceramide (NeuGcLacCer) purified from equine erythrocytes with very high potency. Fimbrial preparations were 253 times more potent than intact organisms, indicating that isolated fimbriae more precisely recognize the structure of NeuGcLacCer than do fimbriae located on the bacterial cell wall. Structurally, the N-glycolyl group of the sialic acid was shown to be essential because substitution of the N-acetyl group for the N-glycolyl group caused the reactivity to completely disappear. The substitution of the O-acetyl group for the C4 hydroxyl group of the sialic acid (4-O-Ac-NeuGcLacCer) also diminished the reactivity by about 500 times, indicating that the fine structure of NeuGc is necessary for recognition. N-Glycolylneuraminyl-neolactotetraosyl-ceramide (NeuGcnLc4Cer) and N-glycolylneuraminyl-neolactohexaosyl-ceramide (NeuGcnLc6Cer), both of which have identical disaccharides at the nonreducing terminal and longer carbohydrate chains, showed reduced reactivity, indicating that the ceramide of NeuGcLacCer is also involved in the recognition. Indeed, NeuGcLac oligosaccharides altered by cleavage of the ceramide or the terminal sialic acid (NeuGc) showed dramatically reduced reactivities. Ten other E. coli strains (isolated from diseased calves) and two strains (isolated from diseased piglets) which possessed the same K99 antigen and various O antigens were used for the recognition test. The results obtained were similar to those mentioned above.
Full text
PDF

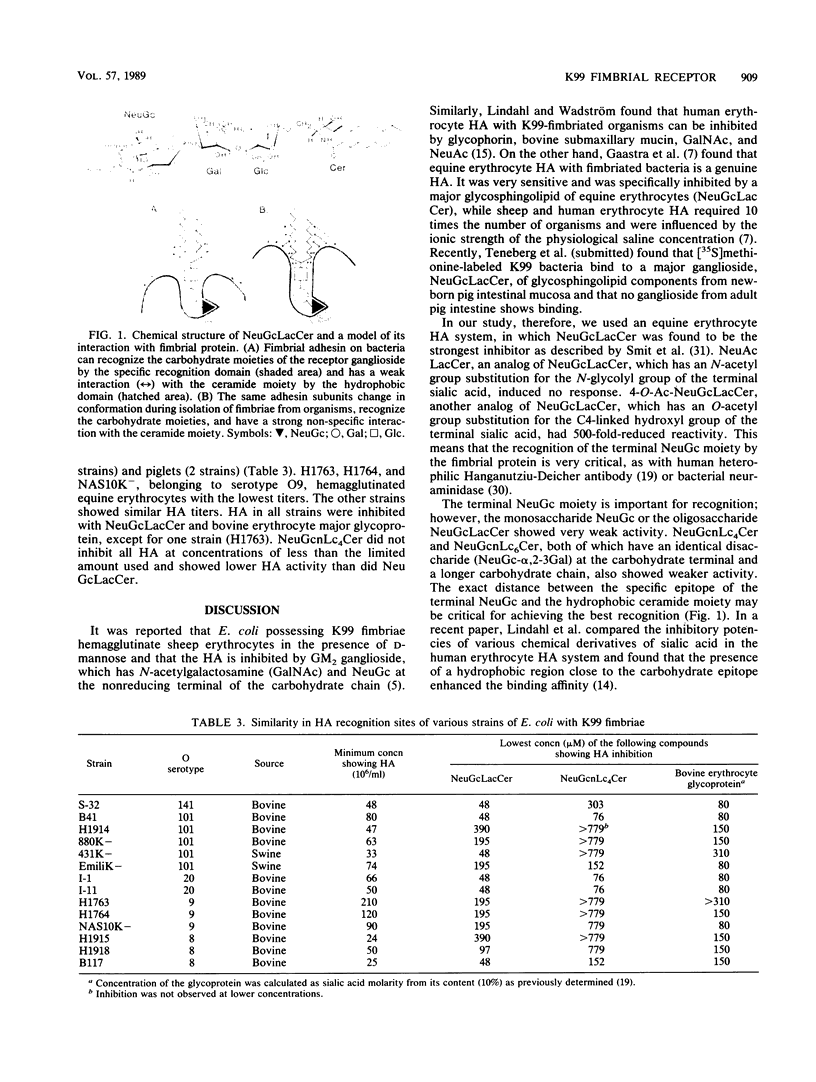


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awad-Masalmeh M., Moon H. W., Runnels P. L., Schneider R. A. Pilus production, hemagglutination, and adhesion by porcine strains of enterotoxigenic Escherichia coli lacking K88, K99, and 987P antigens. Infect Immun. 1982 Jan;35(1):305–313. doi: 10.1128/iai.35.1.305-313.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhours J. F., Glickman R. M. Rat intestinal glycolipids. II. Distribution and biosynthesis of glycolipids and ceramide in villus and crypt cells. Biochim Biophys Acta. 1976 Jul 20;441(1):123–133. doi: 10.1016/0005-2760(76)90287-3. [DOI] [PubMed] [Google Scholar]
- Chien J. L., Li S. C., Laine R. A., Li Y. T. Characterization of gangliosides from bovine erythrocyte membranes. J Biol Chem. 1978 Jun 10;253(11):4031–4035. [PubMed] [Google Scholar]
- Fujii Y., Higashi H., Ikuta K., Kato S., Naiki M. Specificities of human heterophilic Hanganutziu and Deicher (H-D) antibodies and avian antisera against H-D antigen-active glycosphingolipids. Mol Immunol. 1982 Jan;19(1):87–94. doi: 10.1016/0161-5890(82)90250-4. [DOI] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasa S., Makita A., Kinoshita Y. Further study of the chemical structure of the equine erythrocyte hematoside containing O-acetyl ester. J Biol Chem. 1983 Jan 25;258(2):876–881. [PubMed] [Google Scholar]
- Hanson M. S., Brinton C. C., Jr Identification and characterization of E. coli type-1 pilus tip adhesion protein. Nature. 1988 Mar 17;332(6161):265–268. doi: 10.1038/332265a0. [DOI] [PubMed] [Google Scholar]
- Higashi H., Naiki M., Matuo S., Okouchi K. Antigen of "serum sickness" type of heterophile antibodies in human sera: indentification as gangliosides with N-glycolylneuraminic acid. Biochem Biophys Res Commun. 1977 Nov 21;79(2):388–395. doi: 10.1016/0006-291x(77)90169-3. [DOI] [PubMed] [Google Scholar]
- Jacobs A. A., van Mechelen J. R., de Graaf F. K. Effect of chemical modifications on the K99 and K88ab fibrillar adhesins of Escherichia coli. Biochim Biophys Acta. 1985 Nov 29;832(2):148–155. doi: 10.1016/0167-4838(85)90326-7. [DOI] [PubMed] [Google Scholar]
- Jacobs A. A., van den Berg P. A., Bak H. J., de Graaf F. K. Localization of lysine residues in the binding domain of the K99 fibrillar subunit of enterotoxigenic Escherichia coli. Biochim Biophys Acta. 1986 Jul 25;872(1-2):92–97. doi: 10.1016/0167-4838(86)90151-2. [DOI] [PubMed] [Google Scholar]
- Lindahl M., Wadström T. K99 surface haemagglutinin of enterotoxigenic E. coli recognize terminal N-acetylgalactosamine and sialic acid residues of glycophorin and other complex glycoconjugates. Vet Microbiol. 1984 Jul;9(3):249–257. doi: 10.1016/0378-1135(84)90042-7. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Moch T., Hoschützky H., Hacker J., Kröncke K. D., Jann K. Isolation and characterization of the alpha-sialyl-beta-2,3-galactosyl-specific adhesin from fimbriated Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(10):3462–3466. doi: 10.1073/pnas.84.10.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Nagy B., Isaacson R. E., Orskov I. Occurrence of K99 antigen on Escherichia coli isolated from pigs and colonization of pig ileum by K99+ enterotoxigenic E. coli from calves and pigs. Infect Immun. 1977 Feb;15(2):614–620. doi: 10.1128/iai.15.2.614-620.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukuria C. J., Fujii Y., Kato S., Naiki M. Specificities of human heterophile Hanganutziu and Deicher (HD) antibodies to glycosphingolipids and a glycoprotein. J Biochem. 1986 Aug;100(2):469–475. doi: 10.1093/oxfordjournals.jbchem.a121735. [DOI] [PubMed] [Google Scholar]
- Nakazawa M., Nemoto H., Ueda H., Maruyama T. Enteropathogenic Escherichia coli in calves with diarrhea. Nihon Juigaku Zasshi. 1981 Feb;43(1):89–91. doi: 10.1292/jvms1939.43.89. [DOI] [PubMed] [Google Scholar]
- Nakazawa M., Sugimoto C., Isayama Y., Kashiwazaki M. Virulence factors in Escherichia coli isolated from piglets with neonatal and post-weaning diarrhea in Japan. Vet Microbiol. 1987 Apr;13(4):291–300. doi: 10.1016/0378-1135(87)90060-5. [DOI] [PubMed] [Google Scholar]
- Newman R. A., Uhlenbruck G. G. Investigation into the occurrence and structure of lectin receptors on human and bovine erythrocytes milk fat globule and lymphocyte plasma-membrane glycoproteins. Eur J Biochem. 1977 Jun 1;76(1):149–155. doi: 10.1111/j.1432-1033.1977.tb11580.x. [DOI] [PubMed] [Google Scholar]
- Olsson E., Söderlind O. Comparison of different assays for definition of heat-stable enterotoxigenicity of Escherichia coli porcine strains. J Clin Microbiol. 1980 Jan;11(1):6–15. doi: 10.1128/jcm.11.1.6-15.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Smith H. W., Sojka W. J. The establishment of K99, a thermolabile, transmissible escherichia coli K antigen, previously called "Kco", possessed by calf and lamb enteropathogenic strains. Acta Pathol Microbiol Scand B. 1975 Feb;83(1):31–36. doi: 10.1111/j.1699-0463.1975.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Roosendaal E., Boots M., de Graaf F. K. Two novel genes, fanA and fanB, involved in the biogenesis of K99 fimbriae. Nucleic Acids Res. 1987 Aug 11;15(15):5973–5984. doi: 10.1093/nar/15.15.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosendaal E., Jacobs A. A., Rathman P., Sondermeyer C., Stegehuis F., Oudega B., de Graaf F. K. Primary structure and subcellular localization of two fimbrial subunit-like proteins involved in the biosynthesis of K99 fibrillae. Mol Microbiol. 1987 Sep;1(2):211–217. doi: 10.1111/j.1365-2958.1987.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Runnels P. L., Moon H. W., Schneider R. A. Development of resistance with host age to adhesion of K99+ Escherichia coli to isolated intestinal epithelial cells. Infect Immun. 1980 Apr;28(1):298–300. doi: 10.1128/iai.28.1.298-300.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B. The epidemiology of diarrhea due to enterotoxigenic Escherichia coli. J Infect Dis. 1978 May;137(5):639–640. doi: 10.1093/infdis/137.5.639. [DOI] [PubMed] [Google Scholar]
- Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- Smit H., Gaastra W., Kamerling J. P., Vliegenthart J. F., de Graaf F. K. Isolation and structural characterization of the equine erythrocyte receptor for enterotoxigenic Escherichia coli K99 fimbrial adhesin. Infect Immun. 1984 Nov;46(2):578–584. doi: 10.1128/iai.46.2.578-584.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H., Terakado N., Isayama Y., Sakurai K., Nakazawa M. Etiologic studies on enterotoxigenic Escherichia coli among isolates from domestic animals in Japan. Natl Inst Anim Health Q (Tokyo) 1981 Summer;21(2):108–109. [PubMed] [Google Scholar]
- Väisänen-Rhen V., Elo J., Väisänen E., Siitonen A., Orskov I., Orskov F., Svenson S. B., Mäkelä P. H., Korhonen T. K. P-fimbriated clones among uropathogenic Escherichia coli strains. Infect Immun. 1984 Jan;43(1):149–155. doi: 10.1128/iai.43.1.149-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIEGANDT H., BASCHANG G. DIE GEWINNUNG DES ZUCKERANTEILES DER GLYKOSPHINGOLIPIDE DURCH OZONOLYSE UND FRAGMENTIERUNG. Z Naturforsch B. 1965 Feb;20:164–166. [PubMed] [Google Scholar]



