Abstract
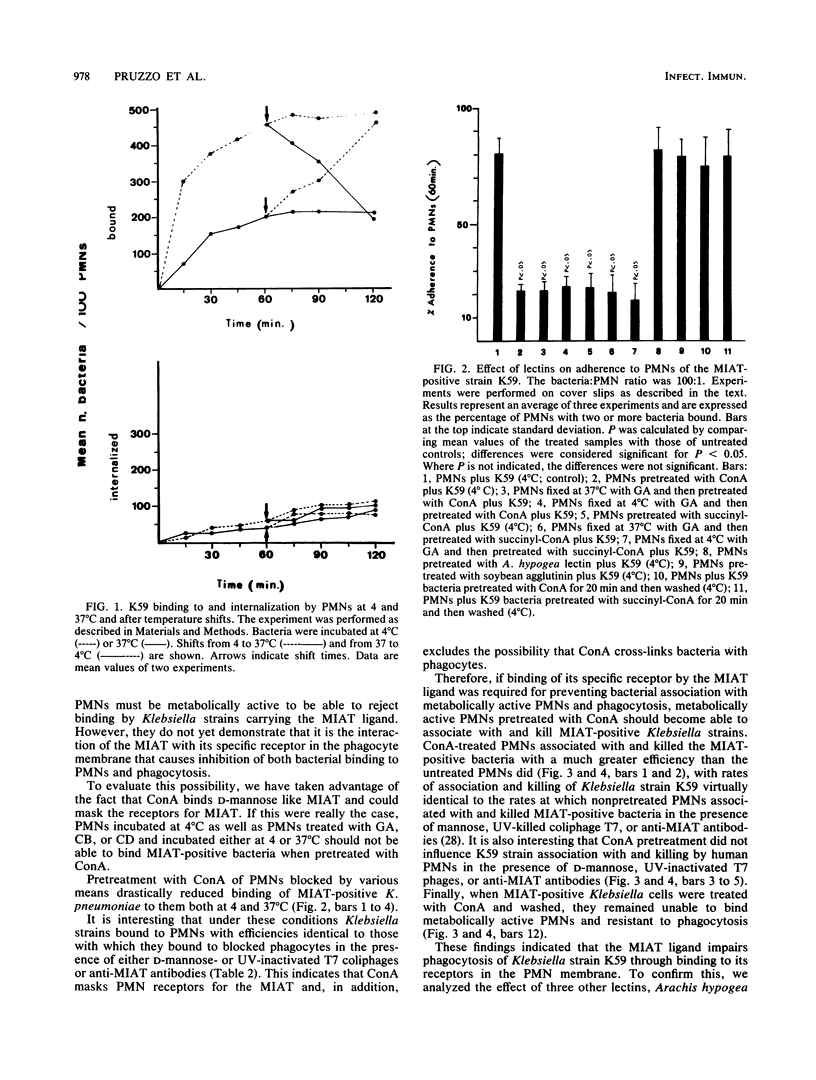
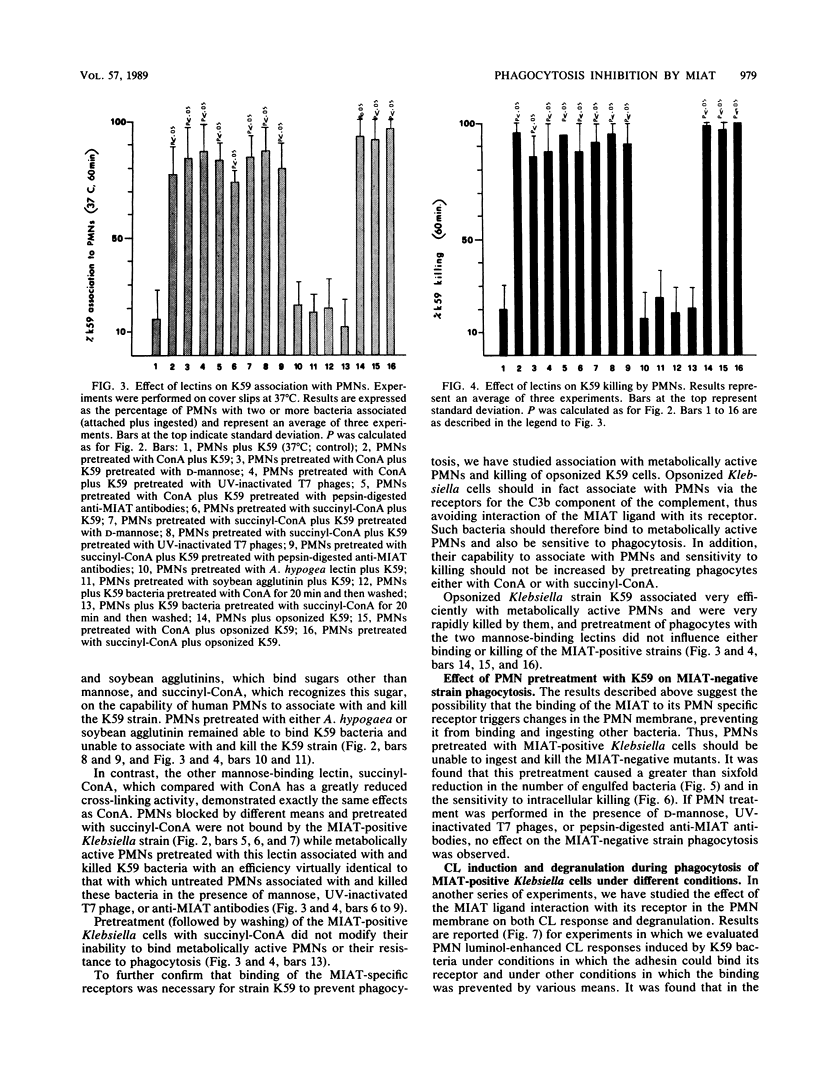
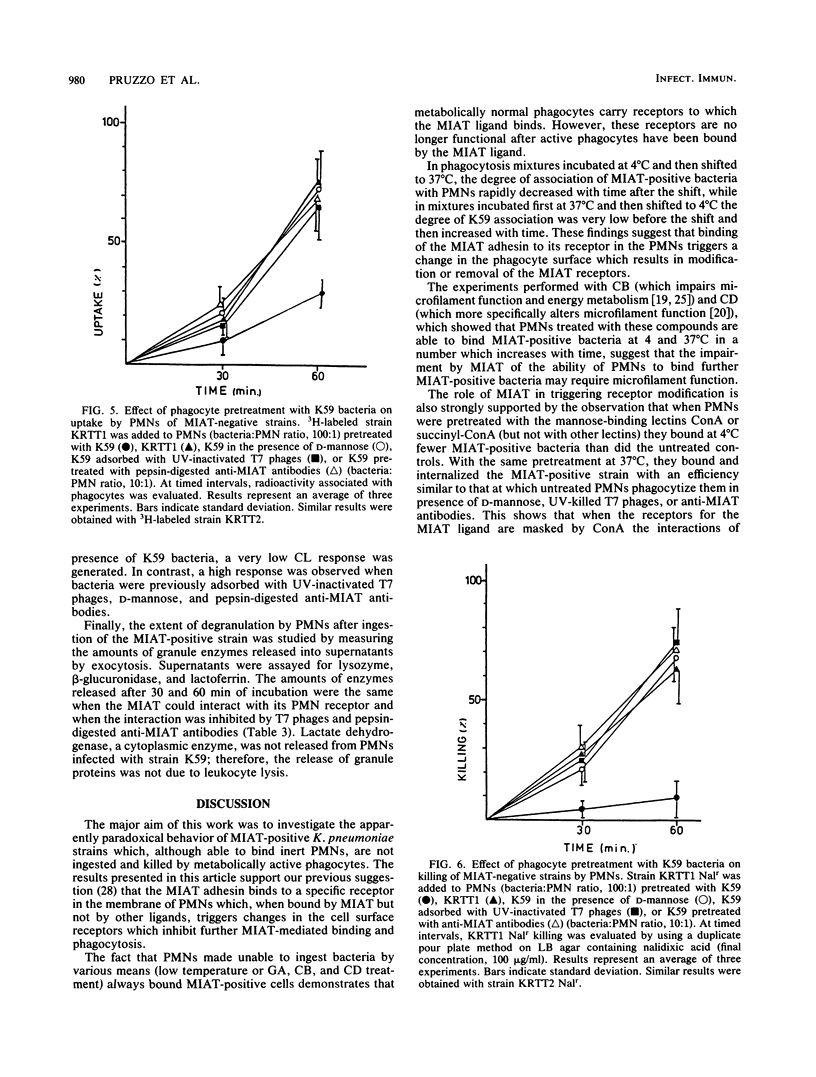
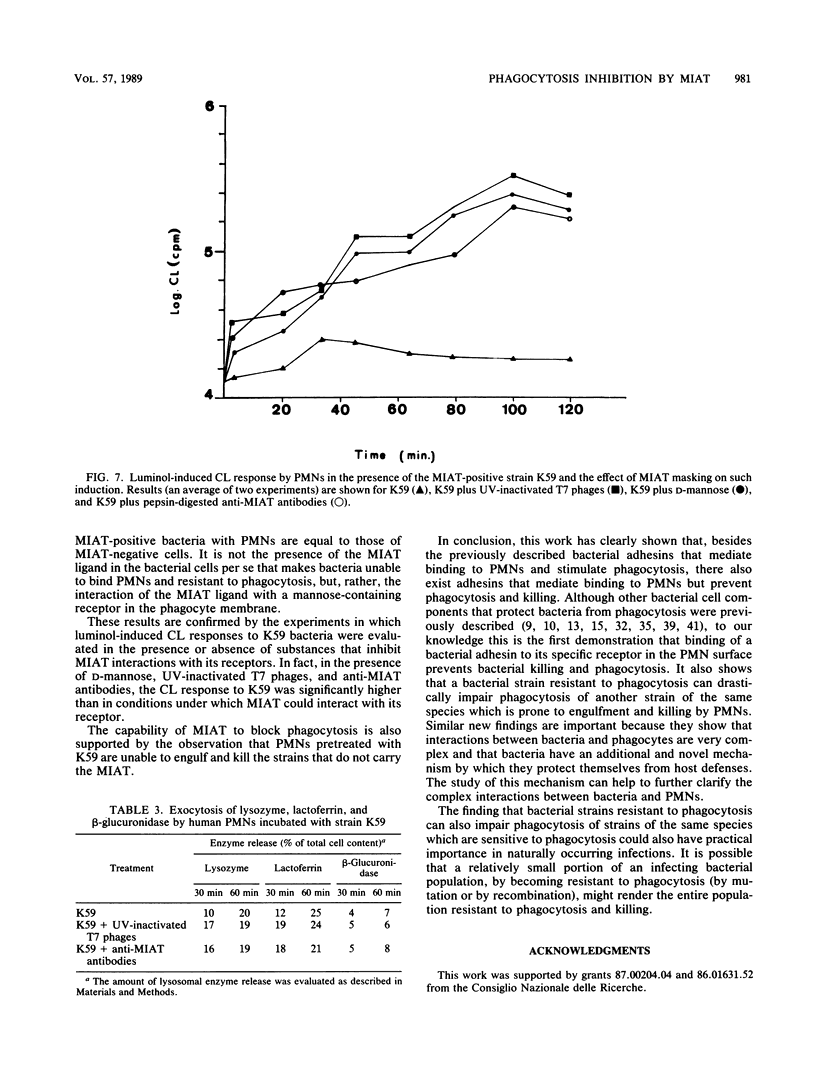
It has been previously shown that Klebsiella pneumoniae K59 carrying the mannose-inhibitable adhesin-T7 receptor (MIAT) efficiently binds to polymorphonuclear leukocytes (PMNs) incubated at 4 degrees C but is not efficiently bound and internalized by phagocytes incubated at 37 degrees C. Pretreatment of K59 with compounds that bind the MIAT ligand (D-mannose, UV-inactivated T7 phages, and pepsin-digested anti-MIAT antibodies) enables PMNs to phagocytize and kill these bacteria. In this article, we show that the incubation temperature has no direct effect on expression of either the MIAT or the PMN receptors. These receptors were always expressed at 37 degrees C when PMNs were treated with substances that impaired their ability to rearrange their surfaces (glutaraldehyde and cytochalasins B and D). Pretreatment of inert PMNs with concanavalin A or succinyl concanavalin A drastically reduced binding of K59 to phagocytes at both 4 and 37 degrees C. The same pretreatment carried out with metabolically active PMNs enabled them to efficiently phagocytize the MIAT-positive strain. When phagocytes were treated with K59 bacteria, they became unable to ingest and kill a K59 mutant not expressing the MIAT which was sensitive to phagocytosis. If this pretreatment was performed in the presence of D-mannose, UV-inactivated T7 phages, and pepsin-digested anti-MIAT antibodies, PMNs maintained their phagocytic activity against the MIAT-negative strain. In the presence of K59 bacteria, a very low chemiluminescence response was generated; in contrast, a significant response was observed when bacteria were previously absorbed with UV-inactivated T7 phages and pepsin-digested anti-MIAT antibodies. These results support our previous suggestion that the MIAT adhesin triggers changes in the cell surface, inhibiting further binding and phagocytosis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit Z., Goldman R., Ofek I., Sharon N., Mirelman D. Mannose-binding activity of Escherichia coli: a determinant of attachment and ingestion of the bacteria by macrophages. Infect Immun. 1980 Aug;29(2):417–424. doi: 10.1128/iai.29.2.417-424.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Björkstén B., Wadström T. Interaction of Escherichia coli with different fimbriae and polymorphonuclear leukocytes. Infect Immun. 1982 Oct;38(1):298–305. doi: 10.1128/iai.38.1.298-305.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenstock E., Jann K. Adhesion of piliated Escherichia coli strains to phagocytes: differences between bacteria with mannose-sensitive pili and those with mannose-resistant pili. Infect Immun. 1982 Jan;35(1):264–269. doi: 10.1128/iai.35.1.264-269.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Cryz S. J. Progress in immunization against Klebsiella infections. Eur J Clin Microbiol. 1983 Dec;2(6):523–528. doi: 10.1007/BF02016559. [DOI] [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Gonococcal interactions with polymorphonuclear neutrophils: importance of the phagosome for bactericidal activity. J Clin Invest. 1978 Dec;62(6):1161–1171. doi: 10.1172/JCI109235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S. H., Rest R. F. Gonococci possessing only certain P.II outer membrane proteins interact with human neutrophils. Infect Immun. 1988 Jun;56(6):1574–1579. doi: 10.1128/iai.56.6.1574-1579.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman W. H., Kato K., Anstiss C. L., Green S. Human serum beta-glucuronidase; its measurement and some of its properties. Clin Chim Acta. 1967 Mar;15(3):435–447. doi: 10.1016/0009-8981(67)90008-3. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A. Phagocytosis of microorganisms. Rev Infect Dis. 1982 Jan-Feb;4(1):104–123. doi: 10.1093/clinids/4.1.104. [DOI] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Fate of human lactoferrin and myeloperoxidase in phagocytizing human neutrophils: effects of immunoglobulin G subclasses and immune complexes coated on latex beads. Infect Immun. 1975 Oct;12(4):813–820. doi: 10.1128/iai.12.4.813-820.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian C. J., Pai C. H. Inhibition of human neutrophil chemiluminescence by plasmid-mediated outer membrane proteins of Yersinia enterocolitica. Infect Immun. 1985 Jul;49(1):145–151. doi: 10.1128/iai.49.1.145-151.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan D. F., Snyder I. S. Mannose-sensitive interaction of Escherichia coli with human peripheral leukocytes in vitro. Infect Immun. 1979 Nov;26(2):520–527. doi: 10.1128/iai.26.2.520-527.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura N., Asano A. Synergistic effect of colchicine and cytochalasin D on phagocytosis by peritoneal macrophages. Nature. 1976 May 27;261(5558):319–321. doi: 10.1038/261319a0. [DOI] [PubMed] [Google Scholar]
- Minion F. C., Abraham S. N., Beachey E. H., Goguen J. D. The genetic determinant of adhesive function in type 1 fimbriae of Escherichia coli is distinct from the gene encoding the fimbrial subunit. J Bacteriol. 1986 Mar;165(3):1033–1036. doi: 10.1128/jb.165.3.1033-1036.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren M., Normark S., Lark D., O'Hanley P., Schoolnik G., Falkow S., Svanborg-Edén C., Båga M., Uhlin B. E. Mutations in E coli cistrons affecting adhesion to human cells do not abolish Pap pili fiber formation. EMBO J. 1984 May;3(5):1159–1165. doi: 10.1002/j.1460-2075.1984.tb01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Sharon N. Lectinophagocytosis: a molecular mechanism of recognition between cell surface sugars and lectins in the phagocytosis of bacteria. Infect Immun. 1988 Mar;56(3):539–547. doi: 10.1128/iai.56.3.539-547.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman L., Hed J., Stendahl O. Interaction between human polymorphonuclear leukocytes and two different strains of type 1 fimbriae-bearing Escherichia coli. J Infect Dis. 1982 Dec;146(6):751–757. doi: 10.1093/infdis/146.6.751. [DOI] [PubMed] [Google Scholar]
- Okuda K. Effects of cytochalasin B on the intrcellular bactericidal activity of human neutrophils. Antimicrob Agents Chemother. 1975 Jun;7(6):736–741. doi: 10.1128/aac.7.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A., Ofek I., Silverblatt F. J. Enhancement of mannose-mediated stimulation of human granulocytes by type 1 fimbriae aggregated with antibodies on Escherichia coli surfaces. Infect Immun. 1983 Mar;39(3):1334–1345. doi: 10.1128/iai.39.3.1334-1345.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzzo C., Debbia E. A., Satta G. Identification of the major adherence ligand of Klebsiella pneumoniae in the receptor for coliphage T7 and alteration of Klebsiella adherence properties by lysogenic conversion. Infect Immun. 1980 Nov;30(2):562–571. doi: 10.1128/iai.30.2.562-571.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzzo C., Debbia E., Satta G. Mannose-inhibitable adhesins and T3-T7 receptors of Klebsiella pneumoniae inhibit phagocytosis and intracellular killing by human polymorphonuclear leukocytes. Infect Immun. 1982 Jun;36(3):949–957. doi: 10.1128/iai.36.3.949-957.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzzo C., Valisena S., Satta G. Laboratory and wild-type Klebsiella pneumoniae strains carrying mannose-inhibitable adhesins and receptors for coliphages T3 and T7 are more pathogenic for mice than are strains without such receptors. Infect Immun. 1983 Feb;39(2):520–527. doi: 10.1128/iai.39.2.520-527.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Lee N., Bowden C. Stimulation of human leukocytes by protein II+ gonococci is mediated by lectin-like gonococcal components. Infect Immun. 1985 Oct;50(1):116–122. doi: 10.1128/iai.50.1.116-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. K., Robertson D. C. Ingestion and intracellular survival of Brucella abortus in human and bovine polymorphonuclear leukocytes. Infect Immun. 1984 Oct;46(1):224–230. doi: 10.1128/iai.46.1.224-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg A. L., Mudrick L. L., Cisar J. O., Brennan M. J., Mergenhagen S. E., Vatter A. E. Type 2 fimbrial lectin-mediated phagocytosis of oral Actinomyces spp. by polymorphonuclear leukocytes. Infect Immun. 1986 Nov;54(2):472–476. doi: 10.1128/iai.54.2.472-476.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Pruzzo C., Debbia E., Fontana R. Close association between shape alteration and loss of immunity to superinfection in a wild-type Klebsiella pneumoniae stable lysogen which can be both immune and nonimmune to superinfection. J Virol. 1978 Dec;28(3):772–785. doi: 10.1128/jvi.28.3.772-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg Edén C., Bjursten L. M., Hull R., Hull S., Magnusson K. E., Moldovano Z., Leffler H. Influence of adhesins on the interaction of Escherichia coli with human phagocytes. Infect Immun. 1984 Jun;44(3):672–680. doi: 10.1128/iai.44.3.672-680.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Young D., King G. Studies on Gonococcus infection. X. Pili and leukocyte association factor as mediators of interactions between gonococci and eukaryotic cells in vitro. Infect Immun. 1975 Jun;11(6):1352–1361. doi: 10.1128/iai.11.6.1352-1361.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott W. M., Hirsh D. C. Demonstration of an outer membrane protein with antiphagocytic activity from Pasteurella multocida of avian origin. Infect Immun. 1988 Jun;56(6):1538–1544. doi: 10.1128/iai.56.6.1538-1544.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Human polymorphonuclear leucocyte receptors for staphylococcal opsonins. Immunology. 1977 Aug;33(2):231–239. [PMC free article] [PubMed] [Google Scholar]
- Whitnack E., Dale J. B., Beachey E. H. Common protective antigens of group A streptococcal M proteins masked by fibrinogen. J Exp Med. 1984 Apr 1;159(4):1201–1212. doi: 10.1084/jem.159.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]


