Abstract
The aim of this review was to discuss the current knowledge on etiopathogenesis, diagnosis and therapeutic management of venous malformations. Venous malformations (VMs) are slow-flow vascular anomalies. They are simple, sporadic or familial (cutaneo-mucosal venous malformation or glomuvenous malformations), combined (e.g. capillaro-venous, capillaro-lymphaticovenous malformations) or syndromic (Klippel-Trenaunay, Blue Rubber Bleb Naevus and Maffucci). Genetic studies have identified causes of familial forms and of 40% of sporadic VMs. Another diagnostic advancement is the identification of elevated D-dimer level as the first biomarker of venous malformations within vascular anomalies. Those associated with pain are often responsive to Low Molecular Weight Heparin which should also be used to avoid disseminated intravascular coagulopathy secondary to intervention, especially if fibrinogen level is low. Finally, development of a modified sclerosing agent, ethylcellulose–ethanol, has improved therapy. It is efficient and safe, and widens indications for sclerotherapy to sensitive and dangerous areas such as hands, feet and periocular area.
Keywords: Klippel-Trenaunay syndrome, Ethylcellulose-Ethanol, Sclerotherapy, D-dimer, Maffuci syndrome
The term » angioma « has been used for a long time for vascular anomalies without precising the diagnosis, leading to improper management. In 1982, a classification was proposed by Mulliken and Glowacki, and accepted by the International Society for the Study of Vascular Anomalies (ISSVA).1-3 This classification is based on clinical, radiological and anatomopathological characteristics, and divides vascular anomalies into vascular tumors and vascular malformations. Hemangioma is the most common vascular tumor, which usually appears postnatally, exhibiting rapid growth due to cellular proliferation, followed by inevitable involution (Infantile Hemangioma) (Figure 1A). Hemangiomas have high-velocity flow in multiple vascular channels. In contrast, vascular malformations are present at birth and grow proportionally with the patient. They are subdivided depending on the affected vessel type into capillary (Figure 1B), venous (Figure 1C), lymphatic (Figure 1D, E) and arterial malformations (Figure 1F). Rheologically, they are slow-flow or fast-flow lesions, discernable with duplex ultrasound scan. When malformations affect more than one vessel type, the combined lesions are named according to the affected vessel, e.g. : capillarovenous and capillaro-lymphatico-venous malformation. Venous malformation can also occur in syndromes such as Klippel-Trenaunay syndrome (capillaro-lymphaticovenous malformation with limb hypertrophy) (Figure 1G) and Maffucci syndrome (Figure 1H) (multiple endochondromas associated with multiple hemangioendothelioma and high incidence of malignancy) (Figure 1) .
Figure 1.
In 1988 another classification (Hamburg classification) of vascular lesions was proposed.4, 5 It includes vascular malformations (called extratruncular lesions) as well as lesions of great vascular channels (called truncular lesions). It is mainly used by vascular surgeons of German and Italian schools. Interdisciplinary teams highly specialized in vascular anomalies follow the ISSVA classification, which is more elaborate and involves etiopathogenetic discoveries for vascular tumors and malformations. The ISSVA classification is based on clinical characteristics, rheology, and ethiopathogenesis, and has helped discovering new subentities. The Hamburg classification is based on a hypothesis of embryologic characteristics without useful clinical applicability. Moreover, since its establishment in 1982, the ISSVA classification is actively replacing old eponyms. The subentities that have been recognized on the basis of clinico-genetic discoveries have been named following the ISSVA classification, rather than by creating new eponyms (e.g. VMCM and CM-AVM).6, 7 (Table 1)
Table 1.
| Tumors | Malformations | Malformation of Major Named Vessels |
|---|---|---|
Hemangioma
Tumors potentially associated with Kasabach-Merritt Phenomenon
Malignant Tumors
|
Simple
Capillary
Venous
Lymphatic
Arterial
Combined
Syndromic
|
Defects of cause, position and number
Valvular Anomalies Aplasia, hypoplasia, obstruction |
1, 2, 3
Legend: gene abbreviations in parenthesis: when on line with disorder = causatif gene; when below = causatif gene for a subcategory of
The clinical diagnosis of a vascular malformation can be difficult even in specialized interdisciplinary centers for vascular anomalies, as these lesions can mimic each other and some malignant tumors. 8-10 As we previously summarized, experienced clinician can make the diagnosis for most patients based on clinical history (presence at birth, growth during life, triggers such as puberty or trauma, and family history) and examination (Table 2). Important clinical clues are color (variations of pink, red, blue and purple), aspect (flat, raised, homogeneous, patchy, hyperkeratotic and ulcerated), localization, size, distribution (uni- or multifocal), palpation (hard, firm, compressible, and presence of a thrill), temperature (warm or normal), painfulness (spontaneous or provoking factors) and auscultation (bruit).11
Table 2.
Venous anomalies : clinical, genetic and histologic characteristics and management
| Venous Anomalies | Genetic | #, localisation, color and palpation | Other features | Histology | Management |
|---|---|---|---|---|---|
| Simple | |||||
|
| |||||
| Unifocal sporadic | somatic activation TIE2 (49%) |
solitary, all tissues & internal organs, normal to bluish color, compressible, phleboliths |
pain at awakening & effort, elevated D-dimer level, local thrombosis (phlebolith), no pulmonary embolism |
enlarged venous channels, flattened layer of endothelial cells, sparse smooth muscle cells |
elastic compression, NSAI, LMWH, sclerotherapy, surgery |
| Multifocal sporadic | somatic activation TIE2 | multifocal, mucosal, cutaneous & muscular, normal to bluish color, less compressible |
|||
| VMCM | germinal activation TIE2 | multifocal, mucosal & cutaneous, bluish color, less compressible |
|||
| Glomuvenous | loss of function Glomulin | multifocal, cutaneous, bluish to purple color, nodular or plaquelike, not compressible,no phlebolith |
pain at compression, normal D- dimer level |
enlarged venous channels & undifferentiated smooth muscle cells = “glomus cells” |
no compression, NSAI, surgery, rarely sclerotherapy |
|
| |||||
| Combined | |||||
|
| |||||
| Capillarovenous | unknown | solitary, cutaneous, subcutaneous, red to bluish-purple color, capillary malformation overlying venous malformation, less compressible |
pain at awakening & effort, elevated D-dimer level |
increased number of dilated capillaries + dilated venous-like channels with relative lack of smooth muscle cells |
laser, elastic compression, NSAI, LMWH, sclerotherapy, surgery |
| Capillary+Venous | unknown | capillary malformation & distant multifocal venous malformations, less compressible |
|||
| Lymphaticovenous | unknown | solitary, bluish-purple color, lymphatic dermal vesicles & subcutaneous venous malformation, not compressible |
lymphatic oozing & infection | lymphatic dermal vesicles + dilated venous-like channels with relative lack of smooth muscle cells |
|
|
| |||||
| Syndromic | |||||
|
| |||||
| Klippel-Trenaunay | unknown | capillaro-lymphaticovenous malformation + limb hypertrophy |
pain, elevated D-dimer level, pulmonary embolism |
elastic compression, NSAI, LMWH, sclerotherapy, surgery |
|
| Blue Rubber Bleb Naevus | unknown | multifocal venous malformations, mucosal & cutaneous, hyperkeratotic bluish blebs on palms & soles |
pain, elevated D-dimers, chronic anemia, GI bleeding |
enlarged venous channels, flattened layer of endothelial cells, sparse smooth muscle cells |
iron supplement, LMWH, sclerotherapy, surgery |
| Maffucci | unknown / no PTHR1 mutation |
multifocal, bluish nodules deforming hands & feet + multiple enchondromas |
pain, normal D-dimer level, severe deformities of hands & feet, spontaneous fractures, malignancies |
spindle cell hemangioendothelioma + enchondromas |
surgery |
NSAI = Non Steroidal anti-inflammatory medication
LMWH =Low Molecular Weight Heparin
# = Number
GI = Gastrointestinal
VMCM: Inherited cutaneomucosal venous malformations
Grey shadows: group of shared features
Clinical and histological presentation of venous malformations
Venous malformations are the most frequent slow-flow vascular malformations referred to specialized centers.11 Most of them are sporadic, and unifocal (VM, 93%), although 1% are multifocal (Table 2). Inherited forms, comprised of cutaneomucosal venous malformations (VMCM, 1%) and glomuvenous malformations (GVMs, 5%) are often multifocal.12, 13 There is no sex preponderance. VMCM and GVM have an age-dependant variation in penetrance, which reaches its maximum by 20 years of age (87% for VMCM and 93% for GVM).14
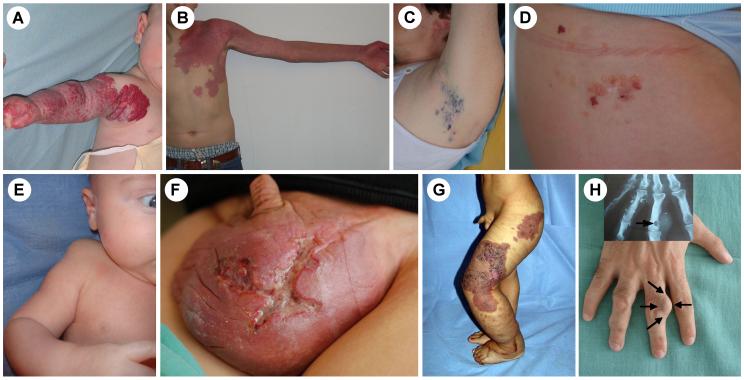
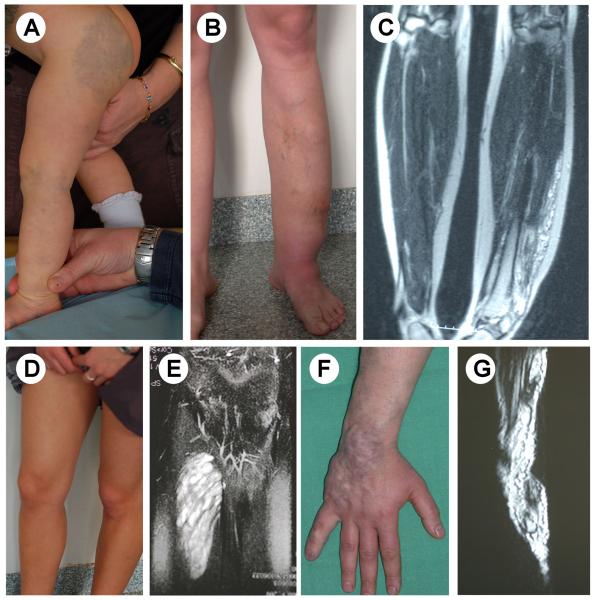
Venous malformations are light-to-dark-blue lesions that can be emptied by compression or in the upright position (Figure 2).15 There is no thrill or bruit, and on palpation, the affected area is not warmer than non-lesional areas. Palpation is not painful unless thrombosis occurs. VMs can affect any tissue or organ, such as cutaneous and subcutaneous tissue (Figure 3A, B, C), muscle (Figure 3D, E), joint (Figure 3F, G) or intestine. Depending on their size and location, and the effort and hormonal status of the patient, VMs can be painful. Migraine is a common feature in facial VM located in the temporal muscle. On extremities, they often cause muscle weakness, and hypo- or hypertrophy (Figure 3). They can also threaten life because of bleeding, expansion or obstruction of vital structures. Pharyngeal or laryngeal location can compromise the airway and cause snoring or even sleeping apnea. Gastrointestinal VM can lead to chronic anemia.
Figure 2.
Figure 3.
Multifocal venous malformations, whether familial or sporadic, are mostly raised, small in diameter (<5cm) and less compressible. Whether sporadic or inherited, patients with multifocal lesions can develop additional VMs by time. In contrast to sporadic VMs, VMCMs are more superficial and seldomly invade muscles. They have never reported to extend within a joint or a bone. Due to their small size, they are often asymptomatic.6, 12, 13, 16-18
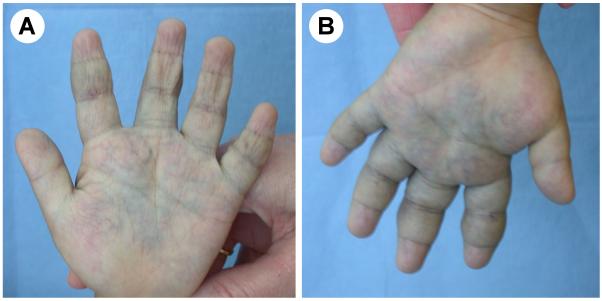
GVMs differ clinically from venous malformations.14, 18, 19 Their color varies from pink-to-purple or dark blue. They are present at birth, raised, cobblestone or plaque-like and slightly hyperkeratotic. In contrast to VMCMs, they are mainly located in the extremities and not compressible. They are more superficial than VMs, involving skin and subcutis, and rarely mucosa. They are painful when compressed and thus, contrary to VMs, elastic compressive garments aggravate pain. They should not be confused with the solitary and painful glomangioma of the nail bed.20
Most VMs undergo a continuous cycle of spontaneous thrombosis and thrombolysis. Persistent thrombi can calcify, resulting in the formation of phleboliths, pathognomonic on plain X-Ray.21-23 VMs do not cause pulmonary embolism because the channels that are thrombosed are sequestered from the main conducting channels.
Histologically, the different types of VMs (unifocal, multifocal sporadic or familial) are characterized by enlarged venous channels lined by a single flattened layer of endothelial cells surrounded by sparse, irregularly distributed smooth muscle cells.16
Glomuvenous malformations, previously known as “glomangiomas”, are characterized by the presence of undifferentiated smooth muscle cells (glomus cells) surrounding convoluted venous channels.24
Clinical presentation of combined and syndromic malformations
Combined vascular malformations (Table 2) most often are capillarovenous malformations (CVM), and capillaro-lymphaticovenous malformations (CLVM). These lesions are composed of a capillary malformation and an underlying venous or lymphatico-venous malformation. They usually involve cutis and subcutis, and rarely muscles. CLVM often causes oozing.10, 25, 26
Some patients have capillary malformations associated with distinct multifocal VMs (CM+VM).
Syndromes with a venous anomaly comprise of Blue Rubber Bleb Naevus syndrome, Klippel-Trenaunay syndrome and Maffucci syndrome. They are all sporadic. Clinical examination, Duplex ultrasound and MRI are necessary to confirm the diagnosis, and to propose a treatment and follow-up.10
The Blue Rubber Bleb Naevus (BRBN) syndrome (Bean syndrome) associates cutaneous and visceral venous malformations. The cutaneous VMs are multiple, small, rubbery often located on the palms and soles.27, 28 There is often a large lesion present at birth, and an increasing number of multiple dark blue lesions disseminated all over the body. The multiple sessile lesions of the intestine, which cause chronic bleeding and anemia, are a diagnostic criterion. In rare cases, BRBN can involve other organs, including bladder, liver, spleen, kidneys and lungs.
The Klippel-Trenaunay syndrome is an eponym for capillary-lymphaticovenous malformation located on an extremity with hypertrophy, often confounded with a pure capillary malformation with a hyper- or hypotrophy of an extremity.29-31
Contrary to Klippel-Trenaunay syndrome, a pure capillary malformation does not affect lymphatic or venous channels. Moreover, tissue hypertrophy is commonly moderate, and not present at birth. Duplex ultrasound is used to detect hypoplasia of the deep venous system, and the presence of ectopic and ectasic subcutaneous veins. Lower extremities are affected in 95% of cases, but upper limbs, abdomen and genitalia can be involved. Hypertrophy of the limb is usually present at birth. Potential complications include bleeding, oozing of the lymphatic vesicles, ulceration, infection, thrombosis and pulmonary embolism.30, 32 These patients need careful management and follow-up to ensure early detection of these complications.
Maffucci syndrome is a rare developmental disorder characterized by multiple enchondromas associated with subcutaneous hemangioendothelioma of the distal extremities.33, 34 The disease starts during childhood with the development of enchondromas of the bones of hands and feet, as well as of the long bones. Deformities and shortening of extremities often occur. The subcutaneous vascular nodules appear later, around puberty, on the fingers and the toes; phleboliths may become present. Histopathological examination shows features of spindle cell hemangioendothelioma, e.g.: nodules of dense spindle cell infiltration in combination with dysplastic vessels.35 These patients have a high incidence of malignancies (40%) mainly of chondrosarcoma, but also glioma, fibrosarcoma and angiosarcoma.36-38
Differential diagnosis of blue lesions on the skin
Patients with blue lesions on the skin are often referred to the interdisciplinary centers as “angioma”. The referred physician must eliminate non-vascular lesions and differentiate within these various blue vascular anomalies. With the clinical history, medical examination and Duplex ultrasound, a clinician can make the diagnosis in most cases. Histopathologic examination is sometimes necessary.
Dermal melanocytic nevi are blue lesions, which arise from dermal melanocytes that have become arrested in the dermis during fetal life and tissue modeling, and have never reached their normal site at the basal layer of the skin.39 The Mongolian spot is a macular blue-grey pigmentation present at birth in infants of darker-skinned races. The lumbosacral region is the most common site but buttocks and shoulders may be affected. Nevi of Ota and Ito are dermal melanocytoses that differ from the Mongolian spot by having a speckled rather than uniform appearance. The nevus of Ota is a unilateral discoloration of the face composed of blue and brown, partially confluent, macular lesions. Ota called the lesion nevus fusocaeruleus ophtalmomaxillaris because of its distribution into the periorbital region, sclera, conjunctiva, temple, forehead, malar area and nose. The nevus of Ito is localized in the supraclavicular, scapular and deltoid regions. The common blue nevus is a well circumscribed blue nodule or macular plaque seen on any site of the body. It appears around puberty.
Subcutaneous hemangioma can mimic a venous malformation as both are blue. Usually hemangiomas grow postnatally and involute spontaneously. However, parents do not always know if the lesion was present at birth, some VMs increase in volume during infancy and spontaneous involution of subcutaneous hemangioma is long (3-4 years). Duplex ultrasound is the best examination to differentiate the fast-flow hemangioma from the slow-flow VM.
Haemorrage within a lymphatic cyst of a lymphatic malformation mimics a venous malformation, as the lymphatic malformation becomes blue. A less compressible feature of the lesion is in favor of a lymphatic malformation. Duplex ultrasound cannot always differentiate these two slow-flow malformations. Histopathological examination of lymphatic malformations will show positive D2-40 staining (podoplanin), a specific marker for lymphatic endothelial cells.33
Imaging studies
Duplex ultrasound is the best examination to confirm slow-flow, to identify the anatomy of feeding vessels and to offer a graphic visual demonstration of vascularity. Venous malformations appear, in 80% of cases, as hypoechoic or heterogeneous and compressible lesions. However, even on experienced radiological hands, differentiation between venous and lymphatic malformations can be difficult.40 The pathognomonic signs of lymphatic malformations, including non compressible, hypo or anechoic cysts, thick septa and liquid levels, are not always present. This can be further complicated by intracystic bleeding.
MRI imaging with spin-echo T1 and T2-weighted sequences is the gold standard for pretherapeutic evaluation of VMs and should not be replaced by MRI-angiography, the flow-sensitive images of which, do not add any useful information. T1 and T2-weighted MRI images depicts the anatomic relation between the vascular lesion and adjacent organs, nerves, tendons and muscles. On T2-weighted sequences with fat saturation, venous malformations show hyperintense channels containing septations.41-43 Goyal and collaborators graded lesions using MR imaging on the basis of the size and margins:44
grade 1: well-defined; ≤5cm in diameter
grade 2A: well-defined; >5cm in diameter
grade 2B: ill-defined; ≤5cm in diameter
grade 3: ill-defined; >5cm in diameter
Another option is to use contrast venograms like Berenguer and collaborators who categorized VMs into 3 major morphologic types.45
lobulated : rounded clusters of vascular spaces with few or no connections to adjacent local veins
varicose: irregular dilated channels recognizable as conducting veins
combined: combination of the two types
The report of Goyal and collaborators demonstrated that small and better defined lesions (grade 1 versus grade 3) had a better therapeutic response to sclerotherapy. Future studies on venous malformations should use these classifications as bases for stratification.
Other imaging studies such as plain X-ray (pathognomonic phleboliths) or CT-scan (intraosseus VM) may be useful. Whole Body Blood Pool scintigraphy (WBBPS), a transvenous angioscan utilizing radioisotope-tagged red blood cells, may be useful for multifocal lesions, to detect associated disseminated venous malformations. However, it necessitates the use of radioisotopes, and has to be coupled with MRI or Duplex scanning to precise the anatomical location.46
Etiopathogenesis
The etiopathogenesis of vascular anomalies has started to be unravelled by genetic studies. Genes and the causative mutations have been identified for several inherited vascular anomalies including cutaneomucosal venous malformation (VMCM), glomuvenous malformation (GVM), capillary malformation-arteriovenous malformation (CM-AVM), hyperkeratotic cutaneous capillarovenous malformation (HCCVM) and cerebral cavernous malformation (CCM).6, 7, 14, 16, 24, 47, 48 Identification of these genetic bases has allowed for better delineation of the entities, therefore optimizing their clinical management.12, 18, 49, 50
Glomuvenous malformations (GVM) are autosomally inherited lesions caused by loss-of-function mutations in glomulin (chromosome 1p21-22), which derails smooth muscle cell differentiation towards rounded “glomus” cells, which are observed around distended vascular channels.14 A somatic intraglomulin deletion was discovered in one resected GVM, suggesting a possible paradominant inheritance. This and the function of glomulin can be studied in the recently generated glomulin deficient knock-out mice (Brouillard et al, unpublished). Although the conventional homozygous knock-out embryos die early in development, the conditional knock-out, with skin specific deletion of glomulin function, hopefully will provide an animal model of GVM.
Inherited VMCM is mediated by germline mutations in the TEK gene (chromosome 9p), which encodes the endothelial cell tyrosine kinase receptor TIE2.16 The mutations result in increased phosphorylation of TIE2 leading to uncoupling between endothelial cells (ECs) and the normal recruitment of smooth muscle cells (SMCs).6, 16 In one patient, for whom tissue was available, a loss-of-function somatic second-hit was identified.50 It is thought that the role of the 2nd hit is to remove the protective wild-type allele, which allows locally the germline mutation to cause dysfunction. In fact, TIE2 forms receptor multimeres. If wild type receptor is present, the function is likely normal.
On the basis of the implication of a somatic mutation in VMCM, it was recently discovered that somatic mutations in TIE2 also cause 40% of sporadic VMs, the lesions frequently encountered in interdisciplinary centers for vascular anomalies.50 The majority of them have the same mutation, which has never been observed as an inherited change in VMCM. This suggests it to be too deleterious for survival when germline.50 Interestingly, 2 sporadic patients with multifocal VMs had the same mutations in lesions of distant sites. This suggests the cellular origin of these lesions to be the same, for example by spreading from a single lesion or from a precursor cell “niche” in the bone marrow or elsewhere. Anyhow, this major discovery pinpoints the TIE2 signaling pathway as a target for development of VM-specific therapies.
In rare cases, small cutaneous VMs can be caused by mutations in the Cerebral Cavernous Malformation (CCM) genes. This occurs when the cutaneous VMs are associated with cerebral cavernous malformation.48, 49, 51, 52 These cutaneous vascular anomalies can also be combined and called hyperkeratotic cutaneous capillarovenous malformation (HCCVM).
For other malformations with a venous component, the etiopathogenic mechanisms remain unknown. For Maffucci syndrome, which combines subcutaneous venous anomalies (spindle cells hemangioendothelioma) with enchondromas, a genetic difference has been found in regard to Ollier disease, which is characterized by multiple enchondromas only. No mutation in the PTHR1 gene, critical for the regulation of endochondral ossification, was found in lesional or leukocyte DNA of patients with Maffucci syndrome in contrast to Ollier patients.53 Thus, these two resembling syndromes have a different etiopathogenic cause. Finally, it was reported that Klippel-Trenaunay syndrome, a capillaro-lymphaticovenous malformation, usually a sporadic condition, would be due to enhanced angiogenic activity due to genetic defects of VG5Q.54 Yet, this change has since been reported to be a polymorphism found in healthy controls.55, 56
A novel diagnostic biomarker
Venous malformations are associated with spontaneous thrombosis and thrombolysis.22, 23, 57 This is witnessed by elevated D-dimer levels (> 0.5μg/ml) in 42% of patients, and associated with size, deepness and presence of palpable phleboliths.58 This phenomenon was named Localized Intravascular Coagulopathy. D-dimer levels are often very high (25% of patients >1.0μg/ml), even if these otherwise healthy patients do not have other conditions to increase D-dimer levels, e.g. cancer, inflammatory disease, thrombophilia, ischemic heart disease, arterial aneurysm or dissection, or pregnancy. We consider this as an important LIC, as levels are twice the upper limit. In other conditions, such as oral contraception, ulceration and old age, D-dimer levels can also mildly increase, but levels are much lower than that reported for venous malformations. In fact, they can still stay within the normal range.59-63 LIC is usually well tolerated during everyday life. However, a few patients with extensive venous malformations, mainly affecting an extremity, have a severe LIC with a very high D-dimer level (>1.8μg/ml) and a low fibrinogen level. These patients are at risk of potential aggravation of LIC to disseminated intravascular coagulopathy (DIC) with dramatic bleeding during a surgical excision, and marked consumption of platelets, coagulation factors and fibrinogen.58 Thus, measurement of D-dimer levels is mandatory for the management of VMs.
Venous malformation is the only disease that can permanently highly increase D-dimer levels in otherwise healthy patients. Thus, it is a biomarker helpful for diagnosis.11 When D-dimer levels are elevated in vascular anomaly patients with no associated pathology, a venous component is present in 96.5% of patients. This is true for pure, isolated VMs (uni or multifocal), as well as for combined and syndromic lesions (e.g.: CVM and Klippel-Trenaunay syndrome = CLVM + hypertrophy). In contrast, sensitivity is lower (42%). Thus, when D-dimer levels are normal, a small VM cannot be ruled out. Among all patients with vascular malformations, D-dimer levels were normal in all GVM, LM, and Maffucci syndrome, as well as in fast-flow lesions, such as AVM and Parkes Weber syndrome (large capillary malformation of the limb associated with multiple arteriolovenular malformations and tissue hypertrophy). Thus, D-dimer measurement is a useful biomarker for the differential diagnosis of VMs. It can help e.g. in differentiating GVMs from other multifocal venous lesions. It can also detect a venous component in combined and syndromic malformations. This is especially interesting for KT because most of them have high D-dimer levels contrary to Parkes Weber syndrome, which is commonly misdiagnosed as KT. Thus, this easy and cheap biomarker test must be used as a routine test in clinical evaluation of vascular anomaly patients.
Management of venous malformations
Management of these lesions needs interdisciplinary discussion. A dedicated team, which comprises at least of a dermatologist, an interventional radiologist, a hematologist, a plastic and/or vascular surgeon and an orthopedic surgeon, reviews all the malformations in regard to diagnosis, clinical and functional disturbance, prognosis, treatment options, and long-term follow-up. Psychological consultation may also be useful for these disfiguring and chronic lesions.
Tailored compression garment is the first line treatment for symptomatic and extensive venous malformations of the extremities to reduce pain and thrombosis. It is contraindicated in glomuvenous malformations, as it increases pain.18 It is not efficient in Maffucci syndrome. Low-dose aspirin and/or anti-inflammatory drugs are proposed if pain is not relieved, or if compression is not anatomically possible. When associated with elevated D-dimer levels, pain can be relieved with Low Molecular Weight Heparin (LMWH) 100AntiXa/kg for 20 days, or longer if pain relapses. Patients with important or severe LIC need careful management to prevent severe bleeding during a surgical procedure. To improve the hematological status and to avoid DIC, preventive treatment with enoxaparin (100AntiXa/kg) should be started 10 days before any surgical procedure for a total of 20 days.58
Most patients with Klippel-Trenaunay syndrome have chronic LIC and need careful follow-up to detect venous thrombosis and/or pulmonary embolism. In these patients, elevated D-dimer level cannot be used to screen for recent thrombosis. Thus, to detect it, Duplex ultrasound is mandatory when clinical signs, e.g. pain, limb inflammation, palpable superficial clot and dyspnea appear.32, 64 Due to this, it may be useful to screen for pulmonary embolism with a few complementary examinations, such as electrocardiogram, echocardiography and pulmonary scintigraphy for future reference.
Curative treatment of VM is rarely possible. Surgical excision is proposed in the following indications: small VM or GVM with possible complete surgical excision, VM with well-defined margins, or VM with post-sclerotherapy fibrosis.65 Partial resection without preceding sclerotherapy is rarely performed because of the risk of relapse and/or surgical morbidity. Surgery is the treatment of choice for GVM because they are more superficial, and invade less the adjacent structures. Classical surgical techniques comprise: fusiform excision, purse-string, skin graft, skin expander and fasciocutaneous flaps, according to the lesion.65 Squeezing technique, using permanent sutures to compress the dilated channels, is sometimes used, to reduce pain and functional impairment.
To diminish the volume of the malformation, percutaneous sclerotherapy is the gold standard treatment.66 The goal is to obliterate the channels by causing damage to the endothelium with subsequent inflammation and fibrosis. Among all the sclerosing agents, absolute ethanol is the most effective one with the lowest recurrence rate, but also with the most serious local and systemic side effects.45, 67 Toxicity is mainly due to the diffuseness of alcohol and the high therapeutic doses needed for the procedures. To reduce diffuseness, absolute ethanol has been mixed with zein and oleum papavaris, leaving behind a non-resorbable mass (Ethibloc®, Ethicon, Hamburg, Germany).45, 68, 69 This product was frequently used in Europe, but not approved by the Food and Drug Administration (FDA, USA), and is no longer commercially available.
Detergent sclerosants, such as polidocanol and sodium morrhuate, are not as aggressive as ethanol, and have a greater tendency for recanalization of the vascular channels.70 Microfoam forms of these detergents have been generated, using air bubbles to increase the volume and surface contact with endothelium, with a lower dose of the solution. However, strokes, headaches, scotoma, and other neurological complications have been reported in 2% of patients, confirming the potential risk of gas embolism.71, 72 Cabrera and collaborators used carbon dioxide and reported a better solubility of the gas with the detergent solution, a better stability of the injected product, and less cardiovascular and neurologic complications due to gas embolism.73 The use of this microfoam agent has not been reported in other centers.
Due to the high frequency of systemic side effects, there is a great need for a sclerosing agent which would be as efficient as absolute ethanol, but less dangerous. Radio-opaque ethylcellulose-ethanol is a newly developped sclerosing gel with a higher viscosity. It can be injected in small quantities, as ethanol is trapped in the malformation, by ethylcellulose.74 As soon as it encounters an aqueous media, a framework of ethylcellulose develops within 150 seconds, and the time of contact of ethanol with the vascular endothelium is prolonged, reducing the quantity of absolute ethanol needed. Ethylcellulose spontaneously dissolves within 2-to-3 months, unless an excessive amount is used.
As it is important to guide sclerotherapy, a radio-opaque oily agent Lipiodol® was added to ethylcellulose-ethanol. The same contrast agent was used with free absolute ethanol in one study, allowing injection of smaller doses of ethanol.75 Thus, visualization of the sclerosing agent increases accuracy and thereby safety, compared to guidance with Duplex ultrasound or fluoroscopy often used for foam sclerotherapy.72, 76, 77
Median total dose of ethylcellulose-ethanol used for injection was only 1cc and, contrary to absolute ethanol, some procedures for superficial lesions could be performed under local anesthesia. Our results of sclerotherapy on pain, functional and esthetic impairment were equivalent to those reported for free ethanol.74
This sclerosing agent was also safe, as no systemic side-effects were encountered, likely due to the small doses of ethanol injected in the malformations. Local side-effects, mainly necrosis and fistulization of ethylcellulose were encountered in 19% of procedures. These lesions were located in sensitive areas (face, mouth, feet and hands).74, 78, 79 The frequency has been reduced by using very small amounts of this agent (0.1-0.3 cc), avoiding excessive ethylcellulose residues.
Conclusion
Recent studies have improved diagnostic accuracy, etiopathogenic knowledge and therapeutic management of venous malformations. These lesions are often chronically painful, cause dysfigurement and dysfunction, and are difficult to treat. Therefore, the improved differential diagnosis using D-dimer level as a biomarker is most helpful for proper management. The identification of the etiopathogenic basis of the inherited forms as well as that of almost half of sporadic VMs, has increased our knowledge on the mechanisms behind the development of these lesions. Animal models can now be generated and specific therapeutic approaches can be envisioned. To this end, the recently developped sclerosing agent, although unspecific, ameliorates treatment and lessens complications. Yet, many questions remain unanswered, such as the cause of the other 50% of VMs, association of D-dimer levels with subtypes of VMs, better subclassification of combined vascular malformations of the extremities etc. Such data should lead to better management of these debilitating lesions.
Acknowledgments
These studies were partially supported by the Interuniversity Attraction Poles initiated by the Belgian Federal Science Policy network 6/05; concerted Research Actions (A.R.C) - Convention N° 07/12-005 of the Belgian French Community Ministry; the National Institute of Health Program Project P01 AR048564; EU FW6 Integrated project LYMPHANGIOGENOMICS, LSHG-CT-2004-503573; the F.R.S.-FNRS (Fonds de Recherche scientifique) (to M.V., a “Maitre de recherche honoraire du F.R.S.-FNRS”) and la Communauté française de Wallonie-Bruxelles et de la Lotterie nationale, Belgium.
The authors thank Ms Liliana Niculescu for secretarial work.
References
- 1.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982 Mar;69(3):412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Mulliken JB, Young AE. Vascular birthmarks: hemangiomas and malformations. WB Saunders; Philadelphia: 1988. [Google Scholar]
- 3.Enjolras O, Mulliken JB. Vascular cutaneous anomalies in children: malformations and hemangiomas. Pediatr Surg Int. 1996;11:290–295. doi: 10.1007/BF00497795. [DOI] [PubMed] [Google Scholar]
- 4.Lee BB, Laredo J, Lee TS, Huh S, Neville R. Terminology and classification of congenital vascular malformations. Phlebology. 2007;22(6):249–252. doi: 10.1177/026835550702200604. [DOI] [PubMed] [Google Scholar]
- 5.Belov S. Classification of congenital vascular defects. Int Angiol. 1990 Jul-Sep;9(3):141–146. [PubMed] [Google Scholar]
- 6.Boon LM, Mulliken JB, Vikkula M, et al. Assignment of a locus for dominantly inherited venous malformations to chromosome 9p. Hum Mol Genet. 1994 Sep;3(9):1583–1587. doi: 10.1093/hmg/3.9.1583. [DOI] [PubMed] [Google Scholar]
- 7.Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003 Dec;73(6):1240–1249. doi: 10.1086/379793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boon LM, Fishman SJ, Lund DP, Mulliken JB. Congenital fibrosarcoma masquerading as congenital hemangioma: report of two cases. J Pediatr Surg. 1995 Sep;30(9):1378–1381. doi: 10.1016/0022-3468(95)90514-6. [DOI] [PubMed] [Google Scholar]
- 9.Dompmartin A, Boon LM, Labbe D. Infantile hemangiomas: differential diagnosis and associated anomalies. Ann Chir Plast Esthet. 2006 Aug-Oct;51(4-5):300–309. doi: 10.1016/j.anplas.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Boon LM, Vikkula M. Vascular Anomalies. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell D, editors. Fitzpatrick’s Dermatology in General Medicine. 7th ed Vol. 2. McGraw-Hill Professional Publishing; 2008. pp. 1651–1666. [Google Scholar]
- 11.Dompmartin A, Bailleux F, Thibon P, et al. Elevated D-dimer level is diagnostic for venous malformations. Arch Dermatol. 2009 Nov;145(1239-44) doi: 10.1001/archdermatol.2009.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brouillard P, Vikkula M. Genetic causes of vascular malformations. Hum Mol Genet. 2007 Oct 15;16:R140–149. doi: 10.1093/hmg/ddm211. Spec No. 2. [DOI] [PubMed] [Google Scholar]
- 13.Limaye N, Boon LM, Vikkula M. From germline towards somatic mutations in the pathophysiology of vascular anomalies. Hum Mol Genet. 2009 Apr 15;18(R1):R65–74. doi: 10.1093/hmg/ddp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”) Am J Hum Genet. 2002 Apr;70(4):866–874. doi: 10.1086/339492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casanova D, Boon LM, Vikkula M. Venous malformations: clinical characteristics and differential diagnosis. Ann Chir Plast Esthet. 2006 Aug-Oct;51(4-5):373–387. doi: 10.1016/j.anplas.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Vikkula M, Boon LM, Carraway KL, 3rd, et al. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996 Dec 27;87(7):1181–1190. doi: 10.1016/s0092-8674(00)81814-0. [DOI] [PubMed] [Google Scholar]
- 17.Vikkula M, Boon LM, Mulliken JB. Molecular genetics of vascular malformations. Matrix Biol. 2001 Sep;20(5-6):327–335. doi: 10.1016/s0945-053x(01)00150-0. [DOI] [PubMed] [Google Scholar]
- 18.Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004 Aug;140(8):971–976. doi: 10.1001/archderm.140.8.971. [DOI] [PubMed] [Google Scholar]
- 19.Mallory SB, Enjolras O, Boon LM, et al. Congenital plaque-type glomuvenous malformations presenting in childhood. Arch Dermatol. 2006 Jul;142(7):892–896. doi: 10.1001/archderm.142.7.892. [DOI] [PubMed] [Google Scholar]
- 20.Song M, Ko HC, Kwon KS, Kim MB. Surgical treatment of subungual glomus tumor: a unique and simple method. Dermatol Surg. 2009 May;35(5):786–791. doi: 10.1111/j.1524-4725.2009.01129.x. [DOI] [PubMed] [Google Scholar]
- 21.Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997 Feb;36(2 Pt 1):219–225. doi: 10.1016/s0190-9622(97)70284-6. [DOI] [PubMed] [Google Scholar]
- 22.Mazoyer E, Enjolras O, Bisdorff A, Perdu J, Wassef M, Drouet L. Coagulation disorders in patients with venous malformation of the limbs and trunk: a case series of 118 patients. Arch Dermatol. 2008 Jul;144(7):861–867. doi: 10.1001/archderm.144.7.861. [DOI] [PubMed] [Google Scholar]
- 23.Hermans C, Dessomme B, Lambert C, Deneys V. Venous malformations and coagulopathy. Ann Chir Plast Esthet. 2006 Aug-Oct;51(4-5):388–393. doi: 10.1016/j.anplas.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Boon LM, Brouillard P, Irrthum A, et al. A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21-22. Am J Hum Genet. 1999 Jul;65(1):125–133. doi: 10.1086/302450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulliken JB, Fishman SJ, Burrows PE. Vascular anomalies. Curr Probl Surg. 2000 Aug;37(8):517–584. doi: 10.1016/s0011-3840(00)80013-1. [DOI] [PubMed] [Google Scholar]
- 26.Tennant LB, Mulliken JB, Perez-Atayde AR, Kozakewich HP. Verrucous hemangioma revisited. Pediatr Dermatol. 2006 May-Jun;23(3):208–215. doi: 10.1111/j.1525-1470.2006.00219.x. [DOI] [PubMed] [Google Scholar]
- 27.Bean W, Thomas Charles C., III . Springfield. Blackwell Scientific Publications; Oxford: 1959. Vascular spiders and related lesions of the skin. [Google Scholar]
- 28.Fishman SJ, Smithers CJ, Folkman J, et al. Blue rubber bleb nevus syndrome: surgical eradication of gastrointestinal bleeding. Ann Surg. 2005 Mar;241(3):523–528. doi: 10.1097/01.sla.0000154689.85629.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berry SA, Peterson C, Mize W, et al. Klippel-Trenaunay syndrome. Am J Med Genet. 1998 Oct 2;79(4):319–326. [PubMed] [Google Scholar]
- 30.Cohen MM., Jr. Klippel-Trenaunay syndrome. Am J Med Genet. 2000 Jul 31;93(3):171–175. doi: 10.1002/1096-8628(20000731)93:3<171::aid-ajmg1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 31.Oduber CE, van der Horst CM, Hennekam RC. Klippel-Trenaunay syndrome: diagnostic criteria and hypothesis on etiology. Ann Plast Surg. 2008 Feb;60(2):217–223. doi: 10.1097/SAP.0b013e318062abc1. [DOI] [PubMed] [Google Scholar]
- 32.Huiras EE, Barnes CJ, Eichenfield LF, Pelech AN, Drolet BA. Pulmonary thromboembolism associated with Klippel-Trenaunay syndrome. Pediatrics. 2005 Oct;116(4):e596–600. doi: 10.1542/peds.2004-1607. [DOI] [PubMed] [Google Scholar]
- 33.Wassef M, Vanwijck R, Clapuyt P, Boon L, Magalon G. Vascular tumours and malformations, classification, pathology and imaging. Ann Chir Plast Esthet. 2006 Aug-Oct;51(4-5):263–281. doi: 10.1016/j.anplas.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Maffucci A. Di un caso encondroma ed angioma multiplo. Contribuzione alla genesi embrionale dei tumori. Movimento medico-chirurgico. 1881;3:399–412. 565–575. Napoli. [Google Scholar]
- 35.Enjolras O, Wassef M, Merland JJ. Maffucci syndrome: a false venous malformation? A case with hemangioendothelioma with fusiform cells. Ann Dermatol Venereol. 1998 Aug;125(8):512–515. [PubMed] [Google Scholar]
- 36.Lewis RJ, Ketcham AS. Maffucci’s syndrome: functional and neoplastic significance. Case report and review of the literature. J Bone Joint Surg Am. 1973 Oct;55(7):1465–1479. [PubMed] [Google Scholar]
- 37.Schwartz HS, Zimmerman NB, Simon MA, Wroble RR, Millar EA, Bonfiglio M. The malignant potential of enchondromatosis. J Bone Joint Surg Am. 1987 Feb;69(2):269–274. [PubMed] [Google Scholar]
- 38.Kaplan RP, Wang JT, Amron DM, Kaplan L. Maffucci’s syndrome: two case reports with a literature review. J Am Acad Dermatol. 1993 Nov;29(5 Pt 2):894–899. doi: 10.1016/0190-9622(93)70265-u. [DOI] [PubMed] [Google Scholar]
- 39.Zembowicz A, Mihm MC. Dermal dendritic melanocytic proliferations: an update. Histopathology. 2004 Nov;45(5):433–451. doi: 10.1111/j.1365-2559.2004.01975.x. [DOI] [PubMed] [Google Scholar]
- 40.Trop I, Dubois J, Guibaud L, et al. Soft-tissue venous malformations in pediatric and young adult patients: diagnosis with Doppler US. Radiology. 1999 Sep;212(3):841–845. doi: 10.1148/radiology.212.3.r99au11841. [DOI] [PubMed] [Google Scholar]
- 41.Hein KD, Mulliken JB, Kozakewich HP, Upton J, Burrows PE. Venous malformations of skeletal muscle. Plast Reconstr Surg. 2002 Dec;110(7):1625–1635. doi: 10.1097/01.PRS.0000033021.60657.74. [DOI] [PubMed] [Google Scholar]
- 42.Dubois J, Soulez G, Oliva VL, Berthiaume MJ, Lapierre C, Therasse E. Soft-tissue venous malformations in adult patients: imaging and therapeutic issues. Radiographics. 2001 Nov-Dec;21(6):1519–1531. doi: 10.1148/radiographics.21.6.g01nv031519. [DOI] [PubMed] [Google Scholar]
- 43.Konez O, Burrows PE. Magnetic resonance of vascular anomalies. Magn Reson Imaging Clin N Am. 2002 May;10(2):363–388. vii. doi: 10.1016/s1064-9689(01)00009-5. [DOI] [PubMed] [Google Scholar]
- 44.Goyal M, Causer PA, Armstrong D. Venous vascular malformations in pediatric patients: comparison of results of alcohol sclerotherapy with proposed MR imaging classification. Radiology. 2002 Jun;223(3):639–644. doi: 10.1148/radiol.2233010025. [DOI] [PubMed] [Google Scholar]
- 45.Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999 Jul;104(1):1–11. discussion 12-15. [PubMed] [Google Scholar]
- 46.Lee BB, Laredo J, Kim YW, Neville R. Congenital vascular malformations: general treatment principles. Phlebology. 2007;22(6):258–263. doi: 10.1177/026835550702200606. [DOI] [PubMed] [Google Scholar]
- 47.Revencu N, Boon LM, Mulliken JB, et al. Parkes Weber syndrome, vein of Galen aneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations. Hum Mutat. 2008 Jul;29(7):959–965. doi: 10.1002/humu.20746. [DOI] [PubMed] [Google Scholar]
- 48.Eerola I, Plate KH, Spiegel R, Boon LM, Mulliken JB, Vikkula M. KRIT1 is mutated in hyperkeratotic cutaneous capillary-venous malformation associated with cerebral capillary malformation. Hum Mol Genet. 2000 May 22;9(9):1351–1355. doi: 10.1093/hmg/9.9.1351. [DOI] [PubMed] [Google Scholar]
- 49.Revencu N, Vikkula M. Cerebral cavernous malformation: new molecular and clinical insights. J Med Genet. 2006 Sep;43(9):716–721. doi: 10.1136/jmg.2006.041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Limaye N, Wouters V, Uebelhoer M, et al. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat Genet. 2009 Jan;41(1):118–124. doi: 10.1038/ng.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toll A, Parera E, Gimenez-Arnau AM, et al. Cutaneous venous malformations in familial cerebral cavernomatosis caused by KRIT1 gene mutations. Dermatology (Basel, Switzerland) 2009;218(4):307–313. doi: 10.1159/000199461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sirvente J, Enjolras O, Wassef M, Tournier-Lasserve E, Labauge P. Frequency and phenotypes of cutaneous vascular malformations in a consecutive series of 417 patients with familial cerebral cavernous malformations. J Eur Acad Dermatol Venereol. 2009 Sep;23(9):1066–1072. doi: 10.1111/j.1468-3083.2009.03263.x. [DOI] [PubMed] [Google Scholar]
- 53.Couvineau A, Wouters V, Bertrand G, et al. PTHR1 mutations associated with Ollier disease result in receptor loss of function. Hum Mol Genet. 2008 Sep 15;17(18):2766–2775. doi: 10.1093/hmg/ddn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian XL, Kadaba R, You SA, et al. Identification of an angiogenic factor that when mutated causes susceptibility to Klippel-Trenaunay syndrome. Nature. 2004 Feb 12;427(6975):640–645. doi: 10.1038/nature02320.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gutierrez S, Magano L, Delicado A, et al. The G397A (E133K) change in the AGGF1 (VG5Q) gene is a single nucleotide polymorphism in the Spanish population. Am J Med Genet A. 2006 Dec 15;140(24):2832–2833. doi: 10.1002/ajmg.a.31532. [DOI] [PubMed] [Google Scholar]
- 56.Barker KT, Foulkes WD, Schwartz CE, et al. Is the E133K allele of VG5Q associated with Klippel-Trenaunay and other overgrowth syndromes? J Med Genet. 2006 Jul;43(7):613–614. doi: 10.1136/jmg.2006.040790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Enjolras O, Mulliken JB. Vascular tumors and vascular malformations. Adv Dermatol. 1997;13:375–423. new issues. [PubMed] [Google Scholar]
- 58.Dompmartin A, Acher A, Thibon P, et al. Association of localized intravascular coagulopathy with venous malformations. Arch Dermatol. 2008;144(7):873–877. doi: 10.1001/archderm.144.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kluft C, Meijer P, LaGuardia KD, Fisher AC. Comparison of a transdermal contraceptive patch vs. oral contraceptives on hemostasis variables. Contraception. 2008 Feb;77(2):77–83. doi: 10.1016/j.contraception.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 60.Johnson JV, Lowell J, Badger GJ, Rosing J, Tchaikovski S, Cushman M. Effects of oral and transdermal hormonal contraception on vascular risk markers: a randomized controlled trial. Obstet Gynecol. 2008 Feb;111(2 Pt 1):278–284. doi: 10.1097/AOG.0b013e3181626d1b. [DOI] [PubMed] [Google Scholar]
- 61.Dompmartin A, Thibon P, Vikkula M, Boon LM. Oral contraceptive and D-dimer level. Arch Dermatol. 2009;145(2):210–211. doi: 10.1001/archdermatol.2009.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Falanga V, Kruskal J, Franks JJ. Fibrin- and fibrinogen-related antigens in patients with venous disease and venous ulceration. Arch Dermatol. 1991 Jan;127(1):75–78. [PubMed] [Google Scholar]
- 63.Cuderman TV, Bozic M, Peternel P, Stegnar M. Hemostasis activation in thrombophilic subjects with or without a history of venous thrombosis. Clin Appl Thromb Hemost. 2008 Jan;14(1):55–62. doi: 10.1177/1076029607304408. [DOI] [PubMed] [Google Scholar]
- 64.Oduber CE, Gerdes VE, van der Horst CM, Bresser P. Vascular malformations as underlying cause of chronic thromboembolism and pulmonary hypertension. J Plast Reconstr Aesthet Surg. 2009 May;62(5):684–689. doi: 10.1016/j.bjps.2007.12.062. discussion 689. [DOI] [PubMed] [Google Scholar]
- 65.Boon LM, Vanwijck R. Medical and surgical treatment of venous malformations. Ann Chir Plast Esthet. 2006 Aug-Oct;51(4-5):403–411. doi: 10.1016/j.anplas.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 66.Rosenblatt M. Endovascular management of venous malformations. Phlebology. 2007;22(6):264–275. doi: 10.1177/026835550702200607. [DOI] [PubMed] [Google Scholar]
- 67.Yakes WF, Haas DK, Parker SH, et al. Symptomatic vascular malformations: ethanol embolotherapy. Radiology. 1989 Mar;170(3 Pt 2):1059–1066. doi: 10.1148/radiology.170.3.2916057. [DOI] [PubMed] [Google Scholar]
- 68.Rivas S, Lopez-Gutierrez JC, Diaz M, Andres AM, Ros Z. Venous malformations. Diagnosis and treatment during the childhood. Cir Pediatr. 2006 Apr;19(2):77–80. [PubMed] [Google Scholar]
- 69.Schumacher M, Ernemann U, Berlis A, Weber J. Treatment of venous malformations--comparison to lymphatic malformations. Lymphology. 2008 Sep;41(3):139–146. [PubMed] [Google Scholar]
- 70.Burrows PE, Mason KP. Percutaneous treatment of low flow vascular malformations. J Vasc Interv Radiol. 2004 May;15(5):431–445. doi: 10.1097/01.rvi.0000124949.24134.cf. [DOI] [PubMed] [Google Scholar]
- 71.Forlee MV, Grouden M, Moore DJ, Shanik G. Stroke after varicose vein foam injection sclerotherapy. J Vasc Surg. 2006 Jan;43(1):162–164. doi: 10.1016/j.jvs.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 72.Li L, Feng J, Zeng XQ, Li YH. Fluoroscopy-guided foam sclerotherapy with sodium morrhuate for peripheral venous malformations: Preliminary experience. J Vasc Surg. 2009 Apr;49(4):961–967. doi: 10.1016/j.jvs.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 73.Cabrera J, Cabrera J, Jr., Garcia-Olmedo MA, Redondo P. Treatment of venous malformations with sclerosant in microfoam form. Archives of dermatology. 2003 Nov;139(11):1409–1416. doi: 10.1001/archderm.139.11.1409. [DOI] [PubMed] [Google Scholar]
- 74.Dompmartin A, Gaillard C, Theron J, et al. Radioopaque ethylcellulose ethanol is a safe and efficient sclerosing agent for slow-flow vascular malformations. Radiology. doi: 10.1007/s00330-011-2213-4. submitted. [DOI] [PubMed] [Google Scholar]
- 75.Suh JS, Shin KH, Na JB, Won JY, Hahn SB. Venous malformations: sclerotherapy with a mixture of ethanol and lipiodol. Cardiovasc Intervent Radiol. 1997 Jul-Aug;20(4):268–273. doi: 10.1007/s002709900150. [DOI] [PubMed] [Google Scholar]
- 76.Yamaki T, Nozaki M, Sakurai H, Takeuchi M, Soejima K, Kono T. Prospective randomized efficacy of ultrasound-guided foam sclerotherapy compared with ultrasound-guided liquid sclerotherapy in the treatment of symptomatic venous malformations. J Vasc Surg. 2008 Mar;47(3):578–584. doi: 10.1016/j.jvs.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 77.Bergan J, Cheng V. Foam sclerotherapy of venous malformations. Phlebology. 2007;22(6):299–302. doi: 10.1258/026835507782655281. [DOI] [PubMed] [Google Scholar]
- 78.Sannier K, Dompmartin A, Théron J, et al. A new sclerosing agent in the treatment of venous malformations. Study on 23 cases. Interven Radiol. 2004;10:113–127. doi: 10.1177/159101990401000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dompmartin A, Labbe D, Theron J, Benateau H, Barrellier MT. The use of an alcohol gel of ethyl cellulose in the treatment of venous malformations. Rev Stomatol Chir Maxillofac. 2000 Jan;101(1):30–32. [PubMed] [Google Scholar]