Abstract
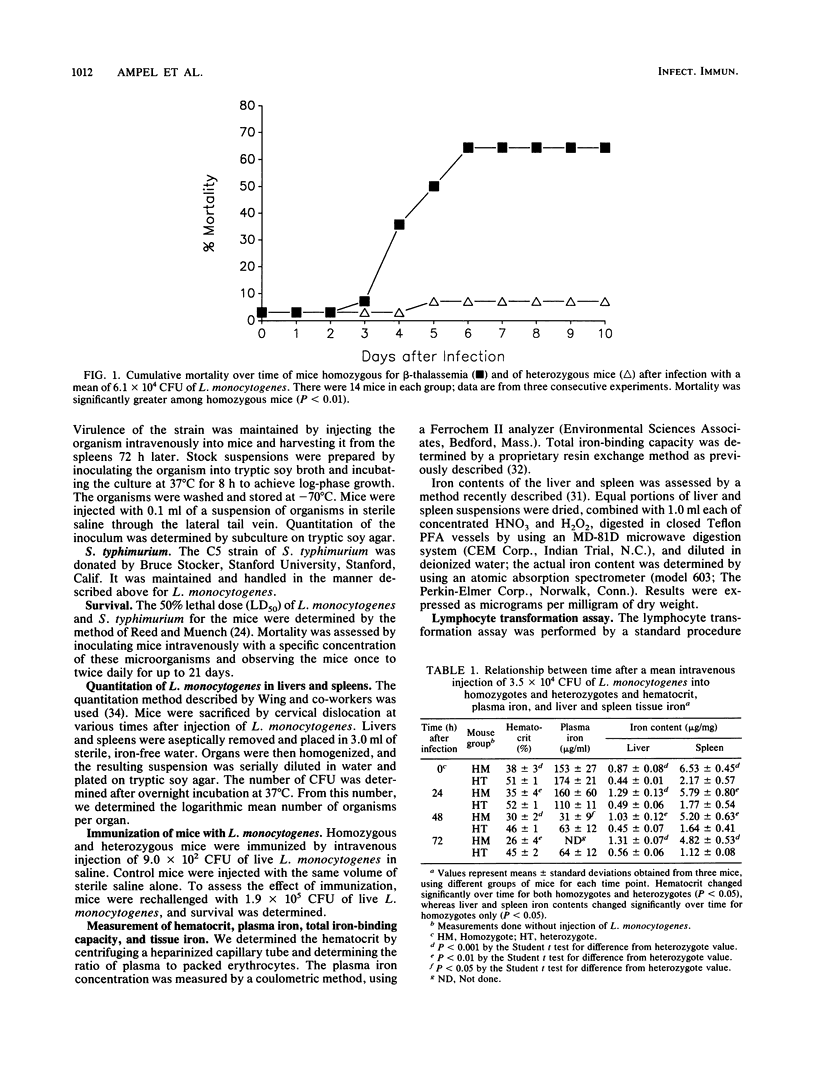
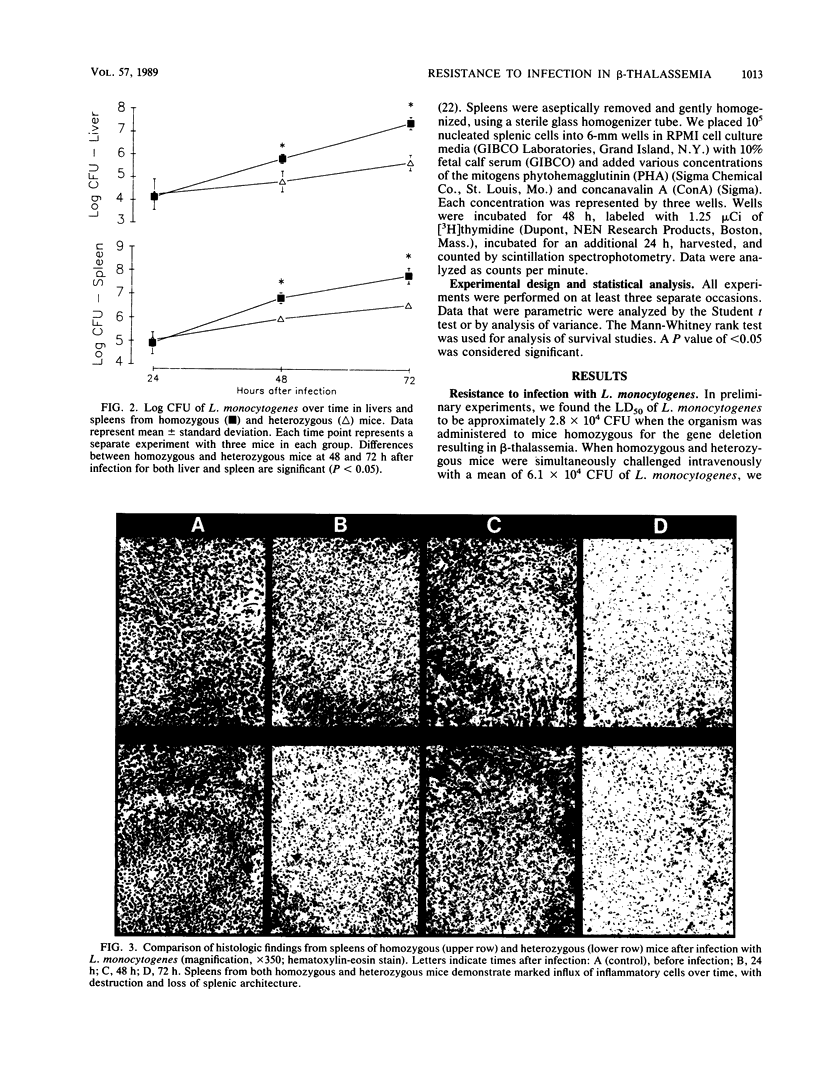
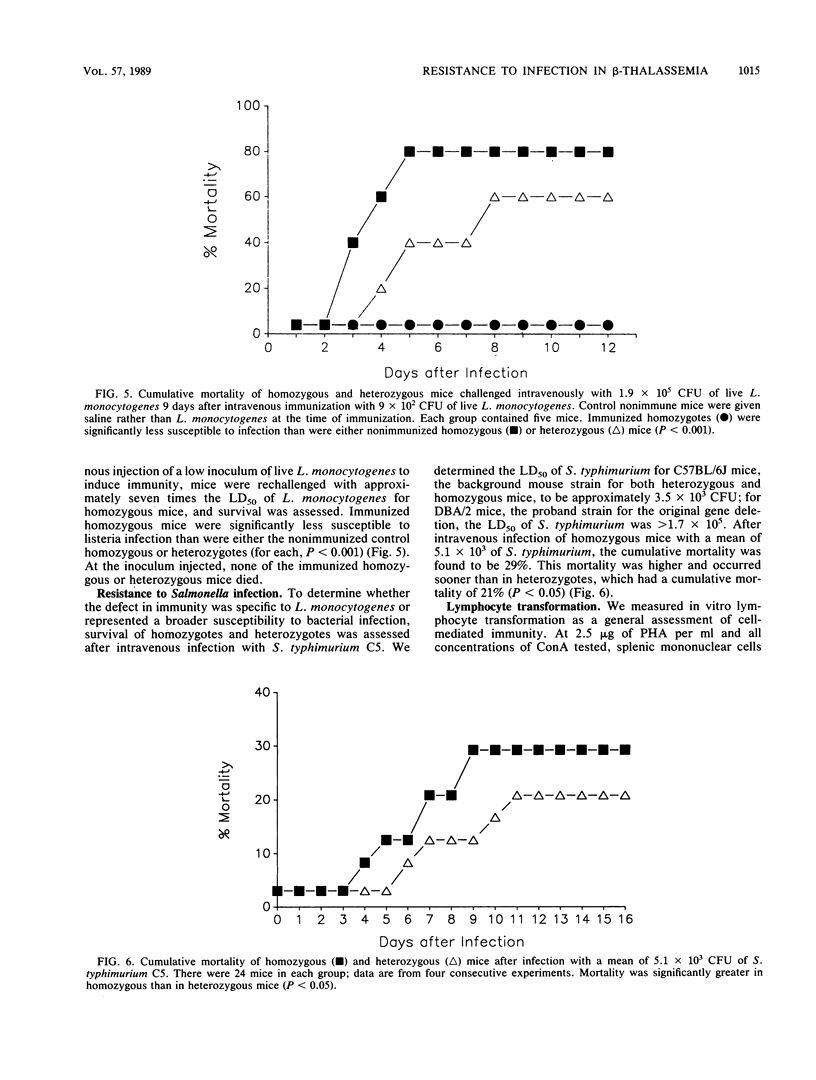
Clinical evidence suggests that individuals with chronic iron overload may be at increased risk of bacterial infection. We studied this question by using a unique model in which mice homozygous for a deletion in the gene encoding for the beta-major globin develop moderate anemia, splenomegaly, and tissue iron overload, a syndrome similar to beta-thalassemia in humans. Mice heterozygous for the gene deletion were phenotypically normal. Homozygous mice were significantly more susceptible to infection with Listeria monocytogenes than were heterozygous mice (P less than 0.01). This increased susceptibility was associated with a greater number of organisms in the liver and spleen than was found in heterozygous mice (P less than 0.05). However, histologic studies demonstrated similar inflammatory responses within these organs in homozygous and heterozygous mice. The increased susceptibility of homozygous mice to infection with L. monocytogenes was not seen when homozygotes were immunized with a low dose of L. monocytogenes. Although the results were not as striking as with L. monocytogenes, homozygous mice were also found to be more susceptible to infection with Salmonella typhimurium than were heterozygous mice (P less than 0.05). Splenic mononuclear cells from homozygous mice demonstrated less responsiveness in vitro to the mitogens concanavalin A and phytohemagglutinin than did those from heterozygotes (P less than 0.05). These data suggest that there is a generalized defect in innate immunity in homozygous mice which makes them more susceptible to infection by L. monocytogenes and S. typhimurium. The site of this immunological defect is not known but is most likely in the mononuclear phagocyte and may be due to tissue iron overload.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG A. S., SWORD C. P. CELLULAR RESISTANCE IN LISTERIOSIS. J Infect Dis. 1964 Jun;114:258–264. doi: 10.1093/infdis/114.3.258. [DOI] [PubMed] [Google Scholar]
- Ballart I. J., Estevez M. E., Sen L., Diez R. A., Giuntoli J., de Miani S. A., Peñalver J. Progressive dysfunction of monocytes associated with iron overload and age in patients with thalassemia major. Blood. 1986 Jan;67(1):105–109. [PubMed] [Google Scholar]
- Barrett-Connor E. Bacterial infection and sickle cell anemia. An analysis of 250 infections in 166 patients and a review of the literature. Medicine (Baltimore) 1971 Mar;50(2):97–112. [PubMed] [Google Scholar]
- Buchanan W. M. Shock in Bantu siderosis. Am J Clin Pathol. 1971 Apr;55(4):401–406. doi: 10.1093/ajcp/55.4.401. [DOI] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Caroline L., Kozinn P. J., Feldman F., Stiefel F. H., Lichtman H. Infection and iron overload in thalassemia. Ann N Y Acad Sci. 1969 Nov 20;165(1):148–155. doi: 10.1111/j.1749-6632.1969.tb27784.x. [DOI] [PubMed] [Google Scholar]
- Gardner I. D., Remington J. S. Age-related decline in the resistance of mice to infection with intracellular pathogens. Infect Immun. 1977 May;16(2):593–598. doi: 10.1128/iai.16.2.593-598.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S. Z., Kuebbing D., Trauber D., Schafer M. P., Lewis S. E., Popp R. A., Anderson W. F. A 66-base pair insert bridges the deletion responsible for a mouse model of beta-thalassemia. J Biol Chem. 1986 Sep 15;261(26):12368–12374. [PubMed] [Google Scholar]
- Hershko C., Peto T. E., Weatherall D. J. Iron and infection. Br Med J (Clin Res Ed) 1988 Mar 5;296(6623):660–664. [PMC free article] [PubMed] [Google Scholar]
- Hormaeche C. E., Harrington K. A., Joysey H. S. Natural resistance to salmonellae in mice: control by genes within the major histocompatibility complex. J Infect Dis. 1985 Nov;152(5):1050–1056. doi: 10.1093/infdis/152.5.1050. [DOI] [PubMed] [Google Scholar]
- Kaye D., Gill F. A., Hook E. W. Factors influencing host resistance to Salmonella infections: the effects of hemolysis and erythrophagocytosis. Am J Med Sci. 1967 Aug;254(2):205–215. doi: 10.1097/00000441-196708000-00011. [DOI] [PubMed] [Google Scholar]
- Kongshavn P. A., Skamene E. The role of natural resistance in protection of the murine host from listeriosis. Clin Invest Med. 1984;7(4):253–257. [PubMed] [Google Scholar]
- Lalonde R. G., Holbein B. E. Role of iron in Trypanosoma cruzi infection of mice. J Clin Invest. 1984 Feb;73(2):470–476. doi: 10.1172/JCI111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo V. G., Lalonde R. G. Role of iron in intracellular growth of Trypanosoma cruzi. Infect Immun. 1984 Sep;45(3):726–730. doi: 10.1128/iai.45.3.726-730.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Mossey R. T., Sondheimer J. Listeriosis in patients with long-term hemodialysis and transfusional iron overload. Am J Med. 1985 Sep;79(3):397–400. doi: 10.1016/0002-9343(85)90322-5. [DOI] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D. Influence of host genes on resistance of inbred mice to lethal infection with Salmonella typhimurium. Curr Top Microbiol Immunol. 1986;124:37–48. [PubMed] [Google Scholar]
- O'Brien A. D., Metcalf E. S. Control of early Salmonella typhimurium growth in innately Salmonella-resistant mice does not require functional T lymphocytes. J Immunol. 1982 Oct;129(4):1349–1351. [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. The critical role of iron in host-bacterial interactions. J Clin Invest. 1978 Jun;61(6):1428–1440. doi: 10.1172/JCI109062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawatzki G., Hoffmann F. A., Kubanek B. Acute iron overload in mice: pathogenesis of Salmonella typhimurium infection. Infect Immun. 1983 Feb;39(2):659–665. doi: 10.1128/iai.39.2.659-665.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamene E., Gros P., Forget A., Kongshavn P. A., St Charles C., Taylor B. A. Genetic regulation of resistance to intracellular pathogens. Nature. 1982 Jun 10;297(5866):506–509. doi: 10.1038/297506a0. [DOI] [PubMed] [Google Scholar]
- Skow L. C., Burkhart B. A., Johnson F. M., Popp R. A., Popp D. M., Goldberg S. Z., Anderson W. F., Barnett L. B., Lewis S. E. A mouse model for beta-thalassemia. Cell. 1983 Oct;34(3):1043–1052. doi: 10.1016/0092-8674(83)90562-7. [DOI] [PubMed] [Google Scholar]
- Sword C. P. Mechanisms of pathogenesis in Listeria monocytogenes infection. I. Influence of iron. J Bacteriol. 1966 Sep;92(3):536–542. doi: 10.1128/jb.92.3.536-542.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L., Heremans J. F. The involvement of lactoferrin in the hyposideremia of acute inflammation. J Exp Med. 1974 Oct 1;140(4):1068–1084. doi: 10.1084/jem.140.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wyck D. B., Schifman R. B., Stivelman J. C., Ruiz J., Martin D. Rapid sample preparation for determination of iron in tissue by closed-vessel digestion and microwave energy. Clin Chem. 1988 Jun;34(6):1128–1130. [PubMed] [Google Scholar]
- Van Wyck D. B., Tancer M. E., Popp R. A. Iron homeostasis in beta-thalassemic mice. Blood. 1987 Nov;70(5):1462–1465. [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Kresefsky-Friedman D. Y. Decreased resistance to Listeria monocytogenes in mice injected with killed corynebacterium parvum: association with suppression of cell-mediated immunity. J Infect Dis. 1980 Feb;141(2):203–211. doi: 10.1093/infdis/141.2.203. [DOI] [PubMed] [Google Scholar]
- van Asbeck B. S., Verbrugh H. A., van Oost B. A., Marx J. J., Imhof H. W., Verhoef J. Listeria monocytogenes meningitis and decreased phagocytosis associated with iron overload. Br Med J (Clin Res Ed) 1982 Feb 20;284(6315):542–544. doi: 10.1136/bmj.284.6315.542. [DOI] [PMC free article] [PubMed] [Google Scholar]