Abstract
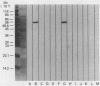
A purified preparation of membranes was obtained by using a unique method of treating Mycoplasma pneumoniae with the ATPase inhibitor, diethylstilbestrol. This method was shown to yield highly purified membranes with little or no cytoplasmic contamination. These membranes were used to immunize mice for subsequent productions of monoclonal antibodies (MAbs). Hybridoma culture supernatants were screened by enzyme-linked immunosorbent assay with whole-cell M. pneumoniae and lipid extract antigens. Four stable MAbs were obtained and characterized. MAb CP3-46F5 reacted with a protein of a molecular weight of approximately 52,000 as determined by Western blot (immunoblot). MAbs CP3-50C2, CP3-53C5, and CP3-53C8 did not react with any antigens on Western blots but did bind to at least 10 distinct glycolipid bands as determined by orcinol staining on thin-layer chromatograms of M. pneumoniae lipid extracts. The MAbs did not react with similarly prepared lipid extracts from Mycoplasma genitalium, Mycoplasma neurolyticum, and Mycoplasma gallisepticum. These MAbs did not inhibit M. pneumoniae metabolism or attachment to WiDr cell cultures. The anti-glycolipid MAbs recognize determinants specific to M. pneumoniae, unlike polyclonal hyperimmune sera against M. pneumoniae, which cross-react with lipid extracts of M. genitalium.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barile M. F., Chandler D. K., Yoshida H., Grabowski M. W., Harasawa R., Razin S. Parameters of Mycoplasma pneumoniae infection in Syrian hamsters. Infect Immun. 1988 Sep;56(9):2443–2449. doi: 10.1128/iai.56.9.2443-2449.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman B. L., Kenny G. E. Immunochemical analysis of serologically active lipids of Mycoplasma pneumoniae. J Bacteriol. 1968 Oct;96(4):1171–1180. doi: 10.1128/jb.96.4.1171-1180.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. K., Barile M. F. Ciliostatic, hemagglutinating, and proteolytic activities in a cell extract of Mycoplasma pneumoniae. Infect Immun. 1980 Sep;29(3):1111–1116. doi: 10.1128/iai.29.3.1111-1116.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. K., Grabowski M. W., Barile M. F. Mycoplasma pneumoniae attachment: competitive inhibition by mycoplasmal binding component and by sialic acid-containing glycoconjugates. Infect Immun. 1982 Nov;38(2):598–603. doi: 10.1128/iai.38.2.598-603.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. K., Grabowski M. W., Rabson A. S., Barile M. F. Further studies on the Mycoplasma pneumoniae extract: ciliostatic and cell recruitment activities. Isr J Med Sci. 1987 Jun;23(6):580–584. [PubMed] [Google Scholar]
- Chandler D. K., Razin S., Stephens E. B., Harasawa R., Barile M. F. Genomic and phenotypic analyses of Mycoplasma pneumoniae strains. Infect Immun. 1982 Nov;38(2):604–609. doi: 10.1128/iai.38.2.604-609.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo V. P., Katzenell A., Rottem S. Sealed vesicles prepared by fusing Mycoplasma gallisepticum membranes and preformed lipid vesicles. Isr J Med Sci. 1987 May;23(5):380–383. [PubMed] [Google Scholar]
- Dunn S. D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986 Aug 15;157(1):144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- ENNY G. E., GRAYSTON J. T. EATON PLEUROPNEUMONIA-LIKE ORGANISM (MYCOPLASMA PNEUMONIAE) COMPLEMENT-FIXING ANTIGEN: EXTRACTION WITH ORGANIC SOLVENTS. J Immunol. 1965 Jul;95:19–25. [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Jacobs E., Schöpperle K., Bredt W. Adherence inhibition assay: a specific serological test for detection of antibodies to Mycoplasma pneumoniae. Eur J Clin Microbiol. 1985 Apr;4(2):113–118. doi: 10.1007/BF02013574. [DOI] [PubMed] [Google Scholar]
- Kenimer J. G., Habig W. H., Hardegree M. C. Monoclonal antibodies as probes of tetanus toxin structure and function. Infect Immun. 1983 Dec;42(3):942–948. doi: 10.1128/iai.42.3.942-948.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny G. E. Serological cross-reaction between lipids of Mycoplasma pneumoniae and Mycoplasma neurolyticum. Infect Immun. 1971 Aug;4(2):149–153. doi: 10.1128/iai.4.2.149-153.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lind K., Lindhardt B. O., Schütten H. J., Blom J., Christiansen C. Serological cross-reactions between Mycoplasma genitalium and Mycoplasma pneumoniae. J Clin Microbiol. 1984 Dec;20(6):1036–1043. doi: 10.1128/jcm.20.6.1036-1043.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker C., Wilson T. H. Sodium and proton transport in Mycoplasma gallisepticum. J Bacteriol. 1985 Sep;163(3):1250–1257. doi: 10.1128/jb.163.3.1250-1257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEnery M. W., Pedersen P. L. Diethylstilbestrol. A novel F0-directed probe of the mitochondrial proton ATPase. J Biol Chem. 1986 Feb 5;261(4):1745–1752. [PubMed] [Google Scholar]
- Morrison-Plummer J., Jones D. H., Baseman J. B. An ELISA to detect monoclonal antibodies specific for lipid determinants of Mycoplasma pneumoniae. J Immunol Methods. 1983 Nov 11;64(1-2):165–178. doi: 10.1016/0022-1759(83)90395-2. [DOI] [PubMed] [Google Scholar]
- Morrison-Plummer J., Leith D. K., Baseman J. B. Biological effects of anti-lipid and anti-protein monoclonal antibodies on Mycoplasma pneumoniae. Infect Immun. 1986 Aug;53(2):398–403. doi: 10.1128/iai.53.2.398-403.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plackett P., Marmion B. P., Shaw E. J., Lemcke R. M. Immunochemical analysis of Mycoplasma pneumoniae. 3. Separation and chemical identification of serologically active lipids. Aust J Exp Biol Med Sci. 1969 Apr;47(2):171–195. doi: 10.1038/icb.1969.19. [DOI] [PubMed] [Google Scholar]
- Pollack J. D., Razin S., Pollack M. E., Cleverdon R. C. Fractionation of mycoplasma cells for enzyme localization. Life Sci. 1965 May;4(9):973–977. doi: 10.1016/0024-3205(65)90200-6. [DOI] [PubMed] [Google Scholar]
- RAZIN S. OSMOTIC LYSIS OF MYCOPLASMA. J Gen Microbiol. 1963 Dec;33:471–475. doi: 10.1099/00221287-33-3-471. [DOI] [PubMed] [Google Scholar]
- Razin S., Prescott B., Caldes G., James W. D., Chanock R. M. Role of Glycolipids and Phosphatidylglycerol in the Serological Activity of Mycoplasma pneumoniae. Infect Immun. 1970 Apr;1(4):408–416. doi: 10.1128/iai.1.4.408-416.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Linker C., Wilson T. H. Proton motive force across the membrane of Mycoplasma gallisepticum and its possible role in cell volume regulation. J Bacteriol. 1981 Mar;145(3):1299–1304. doi: 10.1128/jb.145.3.1299-1304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Razin S. Isolation of mycoplasma membranes by digitonin. J Bacteriol. 1972 May;110(2):699–705. doi: 10.1128/jb.110.2.699-705.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Stein O., Razin S. Reassembly of Mycoplasma membranes disaggregated by detergents. Arch Biochem Biophys. 1968 Apr;125(1):46–56. doi: 10.1016/0003-9861(68)90637-1. [DOI] [PubMed] [Google Scholar]
- Shirvan M. H., Rottem S., Ne'eman Z., Bittman R. Isolation of mycoplasma membranes by dicyclohexylcarbodiimide-induced lysis. J Bacteriol. 1982 Mar;149(3):1124–1128. doi: 10.1128/jb.149.3.1124-1128.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvan M. H., Schuldiner S., Rottem S. Control of sodium fluxes in Mycoplasma gallisepticum. Isr J Med Sci. 1987 May;23(5):384–388. [PubMed] [Google Scholar]
- Taylor-Robinson D., Purcell R. H., Wong D. C., Chanock R. M. A colour test for the measurement of antibody to certain mycoplasma species based upon the inhibition of acid production. J Hyg (Lond) 1966 Mar;64(1):91–104. doi: 10.1017/s0022172400040377. [DOI] [PMC free article] [PubMed] [Google Scholar]