Abstract
Breast tumor cells enter the bloodstream long before the development of clinically-evident metastasis. However, the early presence of such bloodborne cells predicts poor patient outcome. Nearly 90% of human breast tumors arise as carcinomas from mammary epithelial cells, so it is important to study how these cells respond to the detached conditions that they would experience in the bloodstream. We report here that mammary epithelial cell lines produce long and dynamic protrusions of the plasma membrane when detached. Although human and mouse mammary epithelial cell lines die by apoptosis within 16 hours of detachment, this protrusive response persists for days in cells overexpressing either Bcl-2 or Bcl-xL. Unlike actin-dependent invadopodia and podosomes, these protrusions are actually enhanced by actin depolymerization with Cytochalasin-D or Latrunculin-A. Immunofluorescence and Western blotting demonstrate that the protrusions are enriched in detyrosinated Glu-tubulin, a post-translationally modified form of α-tubulin that is found in stabilized microtubules. Video microscopy indicates that these protrusions promote cell-cell attachment, and inhibiting microtubule-based protrusions correlates with reduced extracellular matrix attachment. Since bloodborne metastasis depends on both cell-cell and cell-matrix attachment, microtubule-based protrusions in detached mammary epithelial cells provide a novel mechanism that could influence the metastatic spread of breast tumors.
Keywords: Cytoskeleton, Tubulin, Apoptosis, Protrusions, Breast, Tumor, Actin, Metastasis, Adhesion, Mammary
INTRODUCTION
Breast tumor cells can disseminate prior to significant primary tumor growth and remain dormant in distant tissues for extended periods of time [1–3]. Survival, invasion and reemergence of such disseminated cells are primary determinants of tumor recurrence and patient death [4]. Detachment of epithelial cells from the extracellular matrix of their organ of origin causes cell rounding that leads rapidly to apoptotic cell death, a principle which is thought to limit metastatic spread [5, 6]. In mammary epithelial cells, we have shown that apoptotic resistance allows cells to survive rounding, but additional genetic mutations are required for active tumor growth [7–9]. Resistance to apoptosis by overexpression of survival proteins, like Bcl-2, prevents cell death during dissemination, but cell cycle arrest can still occur through activation of p53 [10, 11]. In solid tumors like breast cancer, detached cells generally remain arrested and must adhere to extracellular matrix in distant tissues to reinitiate growth[1, 2]. So while apoptotic resistance can promote extended bloodborne survival, additional mechanisms are required for tumor cells to escape blood vessels and successfully colonize distant tissues. [2].
In vivo microscopy recently demonstrated that bloodborne tumor cells depend on tubulin polymerization to attach to the walls of capillary blood vessels[12]. However, any specific role for microtubules in this process remains unclear. Surprisingly, this recent study also showed that inhibiting actin polymerization greatly increased binding of tumor cells to blood vessel walls, even though actin depolymerization inhibits the actin-based invadopodia and podosomes that are well-known to affect the invasion of adherent tumor cells[12]. Bloodborne tumor cells therefore attach to capillary vessel walls via a cytoskeletal mechanism that is distinct from that of adherent cells, and is currently not well-characterized.
We report here that mammary epithelial cell lines generate long and dynamic microtubule-driven protrusions of the plasma membrane after detachment. We also determine that detachment produces rapid detyrosination of α-tubulin, and the concentration of detyrosinated tubulin in protrusions. Full length α-tubulin contains a tyrosine residue at its c-terminus, and is termed Tyr-tubulin. Cleavage of this c-terminal tyrosine by a tubulin carboxypeptidase exposes a glutamic acid residue, yielding a detyrosinated form (Glu-tubulin)[13, 14]. Although this α-tubulin modification has been appreciated for nearly thirty years, the identity of the gene encoding tubulin carboxypeptidase remains unknown. Glu-tubulin is postranslationally converted back to Tyr-tubulin, by a tubulin tyrosine ligase (TTL), which has recently been cloned in mice and humans [15]. While microtubules containing Tyr-tubulin have a relatively short half-life, measured in minutes, Glu-tubulin is enriched in a more stable subset of microtubules [16]. Microtubules containing Glu-tubulin can persist for hours and have been observed to remain for as long as 16 hours in nondividing cells [16]. In breast tumor samples, increased levels of Glu-tubulin are associated with poor patient prognosis and an increased risk of cancer-related complications, but the mechanism for this effect is still unknown [17].
We find that the microtubule-based protrusions promote reattachment of mammary epithelial cells to surfaces and each other, and may therefore allow detached cells to escape apoptosis by reattaching. Since this response persists in cells overexpressing Bcl-2 or Bcl-xL, it may promote the dormant dissemination of apoptotically-resistant tumor cells, even though they would not reinitiate growth until a much later time. Our data indicate that these microtubule protrusions do not necessarily originate from a tumor-specific mutation, since they are also observed in nontumorigenic mammary epithelial cell lines of both human and mouse origin. However, the persistence of this inherent microtubule response in apoptotically-resistant cells could have important consequences for the ability of disseminated tumor cells to efficiently adhere to new sites. The increased levels of Glu-tubulin upon detachment and its concentration in plasma membrane protrusions provide novel mechanisms to initiate microtubule-based tumor cell adhesion in blood vessels, and may explain why increased levels of Glu-tubulin in breast tumors predict poor patient survival.
MATERIALS AND METHODS
Cell Lines and Materials
MCF10A human mammary epithelial cells were kindly provided by Fred Miller and Robert Pauley of the Barbara Ann Karmanos Cancer Institute (Detroit. MI) and are a high-passage clone designated MCF10A1. MCF10A cells were grown in DMEM/F12 (Gibco) supplemented with 5% horse serum, insulin (5μg/ml), EGF (20ng/ml), hydrocortisone (500ng/ml), penicillin-streptomycin (100μg/ml each), and L-glutamine (2 mmol/L). EpH4 mouse mammary epithelial cells and those stably expressing pcDNA3.1-Bcl2 (B19) were previously described [7, 18], and maintained in DMEM (Gibco) supplemented with 10% bovine calf serum, penicillin-streptomycin (100μg/ml each), and L-glutamine (2 mmol/L). For all inhibitor and detachment studies, both MCF10A and EpH4 cells were treated in serum-free DMEM.
Immunofluorescence and Microscopy
EpH4 and B19 cells were suspended over 2% agarose coated plates or ultra-low-attachment plates (Corning) to prevent adhesion in either serum-free DMEM or media containing Latrunculin-A (5μM), Colchicine (100μM) or the combination. Single cells were scored blindly for protrusions and photographed with standard phase-contrast microscopy. For co-staining of α-tubulin and actin, suspended cells were then centrifuged onto poly-L-lysine coated coverslips (280g × 5min), fixed in 3.7% formaldehyde in PBS for 10 min, and permeablized with 0.1% Triton X-100 in PBS. Adhered cells were then stained for 1hr at room temperature using FITC-conjugated anti-α-tubulin (1:200; Sigma), Alexa-594 phalloidin (1:100; Molecular Probes), and Hoescht 33342 (1:5000; Sigma).
For visualization of modified tubulin, untreated cells were grown on glass coverslips or suspended in the presence of 5μM LA and centrifuged onto poly-L-lysine coated coverslips. Cells were fixed in ice-cold methanol for 10 min, permeablized in 0.25% Triton X-100 for 10min, and blocked for 1hr at room temperature in PBS containing 5% BSA and 0.5% NP40. Immunostaining for mouse monoclonal antibodies α-tubulin clone DM1A (1:2000; Sigma), tyrosinated tubulin clone TUB1A2 (1:2000; Sigma), and acetylated tubulin clone 611B1 (1:1000; Sigma) were incubated at room temperature for 1hr in PBS containing 2% BSA and 0.5% NP40. Rabbit polyclonal anti-detyrosinated tubulin (Glu; 1:500, Chemicon) was incubated overnight at 4°C. Anti-IgG antibodies conjugated to Alexa-594 (1:1000; Molecular Probes) were used for secondary detection. Live and fixed cell images were collected using an Olympus CKX41 inverted fluorescent microscope equipped with the Olympus F-View II 12-bit CCD digital camera system. Image acquisition and analysis used Olympus MicroSuite software.
Western Blotting
Whole cell lysates were prepared from EpH4 cells that were scraped or suspended in the presence or absence of 5μM Latrunculin-A. Cells were pelleted at 3000rpm × 5min and resuspended in lysis buffer (2% SDS, 100mM Tris-HCl (pH 6.8), 20% Glycerol, 20mM DTT, 1mM phenylmethylsulfonyl fluoride, 1% protease inhibitor cocktail (Sigma, P2714)), and then immediately boiled for 10 min. Protein concentration was measured using a Lowry based assay (Bio-Rad). 17.5μg of protein from each sample was separated by SDS-PAGE on 10% polyacrylamide gels and then transferred to Immuno-Blot PVDF membranes (Bio-Rad, Hercules, CA). The membrane was blocked in 2% milk in TBS with 0.1% Tween for 1hr at room temperature followed by an overnight incubation at 4°C in monoclonal α-tubulin (1:1000), tyrosinated tubulin (1:1000), acetylated tubulin (1:1000), polyclonal detyrosinated tubulin (1:1000) or PARP antibody (1:1000, Santa Cruz Biotechnology - H250) in 1% milk in TBST. Secondary antibodies to IgG conjugated to horseradish peroxidase were used (1:5000; GE Healthcare) and visualized using ECL chemiluminescent detection kit. Chemiluminescent signal was quantitated with an EpiChemi3 Imaging System (UVP, Inc.; Upland, CA) with an attached Hamamatsu CCD camera (Hamamatsu Photonics, Hamamatsu City, Japan) and analyzed with UVP Labworks Image Acquisition and Analysis Software v. 4.6. Signal for each modified tubulin was normalized to total α-tubulin, and then compared to the initial value of untreated, attached cells to allow integration of multiple experiments.
Cell-substratum Attachment Assay
Equivalent numbers of B19 cells were suspended over 6-well plates coated with 2% agarose in serum-free media containing 5μM Latrunculin-A (LA; BioMol), 100μM colchicine (Col; Sigma), 5μg/ml nocodazole (Noc), or 1μg/ml vinblastine (VinB; Sigma) for a duration of 1hr at 37°C. Cells were collected by centrifugation (1000rpm × 5min) and drug containing media was aspirated. Cells were resuspended in EpH4 serum-containing growth media and aliquoted out into 24 wells (8 timepoints × 3 replicates) of a 96-well clear bottom black plate either uncoated or precoated with the extracellular matrix protein laminin (BD Biosciences). After plating, cells were allowed to attach for duration of 15 min, 45 min, 1.5 hr, 3hr, 4hr, 5hr, 6hr. and 24hr at which point the media was aspirated from the replicate wells, washed twice in PBS, and fresh growth media was replaced at each timepoint. After 24hr, all wells were aspirated and the number of viable cells were quantitated by XTT assay (Sigma). Absorbance was measured using a Biotek Synergy HT Multi-Detection Microplate Reader at 450nm. All values are shown as averages + S.D. of triplicate samples from three experiments.
Cell-cell Attachment Assay
B19 cells were seeded onto 10cm dishes until ~ 80% confluency. The media was aspirated and cells were incubated in fresh growth media containing Hoescht 33342 (1:5000) for 30 min at 37°C to label all attached cells. The cells were washed twice in PBS, detached by trypsinization, and an equivalent number of cells were resuspended in serum-free media containing either 5μM Latrunculin A, 100μM Colchicine, 1μg/ml vinblastine, or 5μg/ml nocodazole and plated over 2% agarose-coated 24-well plates or ultra-low-attachment plates (Corning). Methylcellulose (0.3%) was added to the media to slow the process of cell aggregation which otherwise occurred rapidly within 15–30 minutes. The progression of cell-cell adhesion over time was visualized macroscopically by the EpiChemi3 Imaging System (UVP, Inc.; Upland, CA) using an excitation wavelength of 365nm and a blue band pass filter. Images were obtained with an attached Hamamatsu CCD camera (Hamamatsu Photonics, Hamamatsu City, Japan) and analyzed with UVP Labworks Image Acquisition and Analysis Software v. 4.6.
Time-lapse video microscopy
Phase-contrast and fluorescence images of detached cells were captured at one frame per second and are shown with a 5x acceleration. To better visualize protrusions in cell aggregates, cells were transiently transfected with plasma-membrane localized GFP (pAcGFP1-Mem, Clontech, Mountain View, CA). MCF10A cells were transfected using FuGENE-6 (Roche Diagnostics, Switzerland) while EpH4 were transfected using ExGen-500 (Fermentas, Hanover, MD) with 2ug of DNA according to manufacturer’s protocols. After 24hrs expression, cells were trypsinized, suspended in serum-free media and time-lapse images captured as before.
RESULTS
Detachment induces protrusions that persist in apoptotically-resistant cells
We placed MCF10A human mammary epithelial cells and EpH4 mouse mammary epithelial cells in suspension over an agarose surface to more closely examine cytoskeletal rearrangement upon detachment. When deprived of extracellular matrix contact, the nontumorigenic MCF10A human mammary epithelial cell line and EpH4 mouse mammary epithelial cell line produce protrusions of the plasma membrane (Figure 1A, black arrows). Time-lapse video microscopy detects rapid motion in these protrusions and transient probing contact with surfaces (Supplemental movie 1-MCF10A-4h.avi). Identical responses were seen in cells plated over ultra-low-attachment plates (Corning) so the response is not influenced by agarose.
Figure 1.
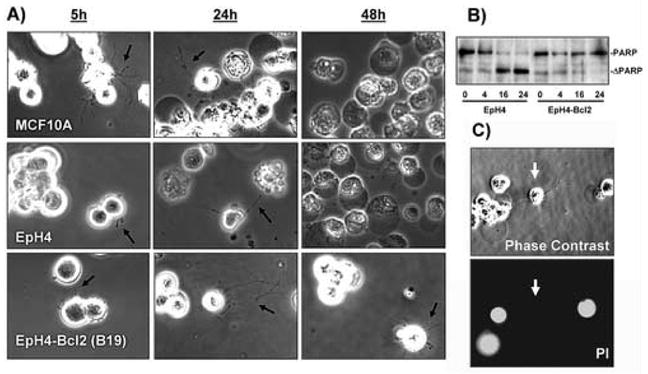
Detachment induces cellular protrusions in both normal and Bcl2-expressing mammary epithelial cells. A) Human MCF10A or mouse EpH4 mammary epithelial cell lines produce protrusions of the plasma membrane when plated over agarose and photographed live (black arrows). Apoptosis becomes apparent in detached MCF10A and EpH4 cells after 24 hours of detachment and almost all cells are dead by 48 hours, as indicated by darkening and condensation of the cells. EpH4 cells that overexpress Bcl-2 (EpH4-Bcl2) remain largely viable at 48 hours and continue to produce protrusions. B) EpH4 cells or those overexpressing Bcl-2 were placed in suspension for the indicated time in hours. Cleavage of PARP to a truncated form (ΔPARP), indicates that apoptosis is largely complete in EpH4 cells after 16 hours, while relatively few EpH4-Bcl2 cells die even after 24 hours. C) EpH4 cells which produce protrusions (white arrows) continue to exclude propidium iodide, indicating an intact plasma membrane. Similar results were obtained with MCF10A cells.
Apoptosis in EpH4 or MCF10A cells causes complete cellular fragmentation by 48 hours (Figure 1A), which obviously limits the time-frame during which such protrusions could affect cell behavior. However, when we used EpH4 cells overexpressing Bcl-2 (B19) that are highly resistant to apoptotic challenge [7], many of the cells remain intact after 48 hours and protrusions persist and continue to move dynamically (Figure 1A). Western blotting for caspase-dependent cleavage of poly-ADP-ribose-polymerase (PARP) indicates that apoptosis is largely complete in EpH4 cells 16 hours after detachment, while very few EpH4-Bcl2 cells undergo cell death, even after 24 hours (Figure 1B). Similar resistance and protrusions are observed in EpH4 or MCF10A cells overexpressing either Bcl-2 or Bcl-xL [8, 9], and protrusions can still be observed in apoptotically-resistant cells for at least seven days following detachment (data not shown). Cells that produce protrusions continue to exclude propidium iodide, indicating that the plasma membrane remains intact (Figure 1C, white arrows).
Protrusions are microtubule-based
In order to characterize the cytoskeletal mechanism underlying these novel detachment-induced protrusions, we treated suspended cells with inhibitors of actin and tubulin polymerization (Figure 2). After 15 minutes of suspension, the protrusions in untreated EpH4 or MCF10A cells remain fairly short (Figure 2A, white arrows). These early protrusions can often be difficult to detect with phase-contrast microscopy, due to interfering light refraction from the plasma membrane. Despite an initial hypothesis that these protrusions were actin-driven, inhibition of actin polymerization with Cytochalasin-D (CD) or Latrunculin-A (LA) did not reduce protrusions and significantly enhanced protrusions (Figure 2A, arrowheads). These protrusions are therefore distinct from the well-known invadopodia or podosomes, both of which are strongly inhibited by actin depolymerization [19, 20]. Inhibition of tubulin polymerization with colchicine prevented protrusions and led to a general blebbing of the plasma membrane. Simultaneous treatment with LA and colchicine generally blocked protrusions, and those that did form appeared fragmented into a “beads-on-a-string” morphology (Figure 2A, black arrow). To quantitate this effect, live cells were blindly scored for protrusions in suspension (Figure 2B). The ability of either Cytochalasin-D or Latrunculin-A to increase protrusion frequency or Colchicine to repress protrusions was statistically-significant (P<0.05, t-test) in MCF10A and EpH4 cells. Colchicine also significantly repressed the induction of protrusions by actin depolymerization (P<0.05, t-test), such that protrusion frequency with the combination of LA+Col or CD+Col was not statistically-significantly different from untreated cells. We performed these cell counts after one hour in live cells to prevent any degradation of these structures during fixation. Extending these counts to longer time points was not possible, since protrusions promote efficient cell-cell attachment and the resulting homotypic aggregation prevents accurate counting (Figure 6A and Supplemental Movies).
Figure 2.

Cytoskeletal inhibitors affect cellular protrusions. A) After 15 minutes of suspension, protrusions in EpH4 and MCF10A cells are quite small (Control, white arrows). Treatment with the actin depolymerizing agent, Cytochalasin-D, increased protrusions (white arrowheads) and this effect was even more pronounced with the actin inhibitor Latrunculin-A (LA, 5μM). The microtubule depolymerizing agent, Colchicine (1μM) prevented protrusions. The combination of LA and Colchicine (LA+Col) prevented protrusion formation, and those that did form appeared to be fragmented (black arrow). B) Populations of live, suspended cells were scored blindly for two or more protrusions longer than the cell radius. Each bar represents the mean +S.D. for three experiments in which at least 100 single cells were counted.
Figure 6.

Microtubule protrusions are required for efficient cell attachment. A) EpH4-Bcl2 cells were suspended over agarose and treated with inhibitors as above. Following treatment, cells were plated into 0.3% methylcellulose media over agarose and the rate of cell-cell attachment was followed by Hoescht DNA staining. While LA-treated cells cluster similarly or even more tightly compared to control cells after 5 hours (black arrow), cells treated with tubulin polymerization inhibitors are signficantly delayed in clustering. Similar results were found in three independent experiments. B) EpH4-Bcl2 cells suspended over agarose for one hour in DMEM or DMEM containing LA (5μM), Colchicine (1μM) or the combination. Reattachment of these cells to either uncoated tissue culture plates or those pre-coated with laminin was followed by XTT cell viability assay. Values represent mean+S.D. of raw XTT values of three separate experiments. While actin depolymerization with LA does not affect cell-surface attachment, depolymerization of tubulin with colchicine significantly prevents initial cell attachment to either uncoated or laminin-coated plates. Additional time-lapse studies of protrusions during cell attachment are available as supplemental data.
These inhibitor results in live cells suggested that the protrusions were microtubule-driven and immunofluorescence of detached cells spun onto coverslips confirmed this hypothesis and the relative lack of actin filaments along the protrusions (Figure 3). Protrusions were more easily visible with immunofluorescence than phase contrast microscopy (Figure 3, white arrows). Although actin filament staining was generally low along the length of the protrusion, dense points of actin were often found at the end of protrusions (Figure 3, arrowheads). The effect of LA to increase the number and length of microtubule protrusions was also evident with immunofluorescence. Colchicine also inhibited protrusions from either untreated cells or those treated with LA.
Figure 3.

Protrusions are microtubule-based. EpH4 cells were suspended in either DMEM (Control) or media containing 5μM Latrunculin-A (LA), 1μM Colchicine or the combination of both drugs for 30 minutes. Cell were then spun onto glass coverslips and fluorescently stained for α-tubulin (green) and polymerized actin (red). Protrusions that are difficult to see by phase contrast are easily discernible with immunostaining for α-tubulin (white arrows). Protrusions contain little polymerized actin, except at points of concentration, which are often at the end (arrowhead). Depolymerization of actin with LA strongly enhances the formation of microtubule protrusions.
Protrusions are enriched in Glu-tubulin
Posttranslationally-modified forms of α-tubulin are differentially localized between the cell body and protrusions, depending on the attachment state of the cells (Figure 4). This and the remaining experiments were performed in EpH4 cells expressing Bcl-2 (B19) to prevent induction of apoptosis during the experiments, but similar results were observed in both MCF10A and EpH4 cells. Immunofluorescence with an antibody that recognizes all forms of α-tubulin shows staining throughout cytoplasmic microtubules in attached cells, and a relatively even distribution between the cell body and protrusions in detached cells. Full-length α-tubulin contains a tyrosine at its c-terminal end (Tyr-tubulin), and is generally found in microtubules which turnover with a half-life of minutes [16]. This dynamic, tyrosinated tubulin is also found throughout cytoplasmic microtubules and specifically extends into lamellipodia in attached cells. In suspended cells, tyrosinated tubulin is mostly concentrated in the cell body and only weakly labels protrusions. Far fewer acetylated microtubules are found in attached cells, showing a mostly perinuclear localization. Acetylated tubulin is also found primarily in the cell body of detached cells, similar to tyrosinated tubulin. Tubulin which has been detyrosinated (Glu-tubulin) labels only a subset of cytoplasmic microtubules in attached cells, and is predominantly found in centrosomes (Figure 4, white arrows). In detached cells, Glu-tubulin remains at the centrosome, but unlike the other forms of α-tubulin, Glu-tubulin is enriched in protrusions relative to the cell body.
Figure 4.
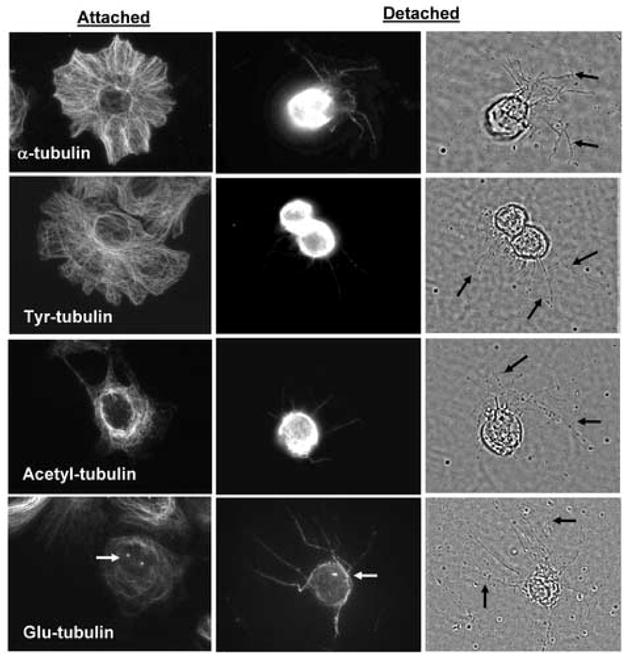
Protrusions are enriched in detyrosinated α-tubulin. EpH4-Bcl2 cells were grown on glass coverslips (Attached) or suspended over agarose for one hour in the presence of LA (5μM) to enhance protrusion formation (Detached). Immunostaining for different posttranslationally-modified forms of tubulin indicates differential localization in attached and suspended cells. Full-length α-tubulin, Tyr-tubulin and Acetyl-tubulin localize predominantly to the cell body in detached cells rather than specifically along the extended protrusions visible by phase-contrast (black arrows). Detyrosinated α-tubulin (Glu-tubulin) is enriched in the protrusions relative to the cell body, and is also found in centrosomes (white arrows).
Measurement of cellular levels of modified α-tubulin shows that detyrosinated Glu-tubulin increases in detached cells (Figure 5). Immediately following detachment, levels of Glu-tubulin increase more than 30-fold, and remain elevated for at least 2 hours. Increases in Glu-tubulin were statistically-significant when compared to attached cells at time zero and also statistically-significant (P<0.05, t-test) when compared to attached controls at matched timepoints either immediately or at one hour (Figure 5B, black asterisks). However, treatment with LA does not significantly increase Glu-tubulin in either attached or suspended cells (P>0.05, t-test). The increased protrusion frequency in response to LA may therefore reflect a decrease in an actin-dependent counteracting force, rather than a direct induction of Glu-tubulin. The relevant stimulus to increase Glu-tubulin is therefore detachment itself. Total cellular levels of α-tubulin, tyrosinated or acetylated tubulin do not significantly increase following detachment (P>0.05, t-test).
Figure 5.
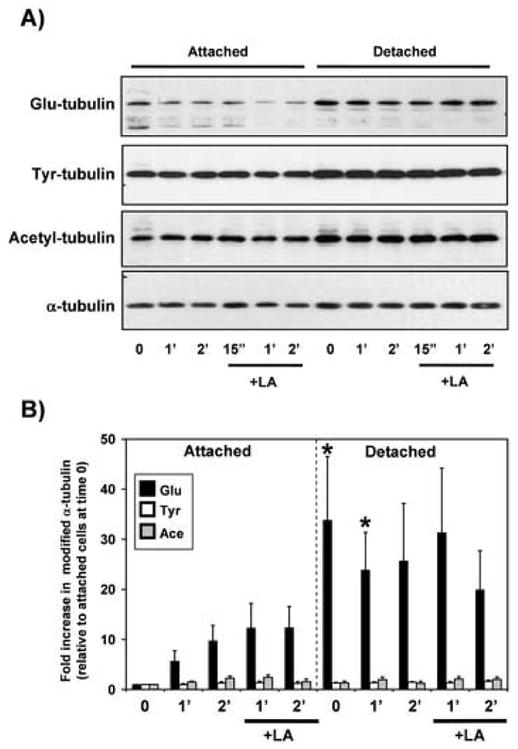
Cellular levels of Glu-tubulin increase in response to detachment. EpH4-Bcl2 cells were grown attached to tissue culture dishes or suspended over agarose for the indicated times in either the absence or presence of LA (5μM). A) Cell lysates were immunoblotted to detect levels of modified tubulin isoforms in the cell populations. B) Modified α-tubulin isoforms were normalized to total α-tubulin and then compared relative to the levels in attached cells at time zero. Bars represent the mean + S.E.M. of four independent experiments. While Glu-tubulin increases significantly in response to detachment (P<0.05, t-test), none of the other tubulin forms increase significantly (P>0.05, t-test). Differences in Glu-tubulin upon detachment were also statistically-significant when compared to matched controls at time zero or one hour (black asterisks). LA treatment does not significantly increase cellular levels of Glu-tubulin in either attached or detached cells (P>0.05, t-test).
Protrusions are required for efficient cell attachment
To determine if these microtubule protrusions influence the ability of cells to form attachments, we observed cells in suspension with time-lapse microscopy. Protrusions extend between cells and become stabilized when they contact adjacent cells (Supplemental Movie 2–10A-Group.avi). Since it is not possible to use phase-contrast microscopy to determine the behavior of individual protrusions as cells cluster together, we transiently transfected cells with GFP to observe how the protrusions in individual cells behave during cell aggregation. In MCF10A cells, protrusions extend around adjacent cells in a cluster and are stabilized compared to those that face away from adjacent cells (Supplemental Movie 3–10A-Untreated.avi). Inhibition of actin polymerization with LA does not prevent these protrusions from promoting cell-cell adhesion (Supplemental Movie 4–10A+LA.avi). Experiments in EpH4 cells showed the same effect (Supplemental Movies 5-EpH4-Untreated.avi and 6-EpH4+LA.avi). These observations in individual cells were confirmed across entire populations of cells by macroscopic observations of cell aggregation (Figure 6A). While actin depolymerization qualitatively speeds cell-cell attachment at 5h (Figure 6A, black arrow), pretreatment with three different microtubule inhibitors slows cell aggregation. In addition, binding to either uncoated or laminin-coated tissue culture surfaces was prevented by tubulin depolymerization (Figure 6B). Actin depolymerization did not significantly increase cell attachment to surfaces (P>0.05, t-test), but did not decrease it, indicating that actin rearrangement is not essential for the initial attachment of suspended cells. Tubulin was strictly required for initial cell attachment, as Colchicine significantly repressed cell binding to surfaces (P<0.05, t-test). Treatment with the tubulin depolymerizers Nocodazole or Vinblastine similarly repressed cell attachment to surfaces (data not shown). Cotreatment with LA and Colchicine also prevented attachment (Figure 6B), even though it only reduced protrusion frequency to control levels (Figure 2B). This deficiency of attachment probably reflects the disrupted structure (beads-on-a-string) that we observe in cells treated with both LA and Colchicine (Figure 2A, black arrow). At this point, our results indicate that these microtubule protrusions simply facilitate contact between cells or with a surface without depending on a specific receptor interaction. Induction of such nonspecific attachment activity in detached mammary epithelial cells could promote tumor cell dissemination, particularly when apoptotic resistance allows this response to continue for extended periods.
DISCUSSION
Elevation of detyrosinated α-tubulin (Glu-tubulin) in detached mammary epithelial cells and its concentration in long cellular protrusions is interesting, given the apparent role of this modified form of tubulin in tumor progression. In breast tumor samples, increased levels of Glu-tubulin are associated with poor patient prognosis and an increased risk of cancer-related complications [17]. Glu-tubulin is postranslationally converted back to Tyr-tubulin, by tubulin tyrosine ligase (TTL)[15]. Reduced expression of TTL increases steady state levels of Glu-tubulin and promotes sarcoma growth [21]. Suppressed TTL expression is also observed in human breast tumors and neuroblastomas of poor prognosis [21].
While microtubules containing Tyr-tubulin have a relatively short half-life, measured in minutes, Glu-tubulin is enriched in a more stable subset of microtubules [16]. Microtubules containing Glu-tubulin can persist for hours and have been observed to remain for as long as 16 hours in nondividing cells [16]. Our observation that the microtubule protrusions of detached cells are enriched in Glu-tubulin is consistent with the stability and persistence of these protrusions. However, microtubules composed of Glu-tubulin are not inherently more stable in vitro [22]. Detyrosination itself is therefore not thought to directly alter the physical properties of tubulin polymers [22], but how microtubules interact with other cellular systems. Glu-tubulin does interact preferentially with kinesin proteins [23], which can transport capping proteins to the plus ends of microtubules to stabilize them [24]. Our results indicate that detachment induces an increase in the total cellular levels of Glu-tubulin and therefore may promote increased microtubule stabilization.
Interestingly, recent data from budding yeast that express only Glu-tubulin show that microtubules composed of Glu-tubulin do not attach to the actin cortex underlying the plasma membrane [25]. Detachment-induced increases in Glu-tubulin could promote plasma membrane protrusions of microtubules by preventing efficient capture at the actin cortex. Our finding that depolymerization of actin with Latrunculin-A promotes the extension of microtubule protrusions is consistent with this hypothesis. Unlike detachment, Latrunculin-A did not increase Glu-tubulin levels. Therefore, our current model is that detachment increases stabilized microtubules enriched in Glu-tubulin, while decreased actin polymerization simply promotes extension of these microtubule protrusions.
Decreased levels of polymerized actin are observed in many different tumor types relative to their untransformed counterparts [26]. In addition, tumor cells often overexpress proteins that favor actin depolymerization, such as Thymosin-β4 [27, 28] and Thymosin-β10 [29]. Elevation of Thymosin-β4 decreases actin filaments in the cytoplasm and at the cortex [28] and is associated with metastatic progression of breast [30] and colon carcinoma [28]. It is possible that widespread depolymerization of cellular actin by proteins such as Thymosin-β4 would enhance microtubule protrusions in a manner similar to Latrunculin-A or Cytochalasin-D, and promote metastasis. Evidence implicating decreased biophysical tension of the actin cortex with the metastatic progression of mammary epithelial cells is especially interesting support for this hypothesis [31]. Extension of microtubule-rich axons at sites of actin cortical instability in neuronal cell lines also supports this proposed mechanism [32–34]. Depolymerization of actin with Cytochalasin-D also promotes microtubule processes in chicken erythrocytes from day 2 embryos[35], again supporting the balanced opposition of actin compression and microtubule extension. Recent experiments in our lab indicate that the frequency of microtubule protrusions varies greatly between different human breast tumor cell lines and correlates roughly with metastatic potential (Whipple, R.A. et al, manuscript in preparation). Although the protrusions in MCF10A and EpH4 cells responded similarly to cytoskeletal inhibitors, the protrusions in MCF10A cells were consistently longer and thicker (Figure 2), suggesting that there are additional determinants of protrusion structure that are yet to be identified.
These extended protrusions are particularly intriguing in light of recent evidence that a microtubule-based phenomenon regulates the ability of tumor cells to arrest in the capillaries of distant tissues [12]. Using in vivo video microscopy, Korb et al demonstrate that initial adherence of intravenously-injected colon carcinoma cells to the walls of liver capillaries is inhibited by microtubule depolymerization [12]. Conversely, inhibition of actin polymerization actually increases binding of the colon carcinoma cells to the capillary wall. Although the reasons for these in vivo effects are not yet clear, both results are consistent with the mechanisms underlying the protrusions that we observe in detached mammary epithelial cells. Successful metastasis of circulating tumor cells also depends on homotypic aggregation [36], a process that we have now shown involves extension of microtubule protrusions between adjacent cells.
Actin polymerization inhibitors did prevent efficient extravasation of tumor cells in vivo [12]. Numerous studies have established that actin-based invadopodia and podosomes are important for tumor cell migration along and through extracellular matrix (see [37] for review). Unlike the protrusions that we observe, invadopodia and podosomes are strongly inhibited by actin depolymerization and unaffected by tubulin depolymerization [19, 20]. On the other hand, microtubule protrusions may be necessary for detached cells to efficiently engage new attachment sites, and are actually enhanced by actin depolymerization. Therefore, we currently favor a two-step model in which microtubule protrusions could promote initial attachment to capillary walls and homotypic aggregation, while actin-based motility is necessary for successful extravasation. To distinguish these microtubule-based structures from actin-based invadopodia and podosomes, we propose the term tubulin microtentacles.
Outside of this mechanism in tumor cells, detachment of untransformed cells also rapidly decreases polymerized actin [38]. Inward tension of actin microfilaments is counteracted by outward expansion of microtubules to stabilize attached cells, in a process termed tensegrity [39]. Residual expansion of microtubules in detached cells, which have lost this actin-mediated compression, could initiate microtubule protrusion formation, without the requirement for any abnormal alteration of the actin cytoskeleton.
Our results showing that detached mammary epithelial cell lines of both human and mouse origin produce microtubule protrusions may indicate that it is a fairly general detachment response. Epithelial cells are tasked with maintaining barrier function in the body through the formation of continuous sheets [40], and are particularly prone to apoptotic cell death when detached from the extracellular matrix [5]. The dynamic protrusions that we observe could provide a selective advantage by promoting cell survival and the maintenance of the epithelial barriers through reattachment. This aggressive reattachment response would normally be controlled through rapid apoptosis in detached cells. In contrast, the persistent microtubule protrusions that we observe in apoptotically-resistant cells could enhance tumor cell attachment at distant sites. Since apoptotic resistance does not directly influence the motility of mammary epithelial cells [7, 9], any advantage would likely arise from the extended opportunity apoptotically-resistant cells have to produce such protrusions. Nearly 90% of human solid tumors arise as carcinomas from epithelial cells [41], so such an aggressive motility response to detachment could have broad implications for metastatic spread. Our data predict that these protrusions would be enhanced by genetic alterations causing reduced actin polymerization, but this is not a strict requirement and could occur without a tumor-specific mutation. Importantly, the persistence of these protrusions requires only apoptotic resistance which is not sufficient to induce primary breast tumor outgrowth [7, 9]. Enhanced attachment of cells via these microtubule protrusions could therefore occur prior to significant tumor outgrowth and promote early spread of bloodborne carcinoma cells to distant tissues.
Supplementary Material
Acknowledgments
This work is supported by a Howard Temin career award (to S.S.M, K01-CA096555) and a Cigarette Restitution Fund Cancer Research Grant (CH 649 CRF) from the State of Maryland Department of Health and Mental Hygiene.
Abbreviations
- CD
Cytochalasin-D
- Col
Colchicine
- EpH4
mouse mammary epithelial cell line
- LA
Latrunculin-A
- MCF10A
human mammary epithelial cell line
- PI
propidium iodide
- TTL
tubulin tyrosine ligase
Footnotes
Supplementary movie files are available at the Experimental Cell Research website.
References
- 1.Naumov GN, MacDonald IC, Weinmeister PM, Kerkvliet N, Nadkarni KV, Wilson SM, Morris VL, Groom AC, Chambers AF. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 2002;62:2162–8. [PubMed] [Google Scholar]
- 2.Naumov GN, MacDonald IC, Chambers AF, Groom AC. Solitary cancer cells as a possible source of tumour dormancy? Semin Cancer Biol. 2001;11:271–6. doi: 10.1006/scbi.2001.0382. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt-Kittler O, Ragg T, Daskalakis A, Granzow M, Ahr A, Blankenstein TJ, Kaufmann M, Diebold J, Arnholdt H, Muller P, Bischoff J, Harich D, Schlimok G, Riethmuller G, Eils R, Klein CA. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci U S A. 2003;100:7737–42. doi: 10.1073/pnas.1331931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 5.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003;3:17–22. doi: 10.1016/s1535-6108(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 7.Pinkas J, Martin SS, Leder P. Bcl-2-mediated cell survival promotes metastasis of EpH4 betaMEKDD mammary epithelial cells. Mol Cancer Res. 2004;2:551–6. [PubMed] [Google Scholar]
- 8.Martin SS, Leder P. Human mcf10a mammary epithelial cells undergo apoptosis following actin depolymerization that is independent of attachment and rescued by bcl-2. Mol Cell Biol. 2001;21:6529–36. doi: 10.1128/MCB.21.19.6529-6536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin SS, Ridgeway AG, Pinkas J, Lu Y, Reginato MJ, Koh EY, Michelman M, Daley GQ, Brugge JS, Leder P. A cytoskeleton-based functional genetic screen identifies Bcl-xL as an enhancer of metastasis, but not primary tumor growth. Oncogene. 2004;23:4641–5. doi: 10.1038/sj.onc.1207595. [DOI] [PubMed] [Google Scholar]
- 10.Nikiforov MA, Hagen K, Ossovskaya VS, Connor TM, Lowe SW, Deichman GI, Gudkov AV. p53 modulation of anchorage independent growth and experimental metastasis. Oncogene. 1996;13:1709–19. [PubMed] [Google Scholar]
- 11.Nikiforov MA, Kwek SS, Mehta R, Artwohl JE, Lowe SW, Gupta TD, Deichman GI, Gudkov AV. Suppression of apoptosis by bcl-2 does not prevent p53-mediated control of experimental metastasis and anchorage dependence. Oncogene. 1997;15:3007–12. doi: 10.1038/sj.onc.1201723. [DOI] [PubMed] [Google Scholar]
- 12.Korb T, Schluter K, Enns A, Spiegel HU, Senninger N, Nicolson GL, Haier J. Integrity of actin fibers and microtubules influences metastatic tumor cell adhesion. Exp Cell Res. 2004;299:236–47. doi: 10.1016/j.yexcr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Argarana CE, Arce CA, Barra HS, Caputto R. In vivo incorporation of [14C]tyrosine into the C-terminal position of the alpha subunit of tubulin. Arch Biochem Biophys. 1977;180:264–8. doi: 10.1016/0003-9861(77)90037-6. [DOI] [PubMed] [Google Scholar]
- 14.Argarana CE, Barra HS, Caputto R. Release of [14C]tyrosine from tubulinyl-[14C]tyrosine by brain extract. Separation of a carboxypeptidase from tubulin-tyrosine ligase. Mol Cell Biochem. 1978;19:17–21. doi: 10.1007/BF00231230. [DOI] [PubMed] [Google Scholar]
- 15.Erck C, MacLeod RA, Wehland J. Cloning and genomic organization of the TTL gene on mouse chromosome 2 and human chromosome 2q13. Cytogenet Genome Res. 2003;101:47–53. doi: 10.1159/000073418. [DOI] [PubMed] [Google Scholar]
- 16.Webster DR, Gundersen GG, Bulinski JC, Borisy GG. Differential turnover of tyrosinated and detyrosinated microtubules. Proc Natl Acad Sci U S A. 1987;84:9040–4. doi: 10.1073/pnas.84.24.9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mialhe A, Lafanechere L, Treilleux I, Peloux N, Dumontet C, Bremond A, Panh MH, Payan R, Wehland J, Margolis RL, Job D. Tubulin detyrosination is a frequent occurrence in breast cancers of poor prognosis. Cancer Res. 2001;61:5024–7. [PubMed] [Google Scholar]
- 18.Lopez-Barahona M, Fialka I, Gonzalez-Sancho JM, Asuncion M, Gonzalez M, Iglesias T, Bernal J, Beug H, Munoz A. Thyroid hormone regulates stromelysin expression, protease secretion and the morphogenetic potential of normal polarized mammary epithelial cells. Embo J. 1995;14:1145–55. doi: 10.1002/j.1460-2075.1995.tb07098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourguignon LY, Gunja-Smith Z, Iida N, Zhu HB, Young LJ, Muller WJ, Cardiff RD. CD44v(3,8–10) is involved in cytoskeleton-mediated tumor cell migration and matrix metalloproteinase (MMP-9) association in metastatic breast cancer cells. J Cell Physiol. 1998;176:206–15. doi: 10.1002/(SICI)1097-4652(199807)176:1<206::AID-JCP22>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Spinardi L, Rietdorf J, Nitsch L, Bono M, Tacchetti C, Way M, Marchisio PC. A dynamic podosome-like structure of epithelial cells. Exp Cell Res. 2004;295:360–74. doi: 10.1016/j.yexcr.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Lafanechere L, Courtay-Cahen C, Kawakami T, Jacrot M, Rudiger M, Wehland J, Job D, Margolis RL. Suppression of tubulin tyrosine ligase during tumor growth. J Cell Sci. 1998;111 (Pt 2):171–81. doi: 10.1242/jcs.111.2.171. [DOI] [PubMed] [Google Scholar]
- 22.Skoufias DA, Wilson L. Assembly and colchicine binding characteristics of tubulin with maximally tyrosinated and detyrosinated alpha-tubulins. Arch Biochem Biophys. 1998;351:115–22. doi: 10.1006/abbi.1997.0510. [DOI] [PubMed] [Google Scholar]
- 23.Kreitzer G, Liao G, Gundersen GG. Detyrosination of tubulin regulates the interaction of intermediate filaments with microtubules in vivo via a kinesin-dependent mechanism. Mol Biol Cell. 1999;10:1105–18. doi: 10.1091/mbc.10.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carvalho P, Gupta ML, Jr, Hoyt MA, Pellman D. Cell cycle control of kinesin-mediated transport of Bik1 (CLIP-170) regulates microtubule stability and dynein activation. Dev Cell. 2004;6:815–29. doi: 10.1016/j.devcel.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Badin-Larcon AC, Boscheron C, Soleilhac JM, Piel M, Mann C, Denarier E, Fourest-Lieuvin A, Lafanechere L, Bornens M, Job D. Suppression of nuclear oscillations in Saccharomyces cerevisiae expressing Glu tubulin. Proc Natl Acad Sci U S A. 2004;101:5577–82. doi: 10.1073/pnas.0307917101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao J, Li N. Microfilament actin remodeling as a potential target for cancer drug development. Curr Cancer Drug Targets. 2004;4:345–54. doi: 10.2174/1568009043332998. [DOI] [PubMed] [Google Scholar]
- 27.Cha HJ, Jeong MJ, Kleinman HK. Role of thymosin beta4 in tumor metastasis and angiogenesis. J Natl Cancer Inst. 2003;95:1674–80. doi: 10.1093/jnci/djg100. [DOI] [PubMed] [Google Scholar]
- 28.Wang WS, Chen PM, Hsiao HL, Ju SY, Su Y. Overexpression of the thymosin beta-4 gene is associated with malignant progression of SW480 colon cancer cells. Oncogene. 2003;22:3297–306. doi: 10.1038/sj.onc.1206404. [DOI] [PubMed] [Google Scholar]
- 29.Liu CR, Ma CS, Ning JY, You JF, Liao SL, Zheng J. Differential thymosin beta 10 expression levels and actin filament organization in tumor cell lines with different metastatic potential. Chin Med J (Engl) 2004;117:213–8. [PubMed] [Google Scholar]
- 30.Magdalena C, Dominguez F, Loidi L, Puente JL. Tumour prothymosin alpha content, a potential prognostic marker for primary breast cancer. Br J Cancer. 2000;82:584–90. doi: 10.1054/bjoc.1999.0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guck J, Schinkinger S, Lincoln B, Wottawah F, Ebert S, Romeyke M, Lenz D, Erickson HM, Ananthakrishnan R, Mitchell D, Kas J, Ulvick S, Bilby C. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J. 2005;88:3689–98. doi: 10.1529/biophysj.104.045476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Etienne-Manneville S. Actin and microtubules in cell motility: which one is in control? Traffic. 2004;5:470–7. doi: 10.1111/j.1600-0854.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 33.Bradke F, Dotti CG. The role of local actin instability in axon formation. Science. 1999;283:1931–4. doi: 10.1126/science.283.5409.1931. [DOI] [PubMed] [Google Scholar]
- 34.Baorto DM, Mellado W, Shelanski ML. Astrocyte process growth induction by actin breakdown. J Cell Biol. 1992;117:357–67. doi: 10.1083/jcb.117.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winckler B, Solomon F. A role for microtubule bundles in the morphogenesis of chicken erythrocytes. Proc Natl Acad Sci U S A. 1991;88:6033–7. doi: 10.1073/pnas.88.14.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glinsky VV, Glinsky GV, Glinskii OV, Huxley VH, Turk JR, Mossine VV, Deutscher SL, Pienta KJ, Quinn TP. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63:3805–11. [PubMed] [Google Scholar]
- 37.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–64. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Mooney DJ, Langer R, Ingber DE. Cytoskeletal filament assembly and the control of cell spreading and function by extracellular matrix. J Cell Sci. 1995;108:2311–20. doi: 10.1242/jcs.108.6.2311. [DOI] [PubMed] [Google Scholar]
- 39.Ingber DE. Cancer as a disease of epithelial-mesenchymal interactions and extracellular matrix regulation. Differentiation. 2002;70:547–60. doi: 10.1046/j.1432-0436.2002.700908.x. [DOI] [PubMed] [Google Scholar]
- 40.Mullin JM, Agostino N, Rendon-Huerta E, Thornton JJ. Keynote review: epithelial and endothelial barriers in human disease. Drug Discov Today. 2005;10:395–408. doi: 10.1016/S1359-6446(05)03379-9. [DOI] [PubMed] [Google Scholar]
- 41.Birchmeier C, Birchmeier W, Brand-Saberi B. Epithelial-mesenchymal transitions in cancer progression. Acta Anat. 1996;156:217–26. doi: 10.1159/000147848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


