Abstract
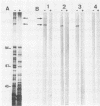
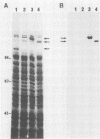
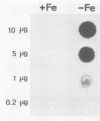
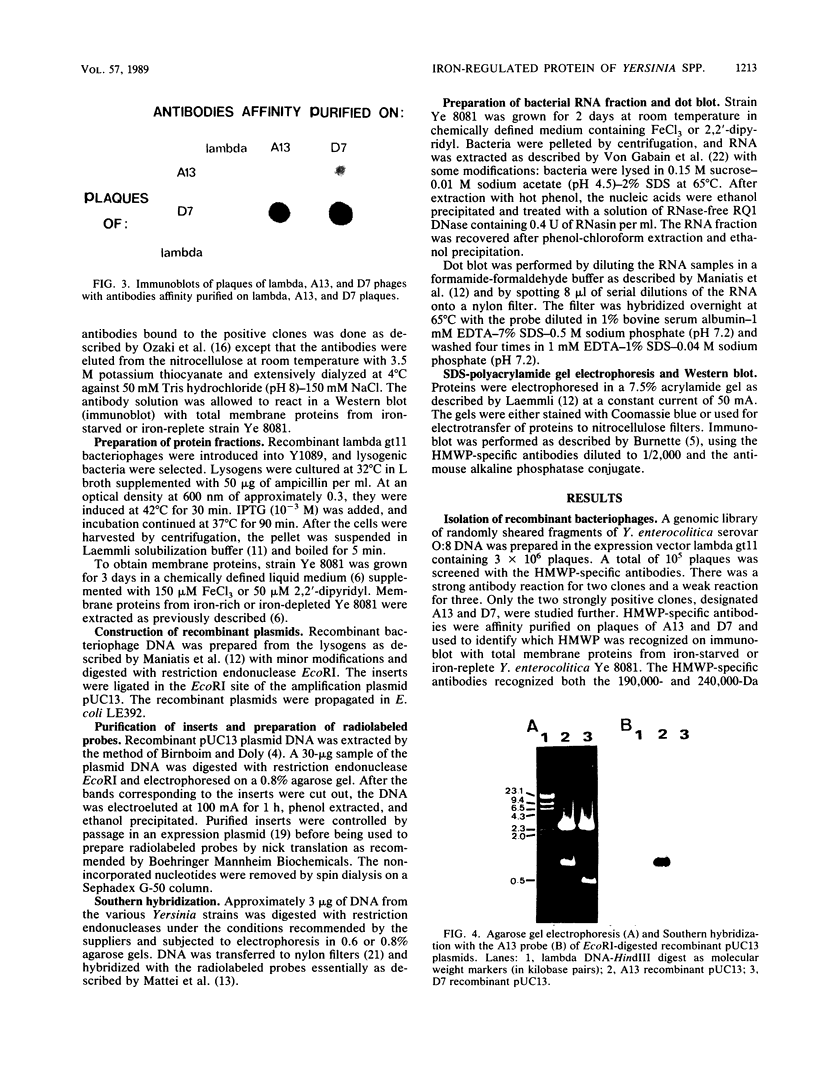
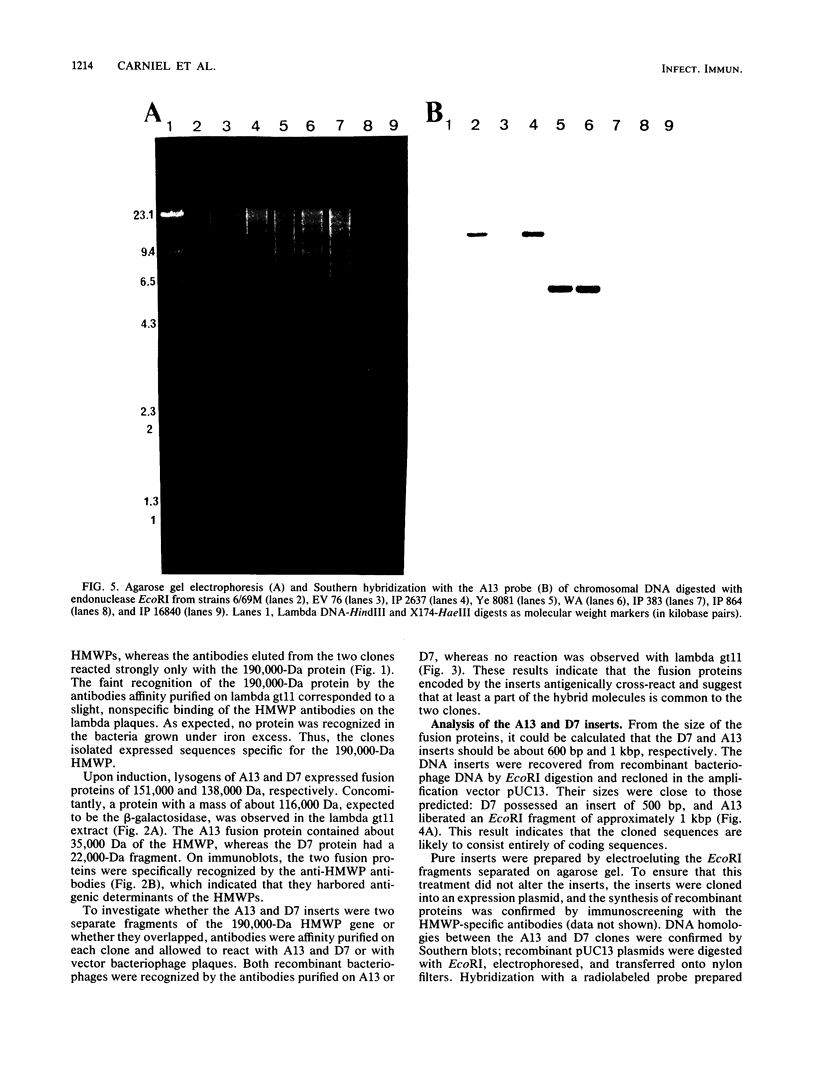
A genomic library containing DNA fragments of 0.5 to 2 kilobase pairs in length from Yersinia enterocolitica serovar O:8 was constructed in a bacteriophage lambda gt11 expression vector. Mouse antibodies specific for the iron-regulated high-molecular-weight proteins (HMWPs) were used to screen the library. Two positive clones of 1 and 0.5 kilobase pairs, designated A13 and D7, respectively, were detected and isolated. They coded for beta-galactosidase fusion proteins of 151,000 and 138,000 daltons (Da). Antibodies affinity purified on the two recombinant lambda gt11 vectors specifically recognized the smaller HMWP (190,000 Da) and not the larger (240,000 Da). The two cloned DNA fragments were used to construct recombinant amplification plasmid pUC13 and to obtain large amounts of purified A13 and D7 inserts. Southern hybridizations performed with the inserts used as probes revealed that: (i) the two cloned DNA fragments overlap; (ii) only one gene hybridizes with the A13 and D7 inserts; (iii) the gene coding for the HMWP is conserved among all highly pathogenic Yersinia species studied; (iv) this gene is missing in the low-virulence and nonvirulent strains; and (v) transcription of the HMWP gene is induced by iron starvation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. Shotgun DNA sequencing using cloned DNase I-generated fragments. Nucleic Acids Res. 1981 Jul 10;9(13):3015–3027. doi: 10.1093/nar/9.13.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Gurion R., Shafferman A. Essential virulence determinants of different Yersinia species are carried on a common plasmid. Plasmid. 1981 Mar;5(2):183–187. doi: 10.1016/0147-619x(81)90019-6. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carniel E., Antoine J. C., Guiyoule A., Guiso N., Mollaret H. H. Purification, location, and immunological characterization of the iron-regulated high-molecular-weight proteins of the highly pathogenic yersiniae. Infect Immun. 1989 Feb;57(2):540–545. doi: 10.1128/iai.57.2.540-545.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carniel E., Mazigh D., Mollaret H. H. Expression of iron-regulated proteins in Yersinia species and their relation to virulence. Infect Immun. 1987 Jan;55(1):277–280. doi: 10.1128/iai.55.1.277-280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber D. M., Brubaker R. R. Plasmids in Yersinia pestis. Infect Immun. 1981 Feb;31(2):839–841. doi: 10.1128/iai.31.2.839-841.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980 Feb;27(2):682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T., Wohlhieter J. A. Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect Immun. 1980 Jun;28(3):1044–1047. doi: 10.1128/iai.28.3.1044-1047.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesemann J., Algermissen B., Laufs R. Genetically manipulated virulence of Yersinia enterocolitica. Infect Immun. 1984 Oct;46(1):105–110. doi: 10.1128/iai.46.1.105-110.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mattei D., Langsley G., Braun-Breton C., Guillotte M., Dubremetz J. F., Mercereau-Puijalon O. The S-antigen of Plasmodium falciparum Palo Alto represents a new S-antigen serotype. Mol Biochem Parasitol. 1988 Jan 15;27(2-3):171–180. doi: 10.1016/0166-6851(88)90036-9. [DOI] [PubMed] [Google Scholar]
- Melby K., Slørdahl S., Gutteberg T. J., Nordbø S. A. Septicaemia due to Yersinia enterocolitica after oral overdoses of iron. Br Med J (Clin Res Ed) 1982 Aug 14;285(6340):467–468. doi: 10.1136/bmj.285.6340.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mofenson H. C., Caraccio T. R., Sharieff N. Iron sepsis: Yersinia enterocolitica septicemia possibly caused by an overdose of iron. N Engl J Med. 1987 Apr 23;316(17):1092–1093. [PubMed] [Google Scholar]
- Ozaki L. S., Mattei D., Jendoubi M., Druihle P., Blisnick T., Guillotte M., Puijalon O., Da Silva L. P. Plaque antibody selection: rapid immunological analysis of a large number of recombinant phage clones positive to sera raised against Plasmodium falciparum antigens. J Immunol Methods. 1986 May 22;89(2):213–219. doi: 10.1016/0022-1759(86)90360-1. [DOI] [PubMed] [Google Scholar]
- Robins-Browne R. M., Prpic J. K. Effects of iron and desferrioxamine on infections with Yersinia enterocolitica. Infect Immun. 1985 Mar;47(3):774–779. doi: 10.1128/iai.47.3.774-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche G., Leheup B., Gérard A., Canton P., Lion C., Leichtmann G., Dureux J. B. Septicémies à Yersinia enterocolitica. Revue générale à propos d'un nouvaeu cas chez une jeune femme présentant une thalassémie majeure. Rev Med Interne. 1982 Mar;3(1):65–74. doi: 10.1016/s0248-8663(82)80010-6. [DOI] [PubMed] [Google Scholar]
- Rüther U., Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli A. C., Fischer D. S., Downs W. G. Use of sarcoma 180/TG to prepare hyperimmune ascitic fluid in the mouse. J Immunol. 1966 Apr;96(4):676–682. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Zink D. L., Feeley J. C., Wells J. G., Vanderzant C., Vickery J. C., Roof W. D., O'Donovan G. A. Plasmid-mediated tissue invasiveness in Yersinia enterocolitica. Nature. 1980 Jan 10;283(5743):224–226. doi: 10.1038/283224a0. [DOI] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]