Abstract
All living organisms depend on dynamic mechanisms that repeatedly reassess the status of amassed energy, in order to adapt energy supply to demand. The AMP-activated protein kinase (AMPK) αβγ heterotrimer has emerged as an important integrator of signals managing energy balance. Control of AMPK activity involves allosteric AMP and ATP regulation, auto-inhibitory features and phosphorylation of its catalytic (α) and regulatory (β and γ) subunits. AMPK has a prominent role not only as a peripheral sensor but also in the central nervous system as a multifunctional metabolic regulator. AMPK represents an ideal second messenger for reporting cellular energy state. For this reason, activated AMPK acts as a protective response to energy stress in numerous systems. However, AMPK inhibition also actively participates in the control of whole body energy homeostasis. In this review, we discuss recent findings that support the role and function of AMPK inhibition under physiological and pathological states.
Keywords: AMP-Activated Protein Kinases, chemistry, metabolism, Animals, Down-Regulation, Energy Metabolism, Enzyme Activation, Humans
Keywords: Energy balance, metabolism, inhibition, AMPK, metabolic diseases
Introduction
The survival of all organisms depends on the dynamic control of energy metabolism during acute or prolonged shortage of nutrient supply. Over the past years, the 5′-adenosine monophosphate-activated protein kinase (AMPK) has emerged as an important regulator of cellular energy homeostasis that coordinates metabolic pathways in order to balance nutrient supply with energy demand in mammalian cells. AMPK is a homolog of Snf1 kinase, a Saccharomyces cerevisiae metabolic stress sensing kinase that is critical for yeast survival under conditions of glucose starvation (Woods et al., 1994). AMPK is an energy-sensing protein complex, activated in response to an increase in the AMP:ATP ratio during hypoxia, starvation, glucose deprivation or muscle contraction (Kahn et al., 2005). AMPK integrates nutritional and hormonal signals to maintain cellular energy balance and execute appropriate metabolic functions (e.g., regulation of fatty acids partitioning between oxidative and biosynthetic pathways) in response to nutritional and environmental variations (Viollet et al., 2009). One mechanism by which AMPK regulates lipid metabolism is phosphorylation and inactivation of acetyl CoA carboxylase (ACC), an important rate-controlling enzyme for the synthesis of malonyl-CoA. ACC is both a critical precursor for biosynthesis of fatty acids and a potent inhibitor of long-chain fatty acyl-CoA transport to mitochondria for β-oxidation. Knockdown/knockout of ACC1 and ACC2 (predominantly expressed in liver and skeletal muscle, respectively), has been reported to cause continuous fatty acid oxidation, increased energy expenditure and reduced fat mass (Abu-Elheiga et al., 2001; Choi et al., 2007; Savage et al., 2006). But recent studies have reported limited effects of ACC2 deletion on fatty acid oxidation in skeletal muscle and overall energy expenditure or adiposity (Hoehn et al., 2010; Olson et al., 2010). These recent reports indicate that increased fatty acid oxidation in skeletal muscle does not cause leanness and raises questions regarding the use of ACC2 inhibitors in the treatment of obesity. However, the use of a small molecule direct AMPK activator A-769662 was shown to have beneficial effects on both hepatic steatosis and insulin resistance, thus emphasizing the potential therapeutic implications for AMPK activation in type 2 diabetes (Cool et al., 2006). Regardless, AMPK activation results in inhibition of energy-consuming biosynthetic pathways (such as fatty acid synthesis and adipocytes, cholesterol synthesis in the liver and insulin secretion from β-cell) and activation of ATP-producing catabolic pathways (such as fatty acid uptake and oxidation in multiple tissues, glycolysis in heart and mitochondrial biogenesis in muscle). AMPK also modulates transcription of specific genes involved in energy metabolism, thereby exerting long-term metabolic control (Viollet et al., 2006). It is also implicated in the central regulation of food intake and energy expenditure in response to hormonal cues including leptin, ghrelin and adiponectin. Thus, AMPK not only governs cellular energy, but regulates overall organismal bio-energetics by coordinating the response in and in-between tissues according to nutritional input. Its position at crossroads of energy metabolism makes AMPK an attractive therapeutic target in metabolic diseases, with its pharmaceutical potential in situations of insulin resistance already confirmed. It has therefore emerged as a promising new drug target for the treatment of metabolic disorders including obesity, Type 2 diabetes and cardiovascular disease.
Due to its role in maintaining energy balance, a dysfunction in AMPK signaling pathway may result in perturbations at the systemic level that contribute to development of metabolic disorders. In support, there is a strong correlation between low AMPK activation state, mainly due to over-nutrition and lack of exercise, and metabolic disorders associated with insulin resistance, obesity and sedentary lifestyle (Ruderman and Prentki, 2004). Furthermore, decreased AMPK activation is implicated in human metabolic disorders associated with increased cancer risk. Numerous studies show links between AMPK and cancer, both at the organism and molecular levels. Although the contribution of AMPK to the etiology of these disorders is unclear, pharmacologic AMPK activators are effective in their treatment. Interestingly, recent evidences indicate that AMPK participates in the regulation of non-metabolic processes such as cell growth, cell cycle progression and organization of the cytoskeleton (Williams and Brenman, 2008). Since, all these are highly energy-consuming processes, AMPK’s involvement is certain, due to its ability to directly sense and regulate cellular energy homeostasis. AMPK signaling pathway serves as a metabolic checkpoint in the cell, arresting cell growth in low energy status, such as low nutrient conditions (Jones et al., 2005). Moreover, recent studies in lower eukaryote demonstrated that AMPK is involved in the regulation of epithelial cell polarity and mitotic cell division (Lee et al., 2007a).
Although AMPK activation is an adaptive response to energy stress in numerous systems, AMPK plays a role in both physiological and pathophysiological states. Here, we review our understanding of AMPK inhibition in response to cellular and organismal energy challenges and describe the beneficial as well as adverse consequences of global AMPK modulation.
A. Structure and regulation of AMPK
1. Structure and subunit composition
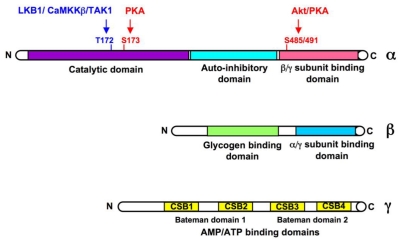
AMPK exists in the cell as a heterotrimeric complex with one catalytic (α, 63 kDa) and two regulatory subunits (β, 30kDa, and γ, 38–63 kDa) in a 1α:1β:1γ ratio (Figure 1). The α subunit contains a conventional serine/threonine kinase domain at the N-terminus followed by an auto-inhibitory domain, and a C-terminus containing the domains required for binding of β and γ subunits (Crute et al., 1998). The β subunit contains two characterized elements, a central domain ensuring the binding of AMPK complexes to glycogen (Hudson et al., 2003; Polekhina et al., 2003) and a C-terminal region acting as a tethering domain for α and γ subunits (Iseli et al., 2005; Townley and Shapiro, 2007). The γ subunit contains a variable N-terminal region followed by four highly conserved cystathionine-β-synthase (CBS) sequence repeats (small motifs found in tandem pairs termed Bateman domains (Bateman, 1997)), capable of binding adenine nucleotides, such as AMP or ATP (Scott et al., 2004; Townley and Shapiro, 2007; Xiao et al., 2007). Further isoforms have been identified for each of the three AMPK subunits (α1, α2, β1, β2, γ1, γ2, γ3 with splice variants for the γ2 and γ3 isoforms adding to the diversity), encoded by distinct genes and theoretically leading to formation of at least 12 different complexes. These combinations confer different properties to the AMPK complexes through differences in subcellular localization and signaling functions (Cheung et al., 2000; Salt et al., 1998a). Thus, the tissue-specific subunit composition may be important to determine a specialized cellular and systemic response to different metabolic stresses. Recent investigation of isoform composition of AMPK complexes in human skeletal muscle found that only 3 of the 12 potential AMPK complexes were present (α2β2γ1≫α2β2γ3=α1β2γ1) and were activated differently, depending on exercise intensity and duration (Birk and Wojtaszewski, 2006). AMPKα1 catalytic subunit expression is relatively distributed across adipose tissue, pancreas, lung, spleen and kidney. Skeletal and cardiac muscles predominantly express AMPKα2 catalytic subunit. While the β1 subunit is ubiquitously expressed, AMPK β2 subunit is abundantly expressed in skeletal muscle and heart. Interestingly, expression of the γ3 subunit appears highly specific to glycolytic skeletal muscle whereas γ1 and γ2 show broad tissue distribution (Cheung et al., 2000).
Figure 1. Domain organization of the catalytic α and regulatory β and γ subunits of AMPK.
Residues phosphorylated by AMPKK (LKB1, CaMKKβ, TAK1), PKA and Akt are shown within the α subunit.
2. Allosteric regulation by AMP and ATP
AMPK is allosterically activated by AMP, which binds to the regulatory γ subunit, resulting in a 2 to 5 fold increase in activity compared to basal activity (Hardie et al., 1999). The degree of activation by AMP is markedly affected by nature of the catalytic α and regulatory γ iso-forms constituting the AMPK complex, illustrating the complexity of AMPK signaling regulation. The greatest scale of activation is observed in AMPK complexes containing the α2 and γ2 subunits, while complexes containing the γ3 isoform are only weakly activated by AMP (Cheung et al., 2000). Binding of AMP to the γ subunit causes direct allosteric activation of the kinase and also induces a conformational change in the kinase domain that protects AMPK from dephosphorylation of Thr-172 (Riek et al., 2008; Sanders et al., 2007; Suter et al., 2006), favoring accumulation of the phosphorylated active form of AMPK (see below). Interestingly, it has been demonstrated that high concentrations of ATP oppose activation of the AMPK complex by AMP, suggesting that the allosteric sites bind AMP and ATP in a mutually exclusive manner (Corton et al., 1995). Thus, AMPK can be considered more as a sensor of the intracellular AMP/ATP ratio, rather than a direct sensor of AMP levels. AMP and ATP vary reciprocally in cells, due to the action of adenylate kinase (AMP + ATP <-> 2ADP [adenosine 5′-diphosphate]), thus, AMP: ATP ratio may be a more sensitive indicator of cellular energy status than ADP: ATP ratio. The finding that AMPK activation is altered in contracting muscles from adenylate kinase-deficient mice also supports a role for adenylate kinase in generation of an AMPK activating signal (Hancock et al., 2006).
The crystal structures of mammalian γ and yeast homologue revealed the structural and conformational elements required for binding the regulatory nucleotides AMP and ATP (Townley and Shapiro, 2007; Xiao et al., 2007). Out of all the CBS domains present, two CBS domains appear to bind AMP or ATP reversibly and may correspond to the two regulatory sites identified from previous binding studies (Scott et al., 2004). A third CBS domain binds AMP very strongly and does not readily exchange with ATP, but its physiological role is unclear. The fourth CBS domain remains unoccupied even in the presence of high concentrations of AMP or ATP (Xiao et al., 2007). Several naturally occurring point mutations in the CBS domains of human γ2 isoform have been reported to cause an inherited syndrome of hypertrophic cardiomyopathy of varying severity associated with excessive glycogen storage in cardio myocytes, accompanied with Wolff–Parkinson–White syndrome, a pre-excitation disorder (Arad et al., 2007). Biochemical studies demonstrate that some of these mutations interfere with the binding of AMP and allosteric activation (Scott et al., 2004) of AMPK. This supports the evidence that Bateman domains (CBS domains) constitute the regulatory binding sites for AMP. Interestingly, although mutations in the γ2 subunit reduces binding of the activating nucleotide AMP, they also appear to increase the basal activity associated with elevated Thr-172 phosphorylation (Arad et al., 2002; Burwinkel et al., 2005). This is presumably due to a concomitant reduction in binding of the inhibitory nucleotide ATP and consequent reduction in phosphatase activity, thus hindering kinase inactivation (see below). Therefore, γ2 mutants, unoccupied by ATP, behave in a partially active conformation and this “gain-of-function” effect could explain the dominant nature of γ subunit mutations (Burwinkel et al., 2005; Hamilton et al., 2001). Hence, AMPK dysregulation could be connected with impaired binding or interaction of both AMP and ATP on the γ subunit.
AMPK is also allosterically inhibited by physiological concentrations of phosphocreatine (Ponticos et al., 1998), consistent with the proposed physiological role of the kinase as a sensor of cellular energy status. As it decreases during muscle contraction, phosphocreatine, rather than AMP, may be the key regulator of the AMPK system during short-term exercise.
3. Autoregulation of AMPK complexes
AMPK, like other protein kinases, autoregulates its own activity through structural elements, that directly block its catalytic site. Within the catalytic α subunit, a region that is C-terminal to the kinase domain appears to act as an auto-inhibitory domain (AID) by interfering with kinase substrate binding and catalytic function (Crute et al., 1998). Detailed mutagenesis studies provide evidence that a conserved short segment of the α subunit [α1-(313–335)], forming an α helix, binds to the kinase domain in an inactive conformation and is responsible for auto-inhibition (Pang et al., 2007). Furthermore, three-dimensional structural studies revealed that hydrophobic contacts between the kinase domain and the AID have a predominant role in the allosteric control by AMP (Chen et al., 2009a). Upon binding of AMP, conformational change between low and high activity forms of AMPK alters the interaction between AID and kinase domains and eventually removes the effect of AID on kinase activation and also Thr172 dephosphorylation (Chen et al., 2009a). This mechanism of AMPK inhibition highlights the potential to develop small compounds that activate AMPK by antagonizing the auto-inhibitory role of AID (Pang et al., 2008). In addition to the AID, it has been suggested that AMPK is also inhibited by an internal auto-inhibitory sequence similar to the consensus recognition motif for AMPK substrates but lacking a phosphoryl-able amino acid. Scott and coworkers proposed that, in the absence of AMP, a pseudo-substrate sequence, within the γ2 CBS2 sub-domain, binds to the catalytic groove of AMPKα, preventing phosphorylation by the upstream kinase and therefore, access to downstream targets (Scott et al., 2007). When AMP binds to the γ subunit, a conformational change prevents the interaction of the pseudo-substrate sequence with the kinase domain and thus, causes activation of AMPK.
4. Regulation by phosphorylation/dephosphorylation
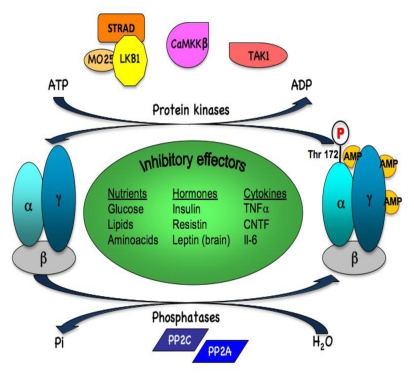
In addition to allosteric activation, AMPK is regulated by reversible phosphorylation (Figure 2). The key step in AMPK activation is its phosphorylation on threonine residue 172 (Thr-172), within the catalytic domain, by upstream kinases. The combination of the allosteric and phosphorylation effects causes >1000-fold increase in kinase activity (compared to up-to fivefold for allosteric activation alone), allowing high sensitivity in responses to small changes in cellular energy status (Suter et al., 2006). Three AMPK upstream kinases (AMPKKs) have been identified to date. The primary AMPKK is a complex between the tumor suppressor, LKB1, and two accessory subunits, STRAD and MO25 (Hawley et al., 2003; Woods et al., 2003a). LKB1 also functions upstream of 12 other kinases (AMPK-related kinases) situated on the same family as AMPK by phylogenetic analysis of kinase domain sequences (Lizcano et al., 2004). The LKB1 protein kinase activity appears to be constitutively active and is not regulated by AMP (Lizcano et al., 2004; Sakamoto et al., 2004). This view was recently challenged with recent studies showing that the subcellular localization of LKB1 and consequently, its activity may be modifiable. It has been suggested that SIRT1, one of the seven mammalian NAD(+)-dependent deacetylases silent mating type information regulator 2 ortholog (sirtuin) genes (Howitz et al., 2003), promotes LKB1-dependent AMPK stimulation through direct deacetylation and increased cytoplasmic/nuclear ratio of LKB1 (Lan et al., 2008). Also, recently it was shown that Fyn kinase phosphorylation of LKB1 on Tyr265 and Tyr365 residues results in cytoplasmic distribution of LKB1 and increased AMPK phosphorylation (Yamada et al., 2010). Binding of AMP to AMPK promotes LKB1-dependent phosphorylation of Thr-172 through inhibition of dephosphorylation (by making AMPK complex a less efficient substrate for protein phosphatases) and produces a large effect on kinase activity by allosterically activating the phosphorylated form of AMPK. In addition, Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) has also been identified as a separate AMPK kinase (Hawley et al., 2005; Hurley et al., 2005; Woods et al., 2005), that phosphorylates and activates AMPK in response to elevated intracellular Ca2+ concentrations, independent of any change in cellular AMP/ATP ratio. TGF-β-activated kinase 1 (TAK1) has also been recently implicated in the regulation of AMPK activity, although the physiological conditions during which TAK1 regulates AMPK are unclear (Momcilovic et al., 2006; Xie et al., 2006).
Figure 2. Regulation of AMPK activation by phosphorylation/dephosphorylation.
Phosphorylation of AMPK at Thr-172 is regulated by the upstream protein kinases LKB1, CaMKKβ and possibly TAK1. Dephosphorylation of AMPK at Thr172 is modulated by protein phosphatases PP2A and PP2C. Multiple effectors (nutrients, hormones and cytokines) regulating AMPK phosphorylation and activity are listed.
While α-Thr-172 is the major AMPK phosphorylation and activation site, α and β subunits are phosphorylated at multiple sites (Mitchelhill et al., 1997; Villen et al., 2007; Woods et al., 2003b), however, the physiological relevance of these sites remain unclear. Recent studies provide evidence that direct phosphorylation of AMPKα1/α2 at Ser485/491 antagonizes its activation and correlates with inhibition of AMPK activity during insulin signaling in the heart (Horman et al., 2006) and cAMP-mediated signaling in an insulin-secreting cell line (Hurley et al., 2006). A hierarchical control by insulin was proposed for the reduction of AMPK activation in ischemic heart via PKB-induced phosphorylation of Ser485/491 (see below). The inhibitory effect of cAMP was linked to reduction in phosphorylation of AMPK at Thr172 and appears to be due, in part, to cAMP-dependent inhibition of the upstream AMPK kinase CaMKK, but not LKB1 (Hurley et al., 2006). On the other hand, cAMPdependent attenuation of AMPK activity has also been correlated with increased phosphorylation of AMPKα1 Ser485/491 by PKA (Hurley et al., 2006). This is in contrast to studies in adipocytes, showing that agents stimulating PKA-mediated cAMP signaling (isoproterenol, isobutylmethylxanthine, forskolin, β-adrenergic agonist and adrenaline) result in increased AMPK activity (Daval et al., 2005; Koh et al., 2007; Moule and Denton, 1998; Omar et al., 2009; Yin et al., 2003). Since, cAMP-stimulated lipolysis in adipocytes was accompanied by an increase in oxidative stress (i.e., an increase AMP:ATP ratio), AMPK activation could be a consequence of lipolysis and the associated relative change in cellular energy balance rather than a direct effect of PKA (Gauthier et al., 2008). Similarly, it has been shown that IL-6 activates AMPK in skeletal muscle by increasing the concentration of cAMP and, secondarily, the AMP:ATP ratio (Kelly et al., 2009). However, potential crosstalk between PKA and AMPK signaling pathways underlying negative action of PKA on AMPK signaling has been recently reported in context of adipocytes. Central to this mechanism is the phosphorylation of AMPKα1 by PKA at Ser173 (Djouder et al., 2010). This site is highly conserved, located directly adjacent to the critical activation loop Thr172 and its phosphorylation may create constraints by steric hindrance or charge incompatible with subsequent phosphorylation at the Thr172 residue. This mechanism is critically important for the control of the lipolytic response. Stimulation of adipocyte lipolysis, via PKA activation, triggers a negative feedback mechanism involving AMPK to restrain the energy depletion and oxidative stress caused by lipolysis (Gauthier et al., 2008). By opposing the activity of AMPK-mediated negative feedback loop, PKA allows fine-tuning of lipolysis (Djouder et al., 2010).
Protein phosphatases have an important role in regulating AMPK phosphorylation at Thr-172 and consequently AMPK activity, although the exact mechanisms that modulate their action remains poorly understood. Their ability to dephosphorylate Thr172 on AMPK is inhibited by AMP binding to the γ subunit (Davies et al., 1995; Sanders et al., 2007). Both protein phosphatases 2A (PP2A) and 2C were shown to dephosphorylate AMPK in vitro (Davies et al., 1995; Kudo et al., 1996). Recent findings revealed the important role of PPs activation in the suppression of AMPK activity by dephosphorylation in different species, organs, and nutrition types (Ravnskjaer et al., 2006; Wang and Unger, 2005; Wu et al., 2007). In support with these results, it has been reported that PP2A is involved in regulating the interaction between AMPK α2 and γ1 (Gimeno-Alcaniz and Sanz, 2003).
5. Regulation by subcellular localization
Intracellular distribution of AMPK complexes appears to shuttle between the nucleus and the cytoplasm in response to specific stimuli. In Hela cells, AMPK translocated to the nucleus upon stimulation by agents inducing cellular stress (Kodiha et al., 2007). In human skeletal muscle, the AMPKα2 subunit translocated to the nucleus following intense exercise (McGee et al., 2003). Interestingly, the two AMPKα subunits, α1 and α2, have been shown to have different localization patterns in mammalian cells, with the α1 subunit being localized to the non-nuclear fraction and the α2 subunit localized to both the nucleus and the non-nuclear fractions. AMPKα1 is hence likely to phosphorylate cytosolic and plasma membrane substrates, whereas AMPKα2 may be primarily involved in the conversion of metabolic signals into transcriptional regulation (Salt et al., 1998a). Another mechanism to localize signaling events is the association with scaffold proteins. The β subunits act as targeting scaffolds, influencing subcellular localization through an N-terminal myristoylation site (Mitchelhill et al., 1997) that can target AMPK to membrane (Gregor et al., 2006; Warden et al., 2001). AMPKα2 bound to AMPKβ1 is anchored in the cytoplasm at the outer mitochondrial membrane through the myristoylation site of β1 subunit. In contrast, AMPKα2 bound to AMPKβ2 translocates to the nucleus in a manner driven by a nuclear localization signal present in AMPKα2 but not in AMPKα1 subunit (Suzuki et al., 2007). The γ1 subunit also exhibits preferential nuclear localization over the other γ subunits (Turnley et al., 1999). These data suggest that activation of AMPK complexes may elicit distinct metabolic as well as signaling effects in tissues and cells depending on the expression of different catalytic and regulatory subunits.
6. Regulation of protein stability
Recent data revealed a new mechanism that regulates AMPK activity independently of AMP and of phosphorylation or dephosphorylation processes. Modulation of AMPK complex stability via ubiquitination-mediating degradation has emerged through a complex containing cell death-inducing DNA fragmentation factor α-like effector A (Cidea) and AMPK (Qi et al., 2008). Cidea and AMPK have been shown to co-localize in the endoplasmic reticulum and form a complex in vivo through specific interaction with the AMPKβ subunit to promote ubiquitin-mediated AMPK degradation and down-regulation of its activity. Truncated AMPKβ proteins lacking the region required for its interaction with Cidea no longer undergo Cidea-mediated protein degradation (Qi et al., 2008).
B. AMPK inhibition in physiology
1. Regulation by nutrient
a. Inhibition by lipid overload
An increasing body of evidence indicates that dysregulation of AMPK activity and its consequential signaling network may have sustained and deleterious effects at the systemic level that underlie the pathogenesis of metabolic syndrome (Ruderman and Prentki, 2004). A strong correlation between low activation state of AMPK and metabolic disorders associated with insulin resistance, obesity and sedentary activities has been established in a variety of rodent models with aspects of the metabolic syndrome (Kelly et al., 2004; Yu et al., 2004). In addition, feeding mice with a high fat diet causes dysregulation of AMPK, associated with impaired AMPK phosphorylation and protein expression in skeletal muscle, heart, liver, aortic endothelium and hypothalamus (Lee et al., 2005; Lessard et al., 2006; Liu et al., 2006; Martin et al., 2006; Muse et al., 2004; Wang and Unger, 2005; Wilkes et al., 2005). Furthermore, inhibition of AMPK was found to occur in mice fed with a high fat diet rich in palmitate (Wu et al., 2007), raising the possibility that chronic exposure to fatty acids inhibits AMPK activation in a feed-forward effect of lipid overload. It was reported that palmitate inhibited AMPK in endothelial cells via ceramide-dependent PP2A activation (Wu et al., 2007). Interestingly, AMPK inhibition by PP2C upregulation was accounted for decreased AMPK activity in the heart of obese rodents with cardiac lipotoxicity (Wang and Unger, 2005). These data provide new insights into the mechanisms of lipo-regulatory dysfunction, leading to lipid metabolism disorders in obesity.
If decreased AMPK activity contributes to the pathogenesis of obesity, as suggested by dysregulation of AMPK signaling in obese rodent models, one would expect that mice lacking AMPK will be more sensitive to deleterious effects of over-nutrition. Consistent with this hypothesis, whole-body ablation of AMPKα2 activity exacerbates high fat diet-induced obesity, while the glucose disposal rates are similar to those of wild-type mice (Villena et al., 2004). The fact that these mice have similar triglycerides contents in liver and muscle, either on high-fat or normal diets, rules out the lipid accumulation in these tissues as a major determinant of their glucose homeostasis (Villena et al., 2004). More recently, Jorgensen and coworkers investigated whether reduced levels of muscle AMPK promoted lipid accumulation and insulin resistance during high-fat diet (Beck Jorgensen et al., 2009). High-fat feeding increased body mass and adiposity, and impaired insulin and glucose tolerance, however, there was no difference between wild-type and transgenic litter-mates overexpressing an AMPKα2 kinase-dead (KD) in muscle. High-fat feeding decreased insulin-stimulated muscle glucose uptake and Akt-phosphorylation, while increasing muscle triacylglycerol, diacylglycerol and ceramide. These effects, as well as obesity-induced lipid accumulation and insulin resistance were not exacerbated in AMPK KD mice, suggesting that reduced levels of muscle AMPKα2 did not promote insulin resistance in the early phase of obesity-related diabetes. Another study by Fujii and coworkers demonstrated that mice overexpressing a muscle-specific KD AMPKα2 Asp157Ala mutation developed more severe muscle insulin resistance after 30 weeks on high-fat diet (Fujii et al., 2008). However, the observation that the genotype effect occurred 26 weeks late than the first evidence of glucose intolerance suggested that AMPK did not play a primary role in the development of insulin resistance. Thus, while AMPK function is impaired with severe obesity, it does not appear to influence the development of insulin resistance in diet-induced obesity.
b. Inhibition by high glucose concentration
AMPK can be negatively regulated by chronic exposure to high glucose. Acute hyperglycemia reduces AMPK activation in muscle, liver (Kraegen et al., 2006) and kidney (Lee et al., 2007b). Decreased AMPK activity observed after glucose infusion does not depend on changes in plasma insulin and FFA levels, as alterations in AMPK activity are also observed following incubation with high glucose concentrations in isolated muscles (Itani et al., 2003) as well as in cultured HepG2 hepatocytes (Zang et al., 2004), human umbilical vein endothelial cells (Ido et al., 2002), β-cells (da Silva Xavier et al., 2003; Gleason et al., 2007; Salt et al., 1998b) and islets (Leclerc et al., 2004). Upon elevation of glucose concentration over the physiological range, AMPK activity is rapidly down-regulated, concomitant with decrease of phosphorylation at Thr172. According to the classic view, glucose-dependent regulation of AMPK activity and phosphorylation is presumably induced by the activation of ATP synthesis and consequent changes in AMP/ATP ratio (da Silva Xavier et al., 2000; da Silva Xavier et al., 2003; Salt et al., 1998b). However, no change in creatine phosphate or adenine nucleotides was reported in muscle incubated with a high concentration of glucose (Itani et al., 2003), indicating that novel regulation mechanisms of AMPK may be operative in response to glucose oversupply. Under circumstances, where no significant change in high-energy phosphate molecules was observed, diminished AMPK activity and phosphorylation were attributed to alterations in phosphorylation and inhibition of AMPK by Akt (Hahn-Windgassen et al., 2005; Lee et al., 2007b), action of specific phosphatases on phosphorylated AMPK (Ravnskjaer et al., 2006), changes in redox state (Rafaeloff-Phail et al., 2004), modification in intracellular free Ca2+ concentration (Leclerc and Rutter, 2004) and alterations in glycogen content (Jorgensen et al., 2004). Regulation of AMPK by glucose might be important to limit glucose uptake into tissues and to protect cells against the adverse effects of sustained hyperglycemia, such as oxidative stress.
Recent work in animal models demonstrated that glucose and fasting/refeeding change AMPK activity in several hypothalamic nuclei (Kim et al., 2004b; Minokoshi et al., 2004). These studies described reduced AMPK activity and phosphorylation state in the basomedial hypothalamus in response to intracerebroventricular (icv) injection of glucose and showed reciprocal effects of AMPK activation or inhibition on feeding behaviour (Kim et al., 2004b; Minokoshi et al., 2004). Hypothalamic neurons appear to mediate the effects of glucose via changes in AMPK activity (Mountjoy et al., 2007). It was established that AMPK responds to changes in blood glucose and functions in transmitting the malonyl-CoA signal (Wolfgang et al., 2007). AMPK activation allows the dephoshorylation/activation of acetyl-CoA carboxylase (ACC) which increases the level of hypothalamic malonyl-CoA resulting in food intake suppression and increased energy expenditure. Interestingly, AMPK has been shown to play an important role in the glucose-sensing mechanism used by the ventromedial hypothalamus, a key brain region involved in the detection of hypoglycemia (Fan et al., 2009). These findings indicate that minute changes in neuron glucose concentration modulate AMP/ATP ratio which can be sensed by AMPK signaling pathway in discrete hypothalamic regions to generate hunger or satiety signals (see below).
c. Inhibition by glycogen accumulation
In skeletal muscle, some studies found that high glycogen content repressed AMPK activation (Derave et al., 2000; Wojtaszewski et al., 2002), suggesting that AMPK system may monitor availability of this energy store by virtue of the glycogen-binding domain on its β subunit (McBride et al., 2009). The degree of AMPK activation was immense during the glycogen-depleted state in both rat and human muscles (Viollet et al., 2003; Wojtaszewski et al., 2002). However, this inverse correlation was not evident under all circumstances. In a human training study, AMPK activity was not found to be directly correlated with muscle glycogen content (McConell et al., 2008). Furthermore, in patients with McArdle’s disease (glycogen storage disease V), the activation of AMPK in response to moderate exercise was exaggerated despite high skeletal muscle glycogen levels (Nielsen et al., 2002). Other paradoxes exist as AMPK can inactivate glycogen synthase (GS) by phosphorylation on Ser7 (site 2) (Jorgensen et al., 2004). Although AMPK is activated by exercise, glycogen synthase was contradictly found dephosphorylated as well as activated after exercise. McBride and coworkers proposed a single hypothesis to explain the physiological role of glycogen binding to AMPK complex based on its structure (McBride et al., 2009). Glycogen preparations with high branching content was found to cause allosteric inhibition of AMPK, due to its binding to the glycogen-binding domain (McBride et al., 2009). It was demonstrated that oligosaccharides with single α1–6 branch points, but not α1–4, are potent allosteric inhibitors of AMPK that also inhibit phosphorylation and activation by upstream kinases. AMPK bound to fully synthesized glycogen particle is probably in an active state due to inaccessibility of internal branch points. This will lead to phosphorylation and inhibition of GS, providing feedback inhibition of further extension of glycogen particles. However, when glycogen is depleted, AMPK becomes inhibited after binding to exposed α1–6 branch points. This allows dephosphorylation of GS on site 2, promoting rapid resynthesis of glycogen. This model implies that different pools of AMPK (glycogen-bound versus glycogen-free) can phosphorylate some targets while not others (Jorgensen et al., 2004).
d. Inhibition by amino acids
Several reports have suggested a possible interplay between the mammalian target of rapamycin (mTOR) and AMPK signaling pathways coordinating amino acids- and energy-sensing. The mTOR pathway has recently emerged as a crucial point of convergence for signaling by amino acids, growth factors and cellular energy (Wullschleger et al., 2006). Whereas mTOR was presumed to be a direct cellular sensor for ATP levels, mounting evidence implicated AMPK in the regulation of mTOR activity. AMPK inhibits mTOR through direct phosphorylation of TSC2 tumor suppressor (Inoki et al., 2003) as well as critical mTOR-binding subunit raptor (Gwinn et al., 2008). Thus, mTOR activation and AMPK activity are inversely related (Aguilar et al., 2007). Recent studies demonstrated that AMPK activity is suppressed by amino acids (Gleason et al., 2007; Leclerc and Rutter, 2004). Treatment of C2C12 myoblast cells with leucine enhanced the phosphorylation of mTOR and concomitantly reduced the phosphorylation of AMPK and inhibited its activity (Du et al., 2007). The ability of leucine to dramatically reduce AMPK activity is linked to a consequent drop in the level of AMP and a subsequent decrease in AMP/ATP ratio. In the liver, the increase of protein intake induces metabolic adaptation characterized by concomitant increase of mTOR phosphorylation and decrease of AMPK phosphorylation (Chotechuang et al., 2009). Similarly, high protein diet decreases AMPK and increases mTOR activity in the hypothalamus, leading to reduction in food intake (Ropelle et al., 2008). Consistent with a cross-regulation between AMPK and mTOR to control food intake, hypothalamic ATP levels are increased and AMP/ATP ratio reduced after high protein feeding.
2. Regulation by hormones and cytokines
a. Inhibition by insulin in the heart
The energy necessary to maintain the myocardial contraction/relaxation cycle is derived from the mitochondrial oxidation of carbohydrates and long chain fatty acids. Under physiological conditions, fatty acid oxidation provides 60–70% of the heart energy requirements (Bertrand et al., 2008). This substrate preference can be attributed to inhibition of glucose uptake and catabolism via the Randle cycle (Randle et al., 1963). Following myocardial infarction, fatty acid oxidation accounts for almost all the heart ATP production (Neely and Morgan, 1974; Opie, 1975). This over-reliance on fatty acid oxidation is detrimental to functional reperfusion recovery of ischemic hearts (Lopaschuk et al., 1990; Lopaschuk et al., 1993). Under such conditions, the beneficial effects of insulin are important for maintaining proper cardiac function. Insulin can increase glucose use by the heart both by activating key steps of glycolysis, namely the recruitment of GLUT-4 to the plasma membrane and the activation of 6-phosphofructo-2-kinase (Bertrand et al., 2008; Rider and Hue, 1984; Russell et al., 1999) and by decreasing the extracellular fatty acid concentration. Also, insulin can directly alter fatty acid oxidation in the normoxic heart. The mechanism behind this involves inactivation of AMPK (Gamble and Lopaschuk, 1997; Kudo et al., 1995) which contributes to accelerated fatty acid oxidation via direct phosphorylation and inactivation of ACC (Carling et al., 1989; Hardie, 1992) resulting in decreased malonyl-CoA, a potent inhibitor of fatty acid transport into the mitochondrial matrix (McGarry et al., 1989). Witters and Kemp have previously observed this inhibitory effect of insulin on AMPK activity in hepatoma cells (Witters and Kemp, 1992).
As insulin is a very potent PKB/Akt activator in the heart (Lefebvre et al., 1996), Kovacic and coworkers investigated if increased PKB/Akt activity could lead to inactivation of AMPK. They demonstrated that hearts from transgenic mice expressing constitutively active PKB/Akt show a dramatic reduction in AMPK phosphorylation, when compared to control hearts that do not express the transgene, indicating that insulin-induced down-regulation of AMPK is mediated by Akt-dependent pathways (Kovacic et al., 2003). PKB/Akt and AMPK have been shown to be inversely correlated in other occurrences too. For example, ischemia in heart causes activation of AMPK and inhibits insulin signaling (Beauloye et al., 2001a), whereas priming of the hearts by insulin pre-treatment in the aerobic period blunts the AMPK response to a subsequent period of ischemia (Beauloye et al., 2001b; Bertrand et al., 2006). The molecular mechanism of the effect of insulin on AMPK signaling pathways has been elucidated as direct phosphorylation of AMPK by PKB/Akt on Ser 485/491 (Horman et al., 2006). This phosphorylation can prevent subsequent activation of AMPK at Th172 by LKB1. It is possible, as suggested by Zou and coworkers, that phosphorylation at Ser 485/491 hinders the physical association of AMPK with LKB1 (Zou et al., 2004). Although insulin inhibits AMPK under ischemia the glycolysis should remain elevated because both insulin and ischemia stimulate glycolysis by activating the same key steps. Physiological relevance of this inhibition in ischemic hearts could also modulate other targets of AMPK, as yet unknown. The ability of PKB/Akt to negatively regulate AMPK activity becomes especially relevant in the physiology of myocardial ischemia-reperfusion. It is possible that PKB/Akt regulates fatty acid oxidation rates secondarily to inhibition of AMPK activity. In addition, PKB/Akt is supposed to be protective by promoting the post-ischemic synthesis of contractile proteins and by inhibiting myocyte apoptosis (Fujio et al., 2000; Miao et al., 2000 ; Ruan et al., 2009), two processes conversely regulated by AMPK (Horman et al., 2003; Meisse et al., 2002). However, the role of PKB/Akt remains controversial and remains to be further investigated, as others argue against the beneficial effects of PKB/Akt negatively regulating AMPK (Nagoshi et al., 2005). Although the metabolic effects of AMPK and PKB/Akt have been largely studied, the ability of PKB/Akt to inhibit AMPK has implications beyond cardiac metabolism. Insulin and IGF-1 have been shown to induce protein synthesis and cardiac hypertrophy via PKB/Akt activation (Proud and Denton, 1997). AMPK antagonizes the stimulating effect of insulin by inhibiting the TSC2/mTOR/p70S6K (Inoki et al., 2003) and eEF2 pathway (Horman et al., 2002). It is therefore possible that the reduction of AMPK activity may be a contributing factor to PKB/Akt-induced cardiac hypertrophy. Studies are ongoing to investigate this relationship.
b. Inhibition by inflammatory signals
Recent studies have suggested AMPK to play a crucial role in the inflammatory signaling pathways. AMPK activity has been shown to be down-regulated upon pro-inflammatory stimulus (LPS) and up-regulated upon anti-inflammatory cytokine stimulation (IL-10 and TGF-β) (Sag et al., 2008). Also, inhibition of AMPK activity or expression increases the production of TNFα, IL-6 and IL-1 upon pro-inflammatory stimulus, whereas overexpression of AMPK results in the dampening of inflammatory response and increases the production of IL-10 (Jeong et al., 2009; Sag et al., 2008). The effects of AMPK deficiency on the regulation of inflammatory status, indicates that the presence of AMPK and its activation is important to counteract inflammation. Furthermore, increasing AMPK activity with AICAR, or by transfection of a constitutively active AMPK catalytic subunit, blunts the ability of free fatty acids (palmitate) or TNFα to activate NFκB (Cacicedo et al., 2004). Accordingly, in vivo AMPK activation decreases severity of LPS-induced lung injury (Zhao et al., 2008) and the expression of pro-inflammatory genes in adipose tissue of obese db/db mice (Bai et al., 2010). A defect in AMPK function has been found in various cells in animals with metabolic diseases. In diabetes and obesity, it is likely that AMPK activation is compromised in inflammation-related cells and leads to the development of inflammatory diseases. Thus, AMPK may be a promising pharmacologic target for the treatment of various chronic inflammatory diseases.
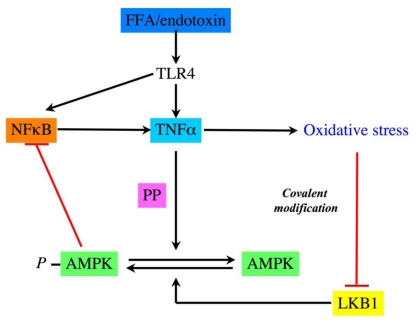
Obesity is a morbid condition characterized by an excess in fat mass and myriad co-morbidities. Among them, it is recognized that insulin resistance promotes the development of type 2 diabetes. Interestingly, insulin resistance varies greatly among obese people, some patients being severely insulin resistant while other remains insulin sensitive despite accumulation of body fat (Brochu et al., 2001; Guilherme et al., 2008). Different hypothesis have been discussed to explain this variability. One of them postulated that obesity related insulin resistance can be recognized as a state of chronic low-grade inflammation (Lumeng et al., 2007; Permana et al., 2006; Rasouli et al., 2005; Xu et al., 2003). Macrophages in obese patients are in an inflammatory state and display increased NFκB and TNFα expression. TNFα induces insulin resistance through the serine phosphorylation of IRS protein by JNK and Iκ/NFκB and increases the expression of STAT3-suppressor of cytokine signaling 3 (SOCS3) (Kern et al., 2001; Shi et al., 2004). Obesity favors increased rates of fatty acid uptake and esterification leading to storage of bioactive lipids such as ceramides, diacylglycerol (DAG) and fatty acyl-CoA in tissues. These lipids contribute to the activation of inflammatory serine threonine kinases such as conventional PKCs, IKK-β and JNK (Schenk et al., 2008). Rates of fatty acid oxidation in skeletal muscle are also reduced in obese humans and rodents and this defect has been correlated with reduced AMPK activity. Nevertheless, the mechanism connecting excess of lipids and decreased AMPK activity in skeletal muscle has not been completely elucidated. To address this question, it has been shown in cultured L6 muscle cells that TNFα reduced AMPK activity without change in LKB1 activity. TNFα suppresses AMPK activity which leads to defective fatty-acid metabolism, an important contributing factor to the development of insulin resistance in obesity (Steinberg et al., 2006a). TNFα mediates its action through TNF receptor (TNFR) 1 to attenuate AMPK activity via transcriptional upregulation of PP2C which results in reduction of ACC phosphorylation, suppressing fatty-acid oxidation, increasing intramuscular diacylglycerol accumulation and causing insulin resistance in skeletal muscle. Using in vitro and in vivo approaches, Steinberg and coworkers provided for the first time conclusive evidence of AMPK as a link between inflammation and metabolic disease. According to these results, ob/ob mice have also reduced muscular AMPK activity, inhibited fatty acid oxidation, increased PP2C expression in their skeletal muscle and reduced muscular insulin sensitivity in vivo. In contrast, AMPK activity is not altered in ob/ob TNFR−/− mice indicating that disruption of TNF signaling prevents AMPK inhibition in this genetically obese mice model. Circulating free fatty acids (FFA) are often increased in obesity and they activate TLR4 signaling-NFκB-inflammation cascade (TNFα production) in adipocytes and macrophages which contributes to insulin resistance in skeletal muscle (Shi et al., 2006). Interestingly, ablation of TLR4 signaling using TLR4 knockout mice protects against high fat diet-induced insulin resistance, due to reduced inflammation, linking innate immune system and metabolism. Activation of TLR4 by endotoxin also leads to loss of AMPK phosphorylation under similar conditions where NFκB pathway is activated in macrophage (Nath et al., 2009). These studies delineate a novel FFA/endotoxin-TLR4-NFκB-TNFα-loss of AMPK-insulin resistance pathway which could be implicated in metabolic disorders (Figure 3). In addition, it has been demonstrated that resistin, an adipocytokine elevated in obesity, inhibited skeletal muscle AMPK activity. Consequent accumulation of lipids and their mediators probably explains resistin-mediated insulin resistance during obesity. When insulin resistance occurs, reduced adiponectin levels can also contribute to continuous suppression of AMPK activity. Because AMPK is a critical factor for mitochondrial biogenesis, long-term reduction of its activity can lead to reduction of mitochondrial density/function in skeletal muscle, as observed in insulin resistance associated with obesity. Supporting this hypothesis, treatment of ob/ob mice by rosiglitazone or by adiponectin reduced TNFα synthesis and increased muscle mitochondrial biogenesis in parallel to metabolic improvement. In summary, it has been evidenced that excess of lipids can inhibit muscular AMPK activity through increased proinflammatory cytokines pathway. Similar conclusions have been obtained in hearts of mice during excess lipids availability. Indeed, acute lipid excess (5 hours of lipids infusion) or diet-induced obesity was both associated with blunted myocardial glucose metabolism concomitantly with reduction of AMPK phosphorylation in heart (Ko et al., 2009). These deleterious effects of long-term or acute exposure to lipids in vivo are based on elevation of inflammatory cytokines (TNFα and IL-6) and increase in their myocardial signaling (Senn et al., 2002). Myocardial levels of STAT3, CD68 and SOCS3, reduction of AMPK activity and down-regulation of myocardial glucose metabolism are attenuated in IL-6 KO mice following high fat diet. This suggests that IL-6 is a key component of the diet-induced myocardial inflammation and subsequent metabolic changes in heart. Chronic exposure of IL-6 (as observed in obesity) promotes insulin resistance both in vitro and in vivo (Nieto-Vazquez et al., 2008). In contrast, during prolonged exercise, IL-6 is released acutely from the skeletal muscle (Febbraio and Pedersen, 2005; Kelly et al., 2004), AMPK is activated (Kelly et al., 2009) and leads to improved peripheral glucose uptake and insulin sensitivity at the whole body level (Glund et al., 2007; Ruderman et al., 2006). This dual effect of IL-6 on insulin sensitivity probably explains some conflicting results recently discussed in more details elsewhere (Nieto-Vazquez et al., 2008).
Figure 3. Regulation of AMPK activity by inflammatory signals.
Activation of TLR4 by endotoxin and free fatty acid (FFA) regulates AMPK phosphorylation status through the action of protein phosphatase (PP).
In general, AMPK functions solely to restore energy balance after depletion of energy stores. However, in T cells, Tamas and coworkers (Tamas et al., 2006) proposed that its unique ability to anticipate energy-consuming processes could be useful for immune cells that need a rapid response to an increased demand for ATP. Activation of AMPK by TCR engagement was shown to be abrogated by CaMKK inhibitor (STO-609) but not when it was activated by AMP/ATP ratio, suggesting two independent pathways for the regulation of AMPK in T cells. Recently, it was reported that the AMPKα1 protein is lost in spleen macrophages, total T cells and their subsets (CD4, CD8 and regulatory T cells) isolated from experimental autoimmune encephalomyelitis (EAE) afflicted animals, compared to control, without affecting its mRNA levels (Nath et al., 2009), suggesting a posttranscriptional modification. Genetic ablation of AMPKα1 in mice exhibited severe disease with profound infiltration of mononuclear cells in central nervous system (CNS) compare to wild type mice. Interestingly, AMPKα2 isoform does not participate in enhancing the severity of the disease.
c. Inhibition of AMPK in the regulation of food intake
Recently, AMPK has emerged as a regulator of appetite. Indeed, hypothalamic AMPK is now recognized not only as a nutrient and glucose sensor in the central nervous system (CNS) but also as a key regulator of appetite. Because the brain has an extremely high metabolic rate and is a high lipid-containing tissue, the distribution of the AMPK isoforms throughout its various areas was considered as an exciting area of research. Turnley and coworkers first reported the cellular distribution of AMPK isoforms in mouse CNS (Turnley et al., 1999). They demonstrated that these are widely expressed in neurons and in activated astrocytes. In addition, several groups showed that AMPK isoforms are expressed in hypothalamus and hindbrain, both areas controlling food intake (Kola, 2008). Studies pertaining to pharmacological or genetic activation as well as inhibition of hypothalamic AMPK lead to a better knowledge of hypothalamic AMPK function as a regulator of food intake. It was first recognized that hypothalamic AMPK activation by AICAR infusion into the third ventricle significantly increased food intake (Andersson et al., 2004). Confirming this first study, expression of dominant negative AMPK in the hypothalamus was reported to be sufficient to reduce food intake and body weight, whereas hypothalamic expression of constitutively active AMPK isoform increased both (Minokoshi et al., 2004). In contrast with these previous studies, some conflicting data came from rodent models, especially α2 catalytic subunit specific knock out in hypothalamic Agouti-related peptide (AgRP) neurons or in hypothalamic pro-opiomelanocortin (POMC) neurons. Indeed, in contrast to what could be expected from the data previously published, AMPK-α2 specific deletion in AgRP neurons did not change food intake nor energy expenditure whereas mice were lean. Furthermore, AMPK-α2 specific deletion in POMC neurons unexpectedly increased body weight and adiposity (Claret et al., 2007). To explain some of these surprising data, it was argued that AICAR or Compound C (as used previously in many studies) were not specific of AMPK pathway and that genetically modified mice models may provide new insights into hypothalamic AMPK functions. In this regard, study from Claret and coworkers clearly suggests that loss of AMPK in orexigenic (AgRP) neurons leads to reduced body weight whereas lost of this enzyme in anorexigenic (POMC) neurons leads to increased body weight. Importantly, electrophysiological studies showed that leptin or insulin action are both preserved in AMPKα2-deficient POMC or AgRP neurons. In consequence, this paper challenged the concept of hypothalamic AMPK as a general sensor and integrator of energy homeostasis in the mediobasal hypothalamus.
Hypothalamic AMPK is regulated by various metabolic signals coming from the periphery (Ahima and Antwi, 2008). It is now well accepted that fasting results in activation of AMPK whereas re-feeding inhibits AMPK activity in multiple hypothalamic regions in mice (Kola, 2008). Specific effects of nutrients and hormones on hypothalamic AMPK activity have been investigated by different groups. Peripheral or central hyperglycaemia is known to inhibit AMPK in all brain areas controlling appetite (such as the arcuate nucleus, the ventro- and dorso-mediobasal hypothalamus, the paraventricular nucleus and the lateral hypothalamus (Kim et al., 2004b; Minokoshi et al., 2004). In contrast, hypothalamic AMPK activity was increased (with greater food intake as a consequence) during insulin-induced hypoglycemia or by inhibition of intracellular glucose utilization (administration of 2-deoxyglucose (2-DG)) (Han et al., 2005; Kim et al., 2004b). These data indicate that intraneuronal glucose concentration is a key modulator of hypothalamic AMPK activity. In order to dissociate the respective effects of glucose and insulin on hypothalamic AMPK activity, i.c.v. insulin infusion can be used to study the effects of insulin without any changes in glucose concentration. It has been demonstrated in this way that insulin inhibits hypothalamic AMPK activity (Minokoshi et al., 2004). In consequence, hyperinsulinaemia and/or hyperglycaemia are now recognized as potent inhibitors of hypothalamic AMPK while hypoglycemia is an activator of this enzyme.
Leptin is a key hormone in the communication between energy stores and the brain. In contrast to what is observed in skeletal muscle, leptin decreases hypothalamic AMPK activity (Minokoshi et al., 2002). Similarly, chronic calorie excess, as observed in diet-induced obese mice, reduced hypothalamic AMPK activity (Martin et al., 2006) probably by the inhibitory effects of combined hyperinsulinaemia, hyperglycaemia and increased secreted leptin. Presumably, leptin promotes loss of body weight by enhancing fat oxidation in peripheral tissues and by decreasing food intake, suggesting that leptin has tissue-specific effects. It is not known if muscular AMPK activation by leptin and concomitant reduction of hypothalamic AMPK activity by leptin are supported by different AMPK isoforms. However, as discussed above, the hypothesis that hypothalamic AMPK could be a key mediator for the control of appetite by leptin has been recently challenged when a normal response to leptin has been described in selective AMPKα2-deficient POMC or AgRP neurons (Claret et al., 2007). Interestingly, it has been shown that like leptin, ciliary neurotrophic factor (CNTF) also suppresses hypothalamic AMPK signaling and reduces food intake (Steinberg et al., 2006b). Importantly, despite the similarities in signaling between leptin and CNTF, CNTF-mediated suppression of hypothalamic AMPK is maintained in diet-induced obesity, whereas the effects of leptin on AMPK signaling are blunted. Thus, the capacity of CNTF to bypass leptin resistance highlights its potential role in the therapeutic treatment of obesity.
AMPK activity is regulated by cellular energetic status, which can be summarized by the intracellular AMP/ATP ratio. Any modification of glucose and/or lipids availability has consequences on AMPK activity. C75 is a fatty acid synthase (FAS) inhibitor which causes weight loss and anorexia. This effect is linked to increased neuronal ATP content by C75 and reduced level of the phosphorylated AMPKα subunit in the hypothalamus (Kim et al., 2004a). Anorectic effect induced by C75 is based on decreased phosphorylation of cAMP response element-binding protein (CREB) in the arcuate nucleus and subsequent reduction in NPY expression (Kim et al., 2004a). Similarly, α-lipoic acid, a cofactor of mitochondrial enzymes that possesses antioxidative, antidiabetic and anorectic properties, inhibits AMPK activity in the hypothalamus (Kim et al., 2004b).
Taking together the effects of nutrients, hormones and compounds described above, it can be postulated that hypothalamic AMPK is a key sensor of whole-body energy status and regulates fuel availability and appetite. Nevertheless, many questions have to be solved. The molecular mechanisms involved in the regulation of food intake by hypothalamic AMPK are not clearly understood. It can be noticed that changes in hypothalamic activity AMPK may contribute to modifications of arcuate neuropeptide expression. Thus, reduction of hypothalamic AMPK activity (by glucose, leptin, insulin, C75, α-lipoic acid and melanocortin 4 receptor agonists) suppresses expression of orexigenic neuropeptides, NPY and AgRP in arcuate nucleus. In contrast, increase in hypothalamic AMPK activity (by hypoglycemia, ghrelin, cannabinoids and adiponectin) enhances the expression of orexigenic NPY and AgRP in arcuate nucleus and melanin-concentrating hormone in the lateral hypothalamus (Minokoshi et al., 2004). In additional studies, it was shown that hypothalamic AMPK and melanocortin pathways are interrelated. Indeed, melanocortin 4 receptor agonists decrease hypothalamic AMPK activity whereas melanocortin receptor antagonists (as AgRP) increase hypothalamic AMPK (Kola, 2008). In these cases, it is difficult to understand if AMPK activity is regulated by AgRP or melanocortin signaling independently of neuronal AMP/ATP ratio changes. Lastly, beyond unspecific effects of AICAR or Compound C, rodent models overexpressing or deleted for hypothalamic AMPK provide evidence of changes of AMPK activity and food intake. However, the extent of physiological relevance of these models could be discussed.
d. Inhibition by resistin: implication for the regulation of glucose homeostasis
Resistin is a 12,5-kDa cysteine-rich protein secreted by adipose tissue of rodents and macrophages of humans (Steppan et al., 2001). The hypothesis that resistin could be a possible link between obesity and insulin resistance is controversial in humans in the light of recent studies (Lee et al., 2003; Nagaev and Smith, 2001). In contrast, consistent findings in rodents suggest that resistin plays a causative role in the development of diet-induced insulin resistance. Additionally, some studies support a link between deleterious metabolic effects of resistin and reduction of AMPK activity. Indeed, a significant correlation has been shown between plasma resistin levels with high fat feeding (or acute infusion of recombinant resistin), hepatic insulin resistance and diminished AMPK phosphorylation in liver (Muse et al., 2004). Conversely, treatment with resistin specific antisense oligodeoxynucleotide reversed these effects. In addition, mice lacking resistin exhibit low blood glucose levels after fasting, due to reduced hepatic glucose production (Banerjee et al., 2004). This is partly mediated by activation of AMPK and decreased expression of gluconeogenic enzymes in the liver. Taken together, these data indicated that resistin is a key promoter of hepatic insulin resistance and that this effect could be partly mediated through reduction of hepatic AMPK activity.
Additional studies suggested that resistin acting on hypothalamus modulates hepatic glucose production. Thus, infusion of resistin in the third cerebral ventricle (icv) or in the mediobasal hypothalamus was sufficient to enhance endogenous glucose production through an increase of TNFα, IL-6, and SOCS-3 expression and a decrease of AMPK phosphorylation in the liver (Muse et al., 2007). This suggested that hypothalamus is an important site of resistin action. It has been also shown that resistin reduces not only insulin-mediated glucose transport in vivo (Satoh et al., 2004) and in isolated muscle cells (Junkin et al., 2009; Niederwanger et al., 2007; Palanivel et al., 2006; Palanivel and Sweeney, 2005); but also AICAR-stimulated glucose uptake in muscle (Jorgensen et al., 2009). Basically, these studies showed that resistin regulates the function of IRS-1 and Akt1 and decreases GLUT4 translocation and glucose uptake in response to insulin. Short-term resistin incubation impairs glycogen synthesis by reducing the rate of glucose-6-phosphate formation by reduction of hexokinase type I activity and reduction of glucose uptake (Niederwanger et al., 2007). Lastly, resistin decreases phosphorylation of muscular AMPK and ACC (Palanivel and Sweeney, 2005). Nevertheless, it can be noted that some studies used supra-physiological concentrations of resistin. This could explain that in a recent study on mouse extensor digitorum longus (EDL), soleus muscles and L6 myotubes, physiological concentrations of resistin impair insulin-stimulated glucose uptake by mechanisms involving reduced plasma membrane GLUT4 translocation but independently of the proximal insulin-signaling cascade, AMPK, and SOCS-3 (Jorgensen et al., 2009).
C. AMPK inhibition in therapeutics
1. Neuroprotection in stroke: slowing down AMPK activation
Lack of blood and oxygen after ischemic stroke causes disruption of cell ion homeostasis and lead to neuronal cell death. To repair the damage and return neurons to homeostasis, a number of energy-consuming processes are activated. Overactivation of these pathways during ischemia can lead to complete energy failure and cell death. Activation of AMPK was initially considered to be an adaptive response due to altered AMP/ATP ratio in response to ischemia, hypoxia, or glucose deprivation (Culmsee et al., 2001; Gadalla et al., 2004; McCullough et al., 2005) but there has been some discordance about the outcome on cell survival and neuroprotection. Some groups proposed that AMPK represents an endogenous neuroprotective pathway conserving cellular energy levels under conditions of intense metabolic stress (Culmsee et al., 2001) or ischemic injury in addition to limiting neuronal injury via excitotoxicity (Kuramoto et al., 2007). Conversely, McCullough and coworkers suggested that AMPK over-activation is detrimental in models of ischemia reperfusion (McCullough et al., 2005). Pharmacological and genetic approaches were used to clarify the role of AMPK in stroke outcome. AMPK inhibition with Compound C or with the fatty acid synthase inhibitor C75 (which reduces AMPK activation indirectly) provided sustained neuroprotection after stroke (Li et al., 2007). Similarly, AMPKα2 knockout mice were protected from stroke damage (Li et al., 2007). Furthermore, the beneficial effect of Compound C was lost in AMPKα2 knockout mice implying that targeting neuronal energy balance during cerebral ischemia may be therapeutic (Li et al., 2007). However, the physiological consequences of AMPK activation after hypoxic stress on cerebral vasculature has been poorly investigated and it is not known if AMPK activation exacerbates or ameliorates cerebral blood flow. In contrast, regarding peripheral vasculartue, many studies confirmed the beneficial effects of pharmacological AMPK activation (Bradley et al., 2010; Davis et al., 2006; Evans et al., 2005; Rubin et al., 2005; Wang et al., 2009), thereby favoring blood flow. Some of the protective actions of AMPK have been related to the activation of endothelial NO synthase (eNOS) and formation of NO, which is a central signaling molecule in the vasculature (Zou and Wu, 2008). AMPK has been shown to enhance eNOS activity by direct phosphorylation of Ser1177 (Chen et al., 2000; Chen et al., 1999), Ser633 (Chen et al., 2009b) and by promoting its association with heat shock protein 90 (Davis et al., 2006) leading to endothelial NO production. In addition, AMPK also produces its regulatory effects in the peripheral vasculature through vascular endothelial growth factor (VEGF)-mediated endothelial angiogenesis (Nagata et al., 2003; Ouchi et al., 2005; Stahmann et al., 2010). Interestingly, a recent study has shown increased phosphorylation of AMPK and eNOS in endothelial cells of cerebral arteries following severe subarachnoid hemorrhage (Osuka et al., 2009). Thus, it is likely that AMPK causes beneficial effects in the brain vasculature through eNOS-mediated acute vasodilatation (Osuka et al., 2009) or VEGF-induced angiogenesis (Lopez-Lopez et al., 2007).
2. AMPK inhibition in cancer: a two-edged sword?
Several recent reports support the idea that the stimulation of AMPK with pharmacological compounds exerts antitumoral effect in various experimental settings (reviewed in (Billaud and Viollet, 2008; Fogarty and Hardie, 2009). Furthermore, epidemiological analyses indicate that treatment with the anti-diabetic drug metformin may reduce the cancer burden in diabetic type 2 patients (reviewed in (Billaud and Viollet, 2008; Fogarty and Hardie, 2009). These findings have led to the conception that pharmacological activators of AMPK may find clinical applications in cancer chemoprevention and therapy. Since the LKB1-AMPK pathway inhibits mTOR, a kinase overactivated in a broad range of tumors, AMPK activators may prove beneficial in a large spectrum of cancers. This idea is reinforced by the observation that metformin is selectively toxic for malignant cells harboring p53-inactivating mutations (Buzzai et al., 2007). However, during specific stages of the tumorigenic process, activation of AMPK might provide a survival advantage to tumor cells. It is clearly documented that nascent cancer cells and their metastatic counterparts are exposed to harsh microenvironmental conditions since they have to cope with extrinsic cellular stresses such as hypoxia, acidosis, shortage of glucose and nutrients. In this context of energetic stress and hypoxia, AMPK is activated and protects cells from apoptosis as demonstrated for pancreas cancer cells (Kato et al., 2002). Also, a recent report has provided evidence that the AMPK catalytic activity is triggered under low-oxygen conditions and is critical to promote the growth of xenografted tumors prepared from Ras-transformed mouse embryonic fibroblasts (Laderoute et al., 2006). It is thus possible that activation of AMPK is a key event at defined steps of the sequential tumorigenic process. For instance, breast cancer cells overcome anoikis, a cell death mechanism that leads to the self-destruction of epithelial cells detaching from the basement membrane, through an increase of glucose uptake that restores the intracellular level of ATP and reduces reactive oxygen species (Schafer et al., 2009). AMPK stimulates the transport of glucose through the GLUT1 transporter and may be involved in the capacity of tumors cells to override cell death induced by loss of extracellular matrix attachment. In a larger prospect, the multiple metabolic pathways regulated by AMPK possibly place this kinase as one of the main actors contributing to the metabolic reprogramming known as Warburg effect, which is a hallmark of malignant cells (Vander Heiden et al., 2009). Thus, it is conceivable that at certain stages of cancer progression, and for some types of malignancies, AMPK inhibition rather than activation may represent a potential way of therapeutic intervention. In any case, the experimental arguments supporting a favoring role for AMPK during oncogenesis call for a cautious evaluation of possible pro-tumoral effects of treatments that aims at activating AMPK in cancer prevention and chemotherapy.
3. Pharmacological AMPK inhibitor: the hidden side of Compound C
Compound C is a cell-permeable pyrrazolopyrimidine compound that can act as a reversible and ATP-competitive inhibitor of AMPK (Zhou et al., 2001). This compound is being used increasingly to inhibit AMPK in cell-based assays. However, several studies have reported inhibition of various biological events by Compound C independently of AMPK inhibition such as inhibition of the hypoxic activation of HIF-1 by suppressing mitochondrial generated reactive oxygen species (ROS) (Emerling et al., 2007) and proliferation of preadipocytes by increasing p21 levels (Nam et al., 2008). Furthermore, Compound C does not inhibit AMPK activation in response to all stimuli. Thus, this pharmacological inhibitor blunted the AICAR-induced but not the dinitrophenol-induced (Fryer et al., 2002) or the LPS-induced (Labuzek et al., 2010) activation of AMPK. Further investigation showed that Compound C inhibits the adenosine transporter (Fryer et al., 2002), the primary transporter for the uptake of AICAR into cells, suggesting that this pharmacological inhibitor should not be used to demonstrate AMPK-dependent effects of AICAR. Lastly, Compound C appears to inhibit a number of other protein kinases with lower IC50 values than AMPK indicating that this compound could certainly have ‘off-target’ effects (Bain et al., 2007). However, despite the uncertain specificity of this pharmacological inhibitor, various reports suggest that in specific circumstances Compound C inhibits AMPK with expected results. For example, the genetic approach combined with the pharmacological approach further confirmed the AMPK-specific action of Compound C during stroke as the effect of this pharmacological inhibitor was lost in AMPKα2 knockout mice (Li et al., 2007).
D. Concluding remarks
Since the initial description of the role of AMPK in modulating energy metabolism (as illustrated by regulation of lipid metabolism), there has been an expanded interest in the role of AMPK in numerous physiological systems. AMPK integrates the activity of several essential processes to maintain energy balance both at the single and the whole body levels. In recent years, additional mechanisms in the AMPK regulation have been discovered and it is clear that multiple pathways for activation or inhibition of AMPK are now possible. These various stimuli include nutrients, hormones, cytokines, physiological state as well as pathological events. A large body of experimental evidence has clearly shown the therapeutic potential of pharmacological activation of AMPK in order to prevent or reverse metabolic disorders associated with the metabolic syndrome. However, understanding the consequence of AMPK inhibition may suggest novel therapeutic targets in a number of disease conditions. Thus, studies using genetic models with AMPK deficiency will be a great help to define the role of AMPK in regulating physiological responses in vivo.
Acknowledgments
This work was supported by the European Commission integrated project (LSHM-CT-2004-005272/exgenesis), Agence Nationale de la Recherche (ANR), Association Française contre les Myopathies (AFM), Association pour l’Etude des Diabètes et des Maladies Métaboliques (ALFEDIAM) and Institut Benjamin Delessert.
We thank Ramandeep Rattan for critical editing of the manuscript.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudiere E, Foretz M, Viollet B, Diolez P, Bastin J, Benit P, Rustin P, Carling D, Sandri M, Ventura-Clapier R, Pende M. S6 Kinase Deletion Suppresses Muscle Growth Adaptations to Nutrient Availability by Activating AMP Kinase. Cell metabolism. 2007;5:476–487. doi: 10.1016/j.cmet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. 2008;37:811–823. doi: 10.1016/j.ecl.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, McGarry K, Seidman JG, Seidman CE. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109:357–362. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circulation research. 2007;100:474–488. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- Bai A, Yong M, Ma Y, Ma A, Weiss C, Guan Q, Bernstein C, Peng Z. Novel Anti-Inflammatory Action of 5-Aminoimidazole-4-carboxamide ribonucleoside with protective effect in DSS-induced acute and chronic colitis. J Pharmacol Exp Ther. 2010 doi: 10.1124/jpet.109.164954. [DOI] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A, Scherer PE, Steppan CM, Ahima RS, Obici S, Rossetti L, Lazar MA. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem Sci. 1997;22:12–13. doi: 10.1016/s0968-0004(96)30046-7. [DOI] [PubMed] [Google Scholar]
- Beauloye C, Bertrand L, Krause U, Marsin AS, Dresselaers T, Vanstapel F, Vanoverschelde JL, Hue L. No-flow ischemia inhibits insulin signaling in heart by decreasing intracellular pH. Circ Res. 2001a;88:513–519. doi: 10.1161/01.res.88.5.513. [DOI] [PubMed] [Google Scholar]
- Beauloye C, Marsin AS, Bertrand L, Krause U, Hardie DG, Vanoverschelde JL, Hue L. Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Lett. 2001b;505:348–352. doi: 10.1016/s0014-5793(01)02788-0. [DOI] [PubMed] [Google Scholar]
- Beck Jorgensen S, O’Neill HM, Hewitt K, Kemp BE, Steinberg GR. Reduced AMP-activated protein kinase activity in mouse skeletal muscle does not exacerbate the development of insulin resistance with obesity. Diabetologia. 2009;52:2395–2404. doi: 10.1007/s00125-009-1483-8. [DOI] [PubMed] [Google Scholar]
- Bertrand L, Ginion A, Beauloye C, Hebert AD, Guigas B, Hue L, Vanoverschelde JL. AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardiomyocytes via the activation of protein kinase B. Am J Physiol Heart Circ Physiol. 2006;291:H239–250. doi: 10.1152/ajpheart.01269.2005. [DOI] [PubMed] [Google Scholar]
- Bertrand L, Horman S, Beauloye C, Vanoverschelde JL. Insulin signalling in the heart. Cardiovasc Res. 2008;79:238–248. doi: 10.1093/cvr/cvn093. [DOI] [PubMed] [Google Scholar]
- Billaud M, Viollet B. Metformin in oncology, new clinical potential for an old remedy ? In: Mithieux G, Wiernsperger N, editors. Metformin: Mechanistic Insights Towards New Applications. Transworld Research Network; Kerala, India: 2008. pp. 241–258. [Google Scholar]
- Birk JB, Wojtaszewski JF. Predominant alpha2/beta2/gamma3 AMPK activation during exercise in human skeletal muscle. J Physiol. 2006;577:1021–1032. doi: 10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley EA, Eringa EC, Stehouwer CD, Korstjens I, van Nieuw Amerongen GP, Musters R, Sipkema P, Clark MG, Rattigan S. Activation of AMP-Activated Protein Kinase by 5-Aminoimidazole-4-Carboxamide-1-{beta}-D-Ribofuranoside in the Muscle Microcirculation Increases Nitric Oxide Synthesis and Microvascular Perfusion. Arterioscler Thromb Vasc Biol. 2010 doi: 10.1161/ATVBAHA.110.204404. [DOI] [PubMed] [Google Scholar]
- Brochu M, Tchernof A, Dionne IJ, Sites CK, Eltabbakh GH, Sims EA, Poehlman ET. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86:1020–1025. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- Burwinkel B, Scott JW, Buhrer C, van Landeghem FK, Cox GF, Wilson CJ, Grahame Hardie D, Kilimann MW. Fatal congenital heart glycogenosis caused by a recurrent activating R531Q mutation in the gamma 2-subunit of AMP-activated protein kinase (PRKAG2), not by phosphorylase kinase deficiency. Am J Hum Genet. 2005;76:1034–1049. doi: 10.1086/430840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- Cacicedo JM, Yagihashi N, Keaney JF, Jr, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;324:1204–1209. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- Carling D, Clarke PR, Zammit VA, Hardie DG. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur J Biochem. 1989;186:129–136. doi: 10.1111/j.1432-1033.1989.tb15186.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Jiao ZH, Zheng LS, Zhang YY, Xie ST, Wang ZX, Wu JW. Structural insight into the autoinhibition mechanism of AMP-activated protein kinase. Nature. 2009a;459:1146–1149. doi: 10.1038/nature08075. [DOI] [PubMed] [Google Scholar]
- Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, Zhu Y, DeFea K, Pan S, Tsai MD, Shyy JY. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009b;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZP, McConell GK, Michell BJ, Snow RJ, Canny BJ, Kemp BE. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab. 2000;279:E1202–1206. doi: 10.1152/ajpendo.2000.279.5.E1202. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J 346 Pt. 2000;3:659–669. [PMC free article] [PubMed] [Google Scholar]
- Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, Zhang D, Cline GW, Wakil SJ, Shulman GI. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A. 2007;104:16480–16485. doi: 10.1073/pnas.0706794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotechuang N, Azzout-Marniche D, Bos C, Chaumontet C, Gausseres N, Steiler T, Gaudichon C, Tome D. mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. Am J Physiol Endocrinol Metab. 2009;297:E1313–1323. doi: 10.1152/ajpendo.91000.2008. [DOI] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose-sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, Marsh K, Kym P, Jung P, Camp HS, Frevert E. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- da Silva Xavier G, Leclerc I, Salt IP, Doiron B, Hardie DG, Kahn A, Rutter GA. Role of AMP-activated protein kinase in the regulation by glucose of islet beta cell gene expression. Proc Natl Acad Sci U S A. 2000;97:4023–4028. doi: 10.1073/pnas.97.8.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J. 2003;371:761–774. doi: 10.1042/BJ20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, Hajduch E, Ferre P, Foufelle F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- Davies SP, Helps NR, Cohen PT, Hardie DG. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- Derave W, Ai H, Ihlemann J, Witters LA, Kristiansen S, Richter EA, Ploug T. Dissociation of AMP-activated protein kinase activation and glucose transport in contracting slow-twitch muscle. Diabetes. 2000;49:1281–1287. doi: 10.2337/diabetes.49.8.1281. [DOI] [PubMed] [Google Scholar]
- Djouder N, Tuerk RD, Suter M, Salvioni P, Thali RF, Scholz R, Vaahtomeri K, Auchli Y, Rechsteiner H, Brunisholz RA, Viollet B, Makela TP, Wallimann T, Neumann D, Krek W. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. Embo J. 2010;29:469–481. doi: 10.1038/emboj.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Shen QW, Zhu MJ, Ford SP. Leucine stimulates mammalian target of rapamycin signaling in C2C12 myoblasts in part through inhibition of adenosine monophosphate-activated protein kinase. J Anim Sci. 2007;85:919–927. doi: 10.2527/jas.2006-342. [DOI] [PubMed] [Google Scholar]
- Emerling BM, Viollet B, Tormos KV, Chandel NS. Compound C inhibits hypoxic activation of HIF-1 independent of AMPK. FEBS Lett. 2007;581:5727–5731. doi: 10.1016/j.febslet.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AM, Mustard KJ, Wyatt CN, Peers C, Dipp M, Kumar P, Kinnear NP, Hardie DG. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J Biol Chem. 2005;280:41504–41511. doi: 10.1074/jbc.M510040200. [DOI] [PubMed] [Google Scholar]
- Fan X, Ding Y, Brown S, Zhou L, Shaw M, Vella MC, Cheng H, McNay EC, Sherwin RS, McCrimmon RJ. Hypothalamic AMP-activated protein kinase activation with AICAR amplifies counterregulatory responses to hypoglycemia in a rodent model of type 1 diabetes. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1702–1708. doi: 10.1152/ajpregu.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev. 2005;33:114–119. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- Fogarty S, Hardie DG. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbapap.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Fryer LG, Parbu-Patel A, Carling D. Protein kinase inhibitors block the stimulation of the AMP-activated protein kinase by 5-amino-4-imidazolecarboxamide riboside. FEBS Lett. 2002;531:189–192. doi: 10.1016/s0014-5793(02)03501-9. [DOI] [PubMed] [Google Scholar]
- Fujii N, Ho RC, Manabe Y, Jessen N, Toyoda T, Holland WL, Summers SA, Hirshman MF, Goodyear LJ. Ablation of AMP-activated Protein Kinase {alpha}2 Activity Exacerbates Insulin Resistance Induced by High-fat Feeding of Mice. Diabetes. 2008;57:2958–2966. doi: 10.2337/db07-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadalla AE, Pearson T, Currie AJ, Dale N, Hawley SA, Sheehan M, Hirst W, Michel AD, Randall A, Hardie DG, Frenguelli BG. AICA riboside both activates AMP-activated protein kinase and competes with adenosine for the nucleoside transporter in the CA1 region of the rat hippocampus. J Neurochem. 2004;88:1272–1282. doi: 10.1046/j.1471-4159.2003.02253.x. [DOI] [PubMed] [Google Scholar]
- Gamble J, Lopaschuk GD. Insulin inhibition of 5′ adenosine monophosphate-activated protein kinase in the heart results in activation of acetyl coenzyme A carboxylase and inhibition of fatty acid oxidation. Metabolism. 1997;46:1270–1274. doi: 10.1016/s0026-0495(97)90229-8. [DOI] [PubMed] [Google Scholar]
- Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem. 2008;283:16514–16524. doi: 10.1074/jbc.M708177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno-Alcaniz JV, Sanz P. Glucose and type 2A protein phosphatase regulate the interaction between catalytic and regulatory subunits of AMP-activated protein kinase. J Mol Biol. 2003;333:201–209. doi: 10.1016/j.jmb.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J Biol Chem. 2007;282:10341–10351. doi: 10.1074/jbc.M610631200. [DOI] [PubMed] [Google Scholar]
- Glund S, Deshmukh A, Long YC, Moller T, Koistinen HA, Caidahl K, Zierath JR, Krook A. Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes. 2007;56:1630–1637. doi: 10.2337/db06-1733. [DOI] [PubMed] [Google Scholar]
- Gregor M, Zeold A, Oehler S, Marobela KA, Fuchs P, Weigel G, Hardie DG, Wiche G. Plectin scaffolds recruit energy-controlling AMP-activated protein kinase (AMPK) in differentiated myofibres. J Cell Sci. 2006;119:1864–1875. doi: 10.1242/jcs.02891. [DOI] [PubMed] [Google Scholar]
- Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081–32089. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- Hamilton SR, Stapleton D, O’Donnell JB, Jr, Kung JT, Dalal SR, Kemp BE, Witters LA. An activating mutation in the gamma1 subunit of the AMP-activated protein kinase. FEBS Lett. 2001;500:163–168. doi: 10.1016/s0014-5793(01)02602-3. [DOI] [PubMed] [Google Scholar]
- Han SM, Namkoong C, Jang PG, Park IS, Hong SW, Katakami H, Chun S, Kim SW, Park JY, Lee KU, Kim MS. Hypothalamic AMP-activated protein kinase mediates counter-regulatory responses to hypoglycaemia in rats. Diabetologia. 2005;48:2170–2178. doi: 10.1007/s00125-005-1913-1. [DOI] [PubMed] [Google Scholar]
- Hancock CR, Janssen E, Terjung RL. Contraction-mediated phosphorylation of AMPK is lower in skeletal muscle of adenylate kinase-deficient mice. J Appl Physiol. 2006;100:406–413. doi: 10.1152/japplphysiol.00885.2005. [DOI] [PubMed] [Google Scholar]
- Hardie DG. Regulation of fatty acid and cholesterol metabolism by the AMP-activated protein kinase. Biochim Biophys Acta. 1992;1123:231–238. doi: 10.1016/0005-2760(92)90001-c. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Salt IP, Hawley SA, Davies SP. AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem J. 1999;338 ( Pt 3):717–722. [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hoehn KL, Turner N, Swarbrick MM, Wilks D, Preston E, Phua Y, Joshi H, Furler SM, Larance M, Hegarty BD, Leslie SJ, Pickford R, Hoy AJ, Kraegen EW, James DE, Cooney GJ. Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. Cell Metab. 2010;11:70–76. doi: 10.1016/j.cmet.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horman S, Beauloye C, Vertommen D, Vanoverschelde JL, Hue L, Rider MH. Myocardial ischemia and increased heart work modulate the phosphorylation state of eukaryotic elongation factor-2. J Biol Chem. 2003;278:41970–41976. doi: 10.1074/jbc.M302403200. [DOI] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, Schlattner U, Wallimann T, Carling D, Hue L, Rider MH. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281:5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr Biol. 2003;13:861–866. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- Hurley RL, Barre LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA. Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem. 2006;281:36662–36672. doi: 10.1074/jbc.M606676200. [DOI] [PubMed] [Google Scholar]
- Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Iseli TJ, Walter M, van Denderen BJ, Katsis F, Witters LA, Kemp BE, Michell BJ, Stapleton D. AMP-activated protein kinase beta subunit tethers alpha and gamma subunits via its C-terminal sequence (186–270) J Biol Chem. 2005;280:13395–13400. doi: 10.1074/jbc.M412993200. [DOI] [PubMed] [Google Scholar]
- Itani SI, Saha AK, Kurowski TG, Coffin HR, Tornheim K, Ruderman NB. Glucose autoregulates its uptake in skeletal muscle: involvement of AMP-activated protein kinase. Diabetes. 2003;52:1635–1640. doi: 10.2337/diabetes.52.7.1635. [DOI] [PubMed] [Google Scholar]
- Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, Kim WS, Kim JB. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–964. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Honeyman J, Oakhill JS, Fazakerley D, Stockli J, Kemp BE, Steinberg GR. Oligomeric resistin impairs insulin and AICAR-stimulated glucose uptake in mouse skeletal muscle by inhibiting GLUT4 translocation. Am J Physiol Endocrinol Metab. 2009;297:E57–66. doi: 10.1152/ajpendo.90744.2008. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, Richter EA, Wojtaszewski JF. The alpha2-5′AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes. 2004;53:3074–3081. doi: 10.2337/diabetes.53.12.3074. [DOI] [PubMed] [Google Scholar]
- Junkin KA, Dyck DJ, Mullen KL, Chabowski A, Thrush AB. Resistin acutely impairs insulin-stimulated glucose transport in rodent muscle in the presence, but not absence, of palmitate. Am J Physiol Regul Integr Comp Physiol. 2009;296:R944–951. doi: 10.1152/ajpregu.90971.2008. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kato K, Ogura T, Kishimoto A, Minegishi Y, Nakajima N, Miyazaki M, Esumi H. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene. 2002;21:6082–6090. doi: 10.1038/sj.onc.1205737. [DOI] [PubMed] [Google Scholar]
- Kelly M, Gauthier MS, Saha AK, Ruderman NB. Activation of AMP-activated protein kinase by interleukin-6 in rat skeletal muscle: association with changes in cAMP, energy state, and endogenous fuel mobilization. Diabetes. 2009;58:1953–1960. doi: 10.2337/db08-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, Giralt M, Hidalgo J, Saha AK, Pedersen BK, Ruderman NB. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun. 2004;320:449–454. doi: 10.1016/j.bbrc.2004.05.188. [DOI] [PubMed] [Google Scholar]
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- Kim EK, Miller I, Aja S, Landree LE, Pinn M, McFadden J, Kuhajda FP, Moran TH, Ronnett GV. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. The Journal of biological chemistry. 2004a;279:19970–19976. doi: 10.1074/jbc.M402165200. [DOI] [PubMed] [Google Scholar]
- Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004b;10:727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- Ko HJ, Zhang Z, Jung DY, Jun JY, Ma Z, Jones KE, Chan SY, Kim JK. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes. 2009;58:2536–2546. doi: 10.2337/db08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodiha M, Rassi JG, Brown CM, Stochaj U. Localization of AMP kinase is regulated by stress, cell density, and signaling through the MEK-->ERK1/2 pathway. Am J Physiol Cell Physiol. 2007;293:C1427–1436. doi: 10.1152/ajpcell.00176.2007. [DOI] [PubMed] [Google Scholar]
- Koh HJ, Hirshman MF, He H, Li Y, Manabe Y, Balschi JA, Goodyear LJ. Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. Biochem J. 2007;403:473–481. doi: 10.1042/BJ20061479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola B. Role of AMP-activated protein kinase in the control of appetite. J Neuroendocrinol. 2008;20:942–951. doi: 10.1111/j.1365-2826.2008.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- Kraegen EW, Saha AK, Preston E, Wilks D, Hoy AJ, Cooney GJ, Ruderman NB. Increased malonyl-CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats. Am J Physiol Endocrinol Metab. 2006;290:E471–479. doi: 10.1152/ajpendo.00316.2005. [DOI] [PubMed] [Google Scholar]
- Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, Lopaschuk GD. Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta. 1996;1301:67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- Kuramoto N, Wilkins ME, Fairfax BP, Revilla-Sanchez R, Terunuma M, Tamaki K, Iemata M, Warren N, Couve A, Calver A, Horvath Z, Freeman K, Carling D, Huang L, Gonzales C, Cooper E, Smart TG, Pangalos MN, Moss SJ. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53:233–247. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuzek K, Liber S, Gabryel B, Buldak L, Okopien B. Ambivalent effects of compound C (dorsomorphin) on inflammatory response in LPS-stimulated rat primary microglial cultures. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:41–57. doi: 10.1007/s00210-009-0472-2. [DOI] [PubMed] [Google Scholar]
- Laderoute KR, Amin K, Calaoagan JM, Knapp M, Le T, Orduna J, Foretz M, Viollet B. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Molecular and cellular biology. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization and activity of LKB1; possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc I, Rutter GA. AMP-activated protein kinase: a new beta-cell glucose sensor?: Regulation by amino acids and calcium ions. Diabetes. 2004;53(Suppl 3):S67–74. doi: 10.2337/diabetes.53.suppl_3.s67. [DOI] [PubMed] [Google Scholar]
- Leclerc I, Woltersdorf WW, da Silva Xavier G, Rowe RL, Cross SE, Korbutt GS, Rajotte RV, Smith R, Rutter GA. Metformin, but not leptin, regulates AMP-activated protein kinase in pancreatic islets: impact on glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2004;286:E1023–1031. doi: 10.1152/ajpendo.00532.2003. [DOI] [PubMed] [Google Scholar]
- Lee JH, Chan JL, Yiannakouris N, Kontogianni M, Estrada E, Seip R, Orlova C, Mantzoros CS. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross-sectional and interventional studies in normal, insulin-resistant, and diabetic subjects. J Clin Endocrinol Metab. 2003;88:4848–4856. doi: 10.1210/jc.2003-030519. [DOI] [PubMed] [Google Scholar]
- Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, Chung J. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007a doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, Foretz M, Viollet B, Weinberg JM, Choudhury GG, Kasinath BS. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007b;292:F617–627. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Lee IK, Kim HS, Kim YM, Koh EH, Won JC, Han SM, Kim MS, Jo I, Oh GT, Park IS, Youn JH, Park SW, Lee KU, Park JY. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:2488–2494. doi: 10.1161/01.ATV.0000190667.33224.4c. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Mechin MC, Louckx MP, Rider MH, Hue L. Signaling pathway involved in the activation of heart 6-phosphofructo-2-kinase by insulin. J Biol Chem. 1996;271:22289–22292. doi: 10.1074/jbc.271.37.22289. [DOI] [PubMed] [Google Scholar]
- Lessard SJ, Chen ZP, Watt MJ, Hashem M, Reid JJ, Febbraio MA, Kemp BE, Hawley JA. Chronic rosiglitazone treatment restores AMPKalpha2 activity in insulin-resistant rat skeletal muscle. Am J Physiol Endocrinol Metab. 2006;290:E251–257. doi: 10.1152/ajpendo.00096.2005. [DOI] [PubMed] [Google Scholar]
- Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38:2992–2999. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wan Q, Guan Q, Gao L, Zhao J. High-fat diet feeding impairs both the expression and activity of AMPKa in rats’ skeletal muscle. Biochem Biophys Res Commun. 2006;339:701–707. doi: 10.1016/j.bbrc.2005.11.068. [DOI] [PubMed] [Google Scholar]
- Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. Embo J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopaschuk GD, Spafford MA, Davies NJ, Wall SR. Glucose and palmitate oxidation in isolated working rat hearts reperfused after a period of transient global ischemia. Circ Res. 1990;66:546–553. doi: 10.1161/01.res.66.2.546. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Wambolt RB, Barr RL. An imbalance between glycolysis and glucose oxidation is a possible explanation for the detrimental effects of high levels of fatty acids during aerobic reperfusion of ischemic hearts. J Pharmacol Exp Ther. 1993;264:135–144. [PubMed] [Google Scholar]
- Lopez-Lopez C, Dietrich MO, Metzger F, Loetscher H, Torres-Aleman I. Disturbed cross talk between insulin-like growth factor I and AMP-activated protein kinase as a possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of Alzheimer’s disease. J Neurosci. 2007;27:824–831. doi: 10.1523/JNEUROSCI.4345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292:E166–174. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem. 2006;281:18933–18941. doi: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009;9:23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConell GK, Manimmanakorn A, Lee-Young RS, Kemp BE, Linden KC, Wadley GD. Differential attenuation of AMPK activation during acute exercise following exercise training or AICAR treatment. J Appl Physiol. 2008;105:1422–1427. doi: 10.1152/japplphysiol.01371.2007. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. The Journal of biological chemistry. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Woeltje KF, Kuwajima M, Foster DW. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes Metab Rev. 1989;5:271–284. doi: 10.1002/dmr.5610050305. [DOI] [PubMed] [Google Scholar]
- McGee SL, Howlett KF, Starkie RL, Cameron-Smith D, Kemp BE, Hargreaves M. Exercise increases nuclear AMPK alpha2 in human skeletal muscle. Diabetes. 2003;52:926–928. doi: 10.2337/diabetes.52.4.926. [DOI] [PubMed] [Google Scholar]
- Meisse D, Van de Casteele M, Beauloye C, Hainault I, Kefas BA, Rider MH, Foufelle F, Hue L. Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells. FEBS Lett. 2002;526:38–42. doi: 10.1016/s0014-5793(02)03110-1. [DOI] [PubMed] [Google Scholar]
- Miao W, Luo Z, Kitsis RN, Walsh K. Intracoronary, adenovirus-mediated Akt gene transfer in heart limits infarct size following ischemia-reperfusion injury in vivo. J Mol Cell Cardiol. 2000;32:2397–2402. doi: 10.1006/jmcc.2000.1283. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Mitchelhill KI, Michell BJ, House CM, Stapleton D, Dyck J, Gamble J, Ullrich C, Witters LA, Kemp BE. Posttranslational modifications of the 5′-AMP-activated protein kinase beta1 subunit. J Biol Chem. 1997;272:24475–24479. doi: 10.1074/jbc.272.39.24475. [DOI] [PubMed] [Google Scholar]
- Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- Moule SK, Denton RM. The activation of p38 MAPK by the beta-adrenergic agonist isoproterenol in rat epididymal fat cells. FEBS Lett. 1998;439:287–290. doi: 10.1016/s0014-5793(98)01392-1. [DOI] [PubMed] [Google Scholar]
- Mountjoy PD, Bailey SJ, Rutter GA. Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity. Diabetologia. 2007;50:168–177. doi: 10.1007/s00125-006-0473-3. [DOI] [PubMed] [Google Scholar]
- Muse ED, Lam TK, Scherer PE, Rossetti L. Hypothalamic resistin induces hepatic insulin resistance. The Journal of clinical investigation. 2007;117:1670–1678. doi: 10.1172/JCI30440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muse ED, Obici S, Bhanot S, Monia BP, McKay RA, Rajala MW, Scherer PE, Rossetti L. Role of resistin in diet-induced hepatic insulin resistance. J Clin Invest. 2004;114:232–239. doi: 10.1172/JCI21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaev I, Smith U. Insulin resistance and type 2 diabetes are not related to resistin expression in human fat cells or skeletal muscle. Biochem Biophys Res Commun. 2001;285:561–564. doi: 10.1006/bbrc.2001.5173. [DOI] [PubMed] [Google Scholar]
- Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278:31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, Hemmings BA, Kass DA, Champion HC, Rosenzweig A. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest. 2005;115:2128–2138. doi: 10.1172/JCI23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam M, Lee WH, Bae EJ, Kim SG. Compound C inhibits clonal expansion of preadipocytes by increasing p21 level irrespectively of AMPK inhibition. Arch Biochem Biophys. 2008;479:74–81. doi: 10.1016/j.abb.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Nath N, Khan M, Rattan R, Mangalam A, Makkar RS, de Meester C, Bertrand L, Singh I, Chen Y, Viollet B, Giri S. Loss of AMPK exacerbates experimental autoimmune encephalomyelitis disease severity. Biochem Biophys Res Commun. 2009;386:16–20. doi: 10.1016/j.bbrc.2009.05.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–459. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- Niederwanger A, Kranebitter M, Ciardi C, Tatarczyk T, Patsch JR, Pedrini MT. Resistin impairs basal and insulin-induced glycogen synthesis by different mechanisms. Mol Cell Endocrinol. 2007;263:112–119. doi: 10.1016/j.mce.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Nielsen JN, Wojtaszewski JF, Haller RG, Hardie DG, Kemp BE, Richter EA, Vissing J. Role of 5′AMP-activated protein kinase in glycogen synthase activity and glucose utilization: insights from patients with McArdle’s disease. J Physiol. 2002;541:979–989. doi: 10.1113/jphysiol.2002.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Vazquez I, Fernandez-Veledo S, de Alvaro C, Lorenzo M. Dual role of interleukin-6 in regulating insulin sensitivity in murine skeletal muscle. Diabetes. 2008;57:3211–3221. doi: 10.2337/db07-1062. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Olson DP, Pulinilkunnil T, Cline GW, Shulman GI, Lowell BB. Gene knockout of Acc2 has little effect on body weight, fat mass, or food intake. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0913492107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar B, Zmuda-Trzebiatowska E, Manganiello V, Goransson O, Degerman E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell Signal. 2009;21:760–766. doi: 10.1016/j.cellsig.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie LH. Metabolism of free fatty acids, glucose and catecholamines in acute myocardial infarction. Relation to myocardial ischemia and infarct size. Am J Cardiol. 1975;36:938–953. doi: 10.1016/0002-9149(75)90086-7. [DOI] [PubMed] [Google Scholar]
- Osuka K, Watanabe Y, Usuda N, Atsuzawa K, Yoshida J, Takayasu M. Modification of Endothelial Nitric Oxide Synthase through AMPK after Experimental Subarachnoid Hemorrhage. J Neurotrauma. 2009;26:1157–1165. doi: 10.1089/neu.2008.0836. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005;96:838–846. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- Palanivel R, Maida A, Liu Y, Sweeney G. Regulation of insulin signalling, glucose uptake and metabolism in rat skeletal muscle cells upon prolonged exposure to resistin. Diabetologia. 2006;49:183–190. doi: 10.1007/s00125-005-0060-z. [DOI] [PubMed] [Google Scholar]
- Palanivel R, Sweeney G. Regulation of fatty acid uptake and metabolism in L6 skeletal muscle cells by resistin. FEBS Lett. 2005;579:5049–5054. doi: 10.1016/j.febslet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Pang T, Xiong B, Li JY, Qiu BY, Jin GZ, Shen JK, Li J. Conserved alpha-helix acts as autoinhibitory sequence in AMP-activated protein kinase alpha subunits. J Biol Chem. 2007;282:495–506. doi: 10.1074/jbc.M605790200. [DOI] [PubMed] [Google Scholar]
- Pang T, Zhang ZS, Gu M, Qiu BY, Yu LF, Cao PR, Shao W, Su MB, Li JY, Nan FJ, Li J. Small molecule antagonizes autoinhibition and activates AMP-activated protein kinase in cells. J Biol Chem. 2008;283:16051–16060. doi: 10.1074/jbc.M710114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permana PA, Menge C, Reaven PD. Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem Biophys Res Commun. 2006;341:507–514. doi: 10.1016/j.bbrc.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, Kemp BE, Stapleton D. AMPK beta subunit targets metabolic stress sensing to glycogen. Curr Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- Ponticos M, Lu QL, Morgan JE, Hardie DG, Partridge TA, Carling D. Dual regulation of the AMP-activated protein kinase provides a novel mechanism for the control of creatine kinase in skeletal muscle. Embo J. 1998;17:1688–1699. doi: 10.1093/emboj/17.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud CG, Denton RM. Molecular mechanisms for the control of translation by insulin. Biochem J. 1997;328 ( Pt 2):329–341. doi: 10.1042/bj3280329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Gong J, Zhao T, Zhao J, Lam P, Ye J, Li JZ, Wu J, Zhou HM, Li P. Downregulation of AMP-activated protein kinase by Cidea-mediated ubiquitination and degradation in brown adipose tissue. Embo J. 2008;27:1537–1548. doi: 10.1038/emboj.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafaeloff-Phail R, Ding L, Conner L, Yeh WK, McClure D, Guo H, Emerson K, Brooks H. Biochemical regulation of mammalian AMP-activated protein kinase activity by NAD and NADH. J Biol Chem. 2004;279:52934–52939. doi: 10.1074/jbc.M409574200. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Rasouli N, Raue U, Miles LM, Lu T, Di Gregorio GB, Elbein SC, Kern PA. Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab. 2005;288:E930–934. doi: 10.1152/ajpendo.00522.2004. [DOI] [PubMed] [Google Scholar]
- Ravnskjaer K, Boergesen M, Dalgaard LT, Mandrup S. Glucose-induced repression of PPARalpha gene expression in pancreatic beta-cells involves PP2A activation and AMPK inactivation. J Mol Endocrinol. 2006;36:289–299. doi: 10.1677/jme.1.01965. [DOI] [PubMed] [Google Scholar]
- Rider MH, Hue L. Activation of rat heart phosphofructokinase-2 by insulin in vivo. FEBS Lett. 1984;176:484–488. doi: 10.1016/0014-5793(84)81223-5. [DOI] [PubMed] [Google Scholar]
- Riek U, Scholz R, Konarev P, Rufer A, Suter M, Nazabal A, Ringler P, Chami M, Muller SA, Neumann D, Forstner M, Hennig M, Zenobi R, Engel A, Svergun D, Schlattner U, Wallimann T. Structural properties of AMP-activated protein kinase: dimerization, molecular shape, and changes upon ligand binding. J Biol Chem. 2008;283:18331–18343. doi: 10.1074/jbc.M708379200. [DOI] [PubMed] [Google Scholar]
- Ropelle ER, Pauli JR, Fernandes MF, Rocco SA, Marin RM, Morari J, Souza KK, Dias MM, Gomes-Marcondes MC, Gontijo JA, Franchini KG, Velloso LA, Saad MJ, Carvalheira JB. A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet-induced weight loss. Diabetes. 2008;57:594–605. doi: 10.2337/db07-0573. [DOI] [PubMed] [Google Scholar]
- Ruan H, Li J, Ren S, Gao J, Li G, Kim R, Wu H, Wang Y. Inducible and cardiac specific PTEN inactivation protects ischemia/reperfusion injury. J Mol Cell Cardiol. 2009;46:193–200. doi: 10.1016/j.yjmcc.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Rubin LJ, Magliola L, Feng X, Jones AW, Hale CC. Metabolic activation of AMP kinase in vascular smooth muscle. J Appl Physiol. 2005;98:296–306. doi: 10.1152/japplphysiol.00075.2004. [DOI] [PubMed] [Google Scholar]
- Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3:340–351. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Keller C, Richard AM, Saha AK, Luo Z, Xiang X, Giralt M, Ritov VB, Menshikova EV, Kelley DE, Hidalgo J, Pedersen BK, Kelly M. Interleukin-6 regulation of AMP-activated protein kinase. Potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes. 2006;55(Suppl 2):S48–54. doi: 10.2337/db06-s007. [DOI] [PubMed] [Google Scholar]
- Russell RR, 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol. 1999;277:H643–649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J. 1998a;334 ( Pt 1):177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt IP, Johnson G, Ashcroft SJ, Hardie DG. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J. 1998b;335 ( Pt 3):533–539. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. The Biochemical journal. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Nguyen MT, Miles PD, Imamura T, Usui I, Olefsky JM. Adenovirus-mediated chronic “hyper-resistinemia” leads to in vivo insulin resistance in normal rats. The Journal of clinical investigation. 2004;114:224–231. doi: 10.1172/JCI20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DB, Choi CS, Samuel VT, Liu ZX, Zhang D, Wang A, Zhang XM, Cline GW, Yu XX, Geisler JG, Bhanot S, Monia BP, Shulman GI. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–824. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Ross FA, Liu JK, Hardie DG. Regulation of AMP-activated protein kinase by a pseudosubstrate sequence on the gamma subunit. Embo J. 2007;26:806–815. doi: 10.1038/sj.emboj.7601542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–3399. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Tzameli I, Bjorbaek C, Flier JS. Suppressor of cytokine signaling 3 is a physiological regulator of adipocyte insulin signaling. J Biol Chem. 2004;279:34733–34740. doi: 10.1074/jbc.M403886200. [DOI] [PubMed] [Google Scholar]
- Stahmann N, Woods A, Spengler K, Heslegrave A, Bauer R, Krause S, Viollet B, Carling D, Heller R. Activation of AMP-activated protein kinase by vascular endothelial growth factor mediates endothelial angiogenesis independently of nitric-oxide synthase. J Biol Chem. 2010;285:10638–10652. doi: 10.1074/jbc.M110.108688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Gorgun CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006a;4:465–474. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Steinberg GR, Watt MJ, Fam BC, Proietto J, Andrikopoulos S, Allen AM, Febbraio MA, Kemp BE. Ciliary neurotrophic factor suppresses hypothalamic AMP-kinase signaling in leptin-resistant obese mice. Endocrinology. 2006b;147:3906–3914. doi: 10.1210/en.2005-1587. [DOI] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- Suter M, Riek U, Tuerk R, Schlattner U, Wallimann T, Neumann D. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. The Journal of biological chemistry. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Okamoto S, Lee S, Saito K, Shiuchi T, Minokoshi Y. Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the alpha2 form of AMP-activated protein kinase. Mol Cell Biol. 2007;27:4317–4327. doi: 10.1128/MCB.02222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, Cantrell DA. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townley R, Shapiro L. Crystal structures of the adenylate sensor from fission yeast AMP-activated protein kinase. Science. 2007;315:1726–1729. doi: 10.1126/science.1137503. [DOI] [PubMed] [Google Scholar]
- Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem. 1999;72:1707–1716. doi: 10.1046/j.1471-4159.1999.721707.x. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villen J, Beausoleil SA, Gerber SA, Gygi SP. Large-scale phosphorylation analysis of mouse liver. Proc Natl Acad Sci U S A. 2007;104:1488–1493. doi: 10.1073/pnas.0609836104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena JA, Viollet B, Andreelli F, Kahn A, Vaulont S, Sul HS. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-alpha2 subunit. Diabetes. 2004;53:2242–2249. doi: 10.2337/diabetes.53.9.2242. [DOI] [PubMed] [Google Scholar]
- Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, Foretz M, Guigas B, Horman S, Dentin R, Bertrand L, Hue L, Andreelli F. Activation of AMP-activated protein kinase in the liver: a new strategy for the management of metabolic hepatic disorders. J Physiol. 2006;574:41–53. doi: 10.1113/jphysiol.2006.108506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, Guigas B, Leclerc J, Hebrard S, Lantier L, Mounier R, Andreelli F, Foretz M. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf) 2009;196:81–98. doi: 10.1111/j.1748-1716.2009.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Unger RH. Role of PP2C in cardiac lipid accumulation in obese rodents and its prevention by troglitazone. Am J Physiol Endocrinol Metab. 2005;288:E216–221. doi: 10.1152/ajpendo.00004.2004. [DOI] [PubMed] [Google Scholar]
- Wang S, Xu J, Song P, Viollet B, Zou MH. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes. 2009;58:1893–1901. doi: 10.2337/db09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden SM, Richardson C, O’Donnell J, Jr, Stapleton D, Kemp BE, Witters LA. Post-translational modifications of the beta-1 subunit of AMP-activated protein kinase affect enzyme activity and cellular localization. Biochem J. 2001;354:275–283. doi: 10.1042/0264-6021:3540275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes JJ, Nguyen MT, Bandyopadhyay GK, Nelson E, Olefsky JM. Topiramate treatment causes skeletal muscle insulin sensitization and increased Acrp30 secretion in high-fat-fed male Wistar rats. Am J Physiol Endocrinol Metab. 2005;289:E1015–1022. doi: 10.1152/ajpendo.00169.2005. [DOI] [PubMed] [Google Scholar]
- Williams T, Brenman JE. LKB1 and AMPK in cell polarity and division. Trends Cell Biol. 2008;18:193–198. doi: 10.1016/j.tcb.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Witters LA, Kemp BE. Insulin activation of acetyl-CoA carboxylase accompanied by inhibition of the 5′-AMP-activated protein kinase. J Biol Chem. 1992;267:2864–2867. [PubMed] [Google Scholar]
- Wojtaszewski JF, Jorgensen SB, Hellsten Y, Hardie DG, Richter EA. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes. 2002;51:284–292. doi: 10.2337/diabetes.51.2.284. [DOI] [PubMed] [Google Scholar]
- Wolfgang MJ, Cha SH, Sidhaye A, Chohnan S, Cline G, Shulman GI, Lane MD. Regulation of hypothalamic malonyl-CoA by central glucose and leptin. Proc Natl Acad Sci U S A. 2007;104:19285–19290. doi: 10.1073/pnas.0709778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003a;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Woods A, Munday MR, Scott J, Yang X, Carlson M, Carling D. Yeast SNF1 is functionally related to mammalian AMP-activated protein kinase and regulates acetyl-CoA carboxylase in vivo. J Biol Chem. 1994;269:19509–19515. [PubMed] [Google Scholar]
- Woods A, Vertommen D, Neumann D, Turk R, Bayliss J, Schlattner U, Wallimann T, Carling D, Rider MH. Identification of phosphorylation sites in AMP-activated protein kinase (AMPK) for upstream AMPK kinases and study of their roles by sitedirected mutagenesis. J Biol Chem. 2003b;278:28434–28442. doi: 10.1074/jbc.M303946200. [DOI] [PubMed] [Google Scholar]
- Wu Y, Song P, Xu J, Zhang M, Zou MH. Activation of protein phosphatase 2A by palmitate inhibits AMP-activated protein kinase. J Biol Chem. 2007;282:9777–9788. doi: 10.1074/jbc.M608310200. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, Walker PA, Haire L, Eccleston JF, Davis CT, Martin SR, Carling D, Gamblin SJ. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL, Taffet GE, Baldini A, Khoury DS, Schneider MD. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17378–17383. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada E, Pessin JE, Kurland IJ, Schwartz GJ, Bastie CC. Fyn-dependent regulation of energy expenditure and body weight is mediated by tyrosine phosphorylation of LKB1. Cell Metab. 2010;11:113–124. doi: 10.1016/j.cmet.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Mu J, Birnbaum MJ. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis In 3T3-L1 adipocytes. J Biol Chem. 2003;278:43074–43080. doi: 10.1074/jbc.M308484200. [DOI] [PubMed] [Google Scholar]
- Yu X, McCorkle S, Wang M, Lee Y, Li J, Saha AK, Unger RH, Ruderman NB. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia. 2004;47:2012–2021. doi: 10.1007/s00125-004-1570-9. [DOI] [PubMed] [Google Scholar]
- Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, Brecher P, Ruderman NB, Cohen RA. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004;279:47898–47905. doi: 10.1074/jbc.M408149200. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zmijewski JW, Lorne E, Liu G, Park YJ, Tsuruta Y, Abraham E. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L497–504. doi: 10.1152/ajplung.90210.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WGt, Schlattner U, Neumann D, Brownlee M, Freeman MB, Goldman MH. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279:43940–43951. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- Zou MH, Wu Y. AMP-activated protein kinase activation as a strategy for protecting vascular endothelial function. Clin Exp Pharmacol Physiol. 2008;35:535–545. doi: 10.1111/j.1440-1681.2007.04851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]