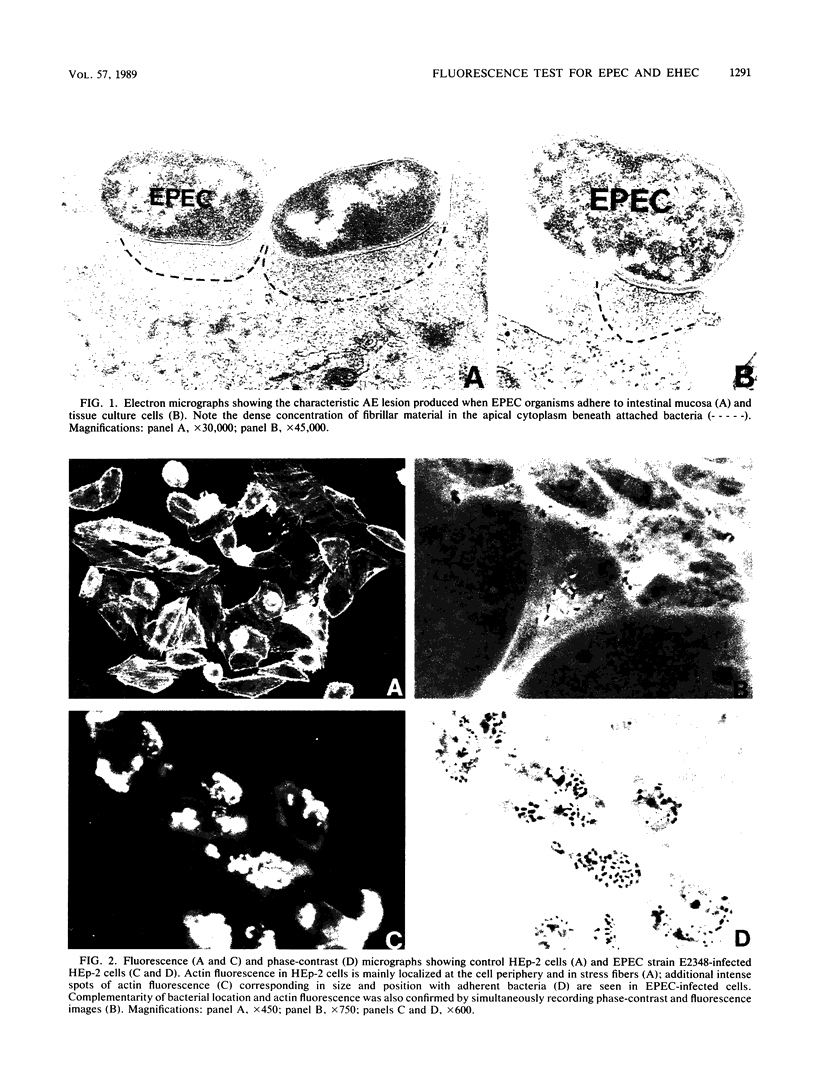
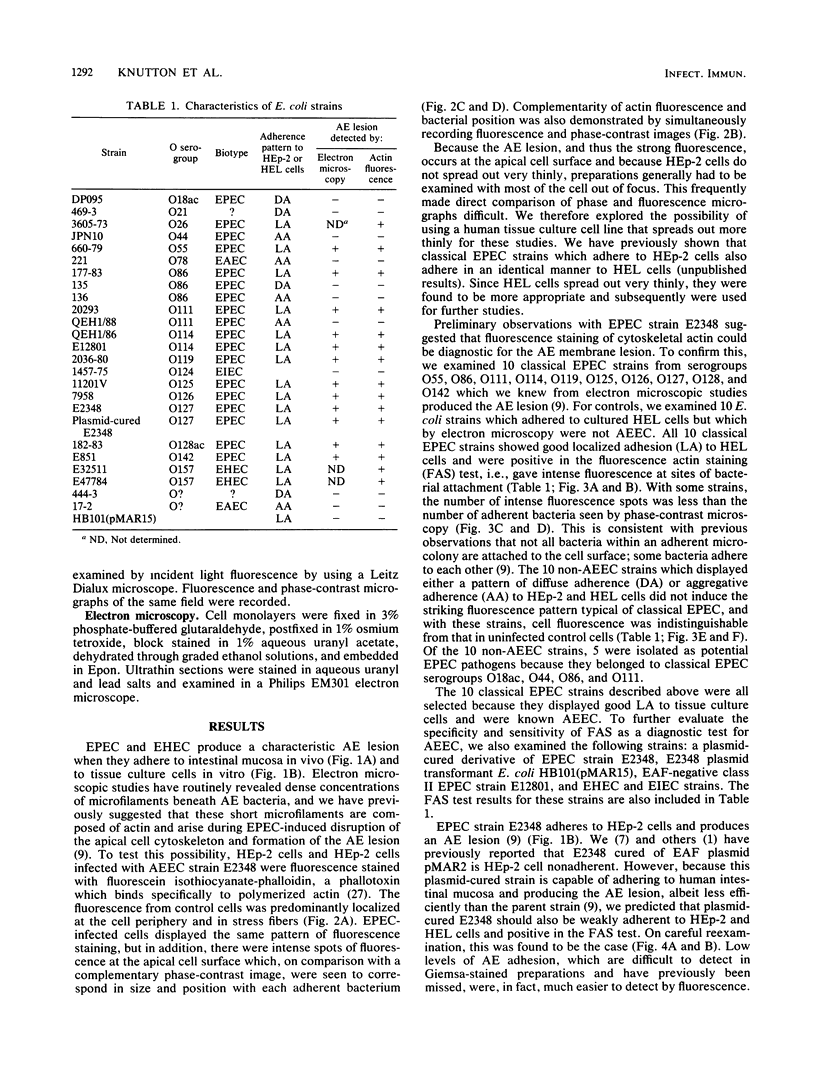
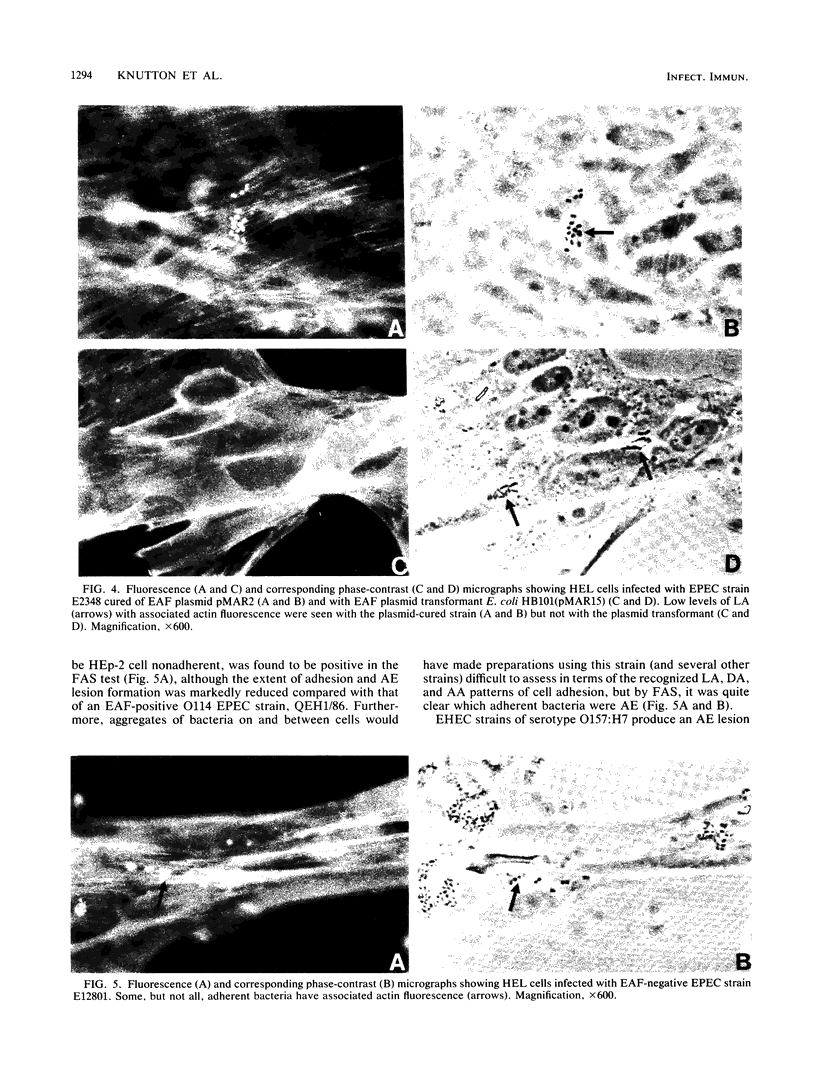
Abstract
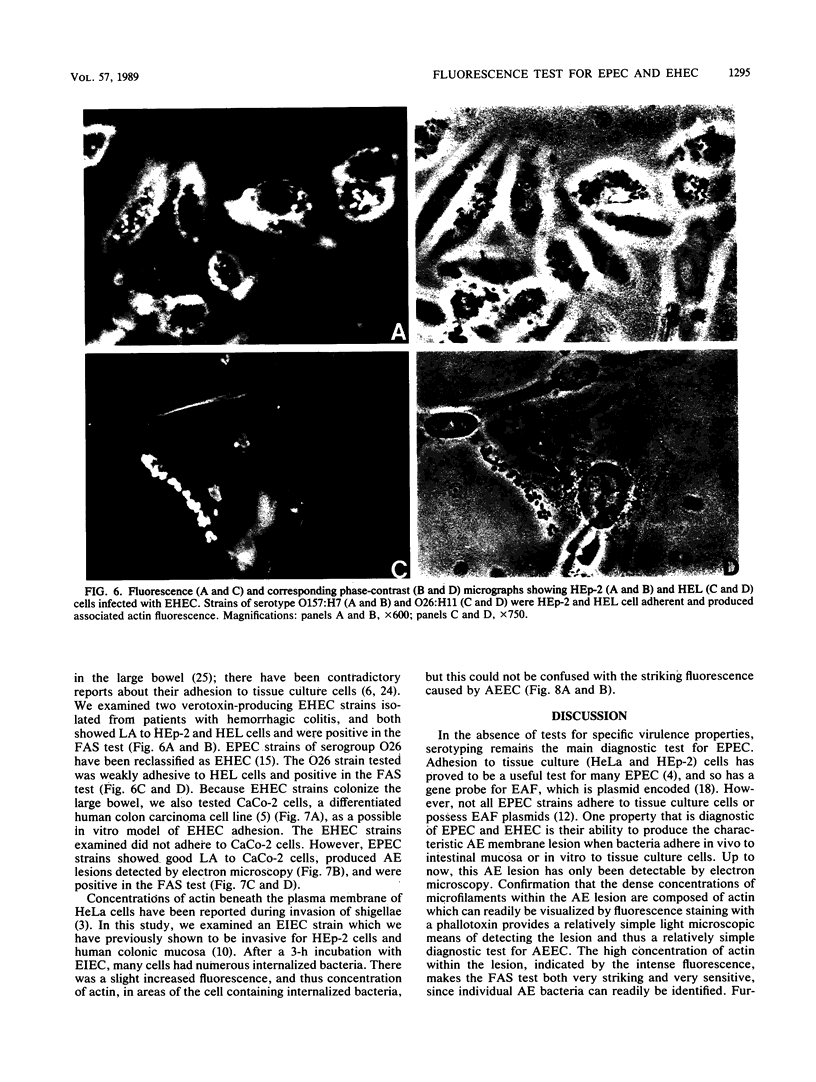
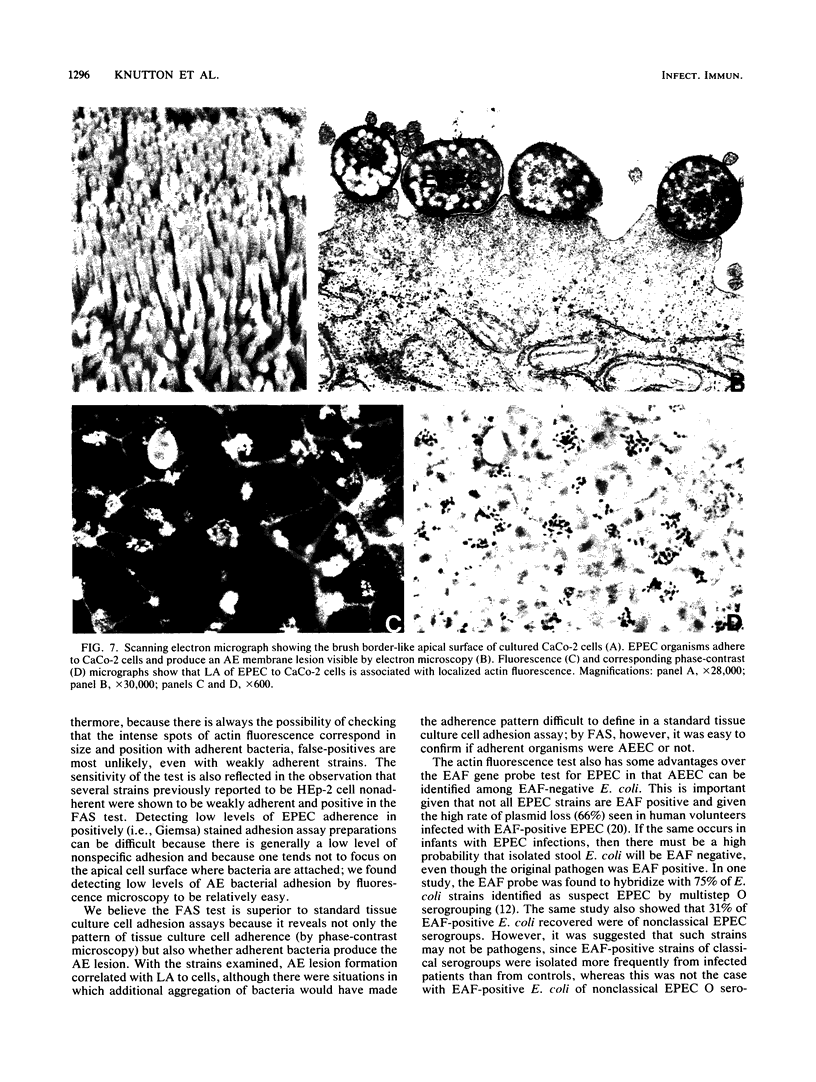
Enteropathogenic Escherichia coli (EPEC) and enterohemorrhagic E. coli (EHEC) adhere to the intestinal mucosa and produce an attaching and effacing (AE) lesion in the brush border microvillous membrane; the AE lesion is characterized by localized destruction of microvilli and intimate attachment of bacteria to the apical enterocyte membrane. A similar lesion is seen when bacteria adhere in vitro to a variety of human tissue culture cell lines. In both cases, dense concentrations of microfilaments are present in the apical cytoplasm beneath attached bacteria. Using a fluorescein-labeled phallotoxin, we have shown that these microfilaments are composed of actin. Cells infected with EPEC and EHEC strains known from electron microscopic studies to produce the AE lesion all exhibited intense spots of fluorescence which corresponded in size and position with each adherent bacterium; cells infected with adherent E. coli strains known not to produce the AE lesion did not produce this striking pattern of fluorescence and were indistinguishable from uninfected control cells. These results indicate that such site-specific concentrations of cytoskeletal actin are characteristic of the AE membrane lesion and can form the basis of a simple, highly sensitive diagnostic test for EPEC and EHEC.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldini M. M., Kaper J. B., Levine M. M., Candy D. C., Moon H. W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2(3):534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- Chart H., Scotland S. M., Willshaw G. A., Rowe B. HEp-2 adhesion and the expression of a 94 kDa outer-membrane protein by strains of Escherichia coli belonging to enteropathogenic serogroups. J Gen Microbiol. 1988 May;134(5):1315–1321. doi: 10.1099/00221287-134-5-1315. [DOI] [PubMed] [Google Scholar]
- Clerc P., Sansonetti P. J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987 Nov;55(11):2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogh J., Fogh J. M., Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977 Jul;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- Knutton S., Baldini M. M., Kaper J. B., McNeish A. S. Role of plasmid-encoded adherence factors in adhesion of enteropathogenic Escherichia coli to HEp-2 cells. Infect Immun. 1987 Jan;55(1):78–85. doi: 10.1128/iai.55.1.78-85.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Baldwin T., Williams P. H., McNeish A. S. New diagnostic test for enteropathogenic Escherichia coli. Lancet. 1988 Jun 11;1(8598):1337–1337. doi: 10.1016/s0140-6736(88)92152-6. [DOI] [PubMed] [Google Scholar]
- Knutton S., Lloyd D. R., McNeish A. S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987 Jan;55(1):69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Williams P. H., Lloyd D. R., Candy D. C., McNeish A. S. Ultrastructural study of adherence to and penetration of cultured cells by two invasive Escherichia coli strains isolated from infants with enteritis. Infect Immun. 1984 Jun;44(3):599–608. doi: 10.1128/iai.44.3.599-608.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Edelman R. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol Rev. 1984;6:31–51. doi: 10.1093/oxfordjournals.epirev.a036274. [DOI] [PubMed] [Google Scholar]
- Levine M. M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987 Mar;155(3):377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Nataro J. P., Karch H., Baldini M. M., Kaper J. B., Black R. E., Clements M. L., O'Brien A. D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985 Sep;152(3):550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Xu J. G., Kaper J. B., Lior H., Prado V., Tall B., Nataro J., Karch H., Wachsmuth K. A DNA probe to identify enterohemorrhagic Escherichia coli of O157:H7 and other serotypes that cause hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1987 Jul;156(1):175–182. doi: 10.1093/infdis/156.1.175. [DOI] [PubMed] [Google Scholar]
- Mathewson J. J., Johnson P. C., DuPont H. L., Morgan D. R., Thornton S. A., Wood L. V., Ericsson C. D. A newly recognized cause of travelers' diarrhea: enteroadherent Escherichia coli. J Infect Dis. 1985 Mar;151(3):471–475. doi: 10.1093/infdis/151.3.471. [DOI] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NETER E. Enteritis due to enteropathogenic Escherichia coli; present-day status and unsolved problems. J Pediatr. 1959 Aug;55(2):223–239. doi: 10.1016/s0022-3476(59)80091-3. [DOI] [PubMed] [Google Scholar]
- Nataro J. P., Baldini M. M., Kaper J. B., Black R. E., Bravo N., Levine M. M. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J Infect Dis. 1985 Sep;152(3):560–565. doi: 10.1093/infdis/152.3.560. [DOI] [PubMed] [Google Scholar]
- Nataro J. P., Kaper J. B., Robins-Browne R., Prado V., Vial P., Levine M. M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987 Sep;6(9):829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Nataro J. P., Scaletsky I. C., Kaper J. B., Levine M. M., Trabulsi L. R. Plasmid-mediated factors conferring diffuse and localized adherence of enteropathogenic Escherichia coli. Infect Immun. 1985 May;48(2):378–383. doi: 10.1128/iai.48.2.378-383.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins-Browne R. M. Traditional enteropathogenic Escherichia coli of infantile diarrhea. Rev Infect Dis. 1987 Jan-Feb;9(1):28–53. doi: 10.1093/clinids/9.1.28. [DOI] [PubMed] [Google Scholar]
- Rothbaum R., McAdams A. J., Giannella R., Partin J. C. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982 Aug;83(2):441–454. [PubMed] [Google Scholar]
- Sherman P., Soni R., Petric M., Karmali M. Surface properties of the Vero cytotoxin-producing Escherichia coli O157:H7. Infect Immun. 1987 Aug;55(8):1824–1829. doi: 10.1128/iai.55.8.1824-1829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Wachsmuth I. K., Chapman C., Birden R., Brittingham J., Jackson C., Hogg J. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic piglets. J Infect Dis. 1986 Oct;154(4):712–716. doi: 10.1093/infdis/154.4.712. [DOI] [PubMed] [Google Scholar]
- Ulshen M. H., Rollo J. L. Pathogenesis of escherichia coli gastroenteritis in man--another mechanism. N Engl J Med. 1980 Jan 10;302(2):99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]