Abstract
The highly interconnected local and large-scale networks of the neocortical sheet rapidly and dynamically modulate their functional connectivity according to behavioral demands. This basic operating principle of the neocortex is mediated by the continuously changing flow of excitatory and inhibitory synaptic barrages that not only control participation of neurons in networks but also define the networks themselves. The rapid control of neuronal responsiveness via synaptic bombardment is a fundamental property of cortical dynamics that may provide the basis of diverse behaviors, including sensory perception, motor integration, working memory, and attention.
Introduction
Typical diagrams of the neocortical sheet suggest a static, compartmentalized structure in which signals travel in a well-prescribed manner, flowing across and within layers in a manner dictated predominantly by patterns of anatomical connectivity. While these relatively hard-wired pathways certainly establish routes for information transfer within cortical networks, it is the dynamic, moment-to-moment fluctuations in activity traversing these pathways that determine the functional connectivity of cortical networks. Since an individual cortical neuron receives input from tens of thousands of other cortical neurons, and in turn projects its output activity patterns to thousands of recipient neurons, the influence of one neuron (or group of neurons) upon another depends critically upon the activity state of all of the neurons that are interconnected with the cells or networks under consideration. In other words, although there is a strong anatomical bias to neuronal interactions, exactly which neuronal subpopulations actively communicate at any particular moment in time (i.e., functional connectivity) depends upon the state of activity in the network itself and can change rapidly to meet behavioral demands. Although this framework of functional cortical connectivity is by no means new (Hebb, 1949; Lorente de No, 1938), it nonetheless has motivated a considerable number of studies that collectively have yielded insights into the subtle spatial and temporal dynamics of local recurrent excitatory and inhibitory cortical networks (e.g., see Douglas and Martin, 2007a; Gilbert and Sigman, 2007; Thomson et al., 2002). Through such work, it is becoming clear that a basic operation of the cerebral cortex is to control the flow of neuronal communication by transiently linking specific groups of neurons (subnetworks) together by dynamic modulation of neuronal responsiveness. These rapid changes in responsiveness (over milliseconds to seconds) occur largely through alterations in ongoing synaptic activity generated by local, as well as long-range, neuronal interactions and may underlie changes in functional connectivity that enable the flexibility of sensory-motor behaviors at these same timescales.
The purpose of this review is to provide a brief overview of rapid (milliseconds to seconds) network dynamics in the cortex that may be mediated by changes in neuronal responsiveness and interactions resultant from synaptic bombardment. Many excellent reviews have been written on other aspects of cortical dynamics, including the roles of synaptic plasticity, intrinsic membrane properties, neuromodulators, and changes in anatomical connectivity (Alvarez and Sabatini, 2007; Bean, 2007; Caporale and Dan, 2008; Kerchner and Nicoll, 2008; Llinas, 1988; Luo et al., 2008; Magee and Johnston, 2005; Malenka and Bear, 2004; McCormick, 1992; Sjostrom et al., 2008). Here, we begin by considering the main cellular and network mechanisms that may rapidly alter the responsiveness and functional connectivity of cortical neurons and examine the predictions of computational models regarding the impact of synaptic barrages on neuronal input-output relations in vivo. We will then review experimental work demonstrating that rapid alterations in excitatory and inhibitory synaptic barrages generated within specific subnetworks mediate fast changes in cortical excitability and information flow. We will take these insights and evaluate how such state changes via synaptic bombardment affect the sensory response properties of cortical neurons in vivo and attempt to link the described cellular and network mechanisms to recent evidence for cortical activity states observed in awake and behaving animals. We will conclude by outlining some key experiments dissecting rapid changes in functional connectivity that may be testable in the near future with newly emerging techniques. Our overall synthesis suggests that concerted changes in synaptic activity in local networks may serve as the key mechanism for determining not only action potential rate and timing in single neurons but also serve as a context of past and present network activity, linking ensembles of neurons together in a behaviorally relevant fashion. The dynamic control of neuronal interactions through synaptic barrages generated in the local network may be a unifying principle underlying diverse phenomena such as attention, sensory-motor integration, working memory, and the sleep-wake cycle.
Modulating Responsiveness Dynamically Reconfigures Functional Connectivity
Neurons in the neocortex are interconnected directly with each other and indirectly through other interposed neurons. Consequently, a major mechanism by which neocortical subnetworks are rapidly formed and broken is through the modulation of neuronal excitability (Figure 1). Of course, changes in nearly any property of the cortical network will affect neuronal responsiveness and network interactions. Classic examples include changes in ionic concentrations and currents (Bean, 2007), synaptic plasticity (Dan and Poo, 2006), and the actions of neuromodulators (McCormick, 1992). Here, we will focus on rapid changes in network dynamics (milliseconds to seconds) and the subsequent changes in synaptic barrages in recipient neurons as a possible mediator of response modulation, since network reconfiguration at this timescale is likely to be of utmost importance to active waking behavior.
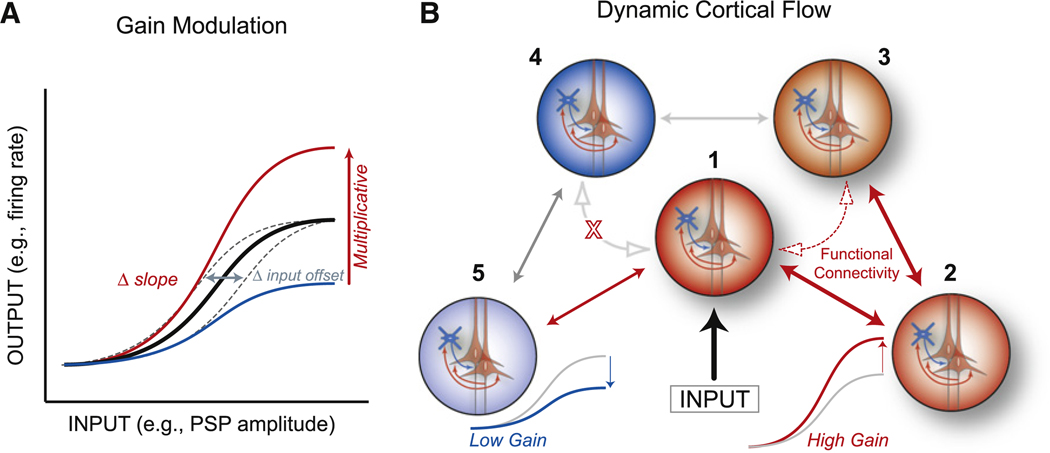
Figure 1. Rapid Cortical Network Dynamics Are Mediated by Modulation of Neuronal Gain.
(A) Schematic of various forms of response modulation. Input gain (dashed gray) shifts the threshold and saturation points without altering the ratio of input to output. Multiplicative gain increases (red) or divisively decreases (blue) the responsiveness at all levels of input by a similar percentage and results in a change in the slope of the input-output function.
(B) Schematic diagram illustrating five sets of neurons or neuronal groups that are anatomically interconnected (double-headed arrows), with the middle group (1) receiving an input. If neuronal group2 has high gain, then groups 1, 2, and 3 exhibit enhanced functional connectivity (dashed arrows), with activity flowing easily between groups 1 and 3. Likewise, if group 5 has low gain, then the flow of information and interactions between groups 1, 4, and 5 will be limited. Note that the responsiveness (gain) within and among neuronal groups is determined by local recurrent excitation and inhibition.
Let us first consider the relationship of neuronal discharge to the amplitude of incoming synaptic barrages. The relationship between neuronal response magnitude as a function of input amplitude, or input-output curve, generally exhibits a sigmoidal shape such that increasing the amplitude of weak inputs causes a gradual increase in action potential response, while increasing the amplitude of intermediate-sized inputs results in a steep increase in spike response. Larger inputs typically elicit response saturation (Figure 1A). To alter neuronal responsiveness to an input, this whole curve may move leftward or rightward along the input axis, altering the threshold and response saturation points, while preserving the shape of the input-output function (Figure 1A, dashed lines). Alternatively, the input-output curve may shift up or down along the output axis, changing the sensitivity of the neuron and the maximal response rate. Finally, the ratio of response magnitude relative to input amplitude may change by a constant gain factor (i.e., stretch along the output axis), producing a multiplicative (Figure 1A, red) or divisive (blue) change, thus varying the slope of the input-output curve. Although all of these alterations represent changes in neural responsiveness, the last, a multiplicative change of the input-output curve, is typically referred to as gain modulation (Cardin et al., 2008; Chance et al., 2002; McAdams and Maunsell, 1999; Salinas and Abbott, 1996; Treue and Martinez Trujillo, 1999).
How might cortical neurons rapidly alter the responsiveness, or gain, of other cortical neurons? The major mechanism by which neurons influence one another is through the generation of action potentials that are visible in target structures as postsynaptic potentials. Bombardment of neurons by synaptic potentials strongly influences neuronal excitability by controlling membrane potential, membrane conductance, and membrane potential variance. Together, these factors ultimately control not only spike number but also spike timing (Chance et al., 2002; Ho and Destexhe, 2000; Kuhn et al., 2004; Rudolph and Destexhe, 2003; Shu et al., 2003a). In other words, patterns of synaptic bombardment control, on a moment-to-moment basis, the probability of spike generation in the recipient neurons. These changes in spike probability are highly dynamic in time and provide “windows of opportunity” for interactions of cells with coactivated groups of cortical and subcortical neurons (Figure 2). These variations in neuronal interactions through synaptic bombardment occur at multiple timescales, from hours in the case of the sleep-wake cycles to milliseconds in the case of higher-frequency fluctuations in membrane potential (Buzsaki and Draguhn, 2004). The changing form of ongoing synaptic barrages continuously determines whether or not a neuron responds to incoming synaptic inputs, particularly those of small to medium amplitudes (Figure 1A), as typically found between single cortical neurons. If the modulated neuron or group of neurons is interposed between other cells, then its level of responsiveness will in turn determine the degree to which the other neurons interact (Figure 1B). When the window of opportunity becomes relatively short (within the integration time constant of a single neuron, e.g., window 3 in Figure 2), then the precise timing of spikes may play an important role in the interactions within and between cortical networks.
Figure 2. Synaptic Bombardment in Active Cortical Networks In Vivo.
Illustrated are simultaneous recordings of the extracellular local field potential (LFP, top), the multiple unit activity (MU, middle), and intracellular membrane potential (bottom) from a cortical pyramidal cell during the generation of one Up state (bordered by two Down states). Note that the Up state is associated with a marked increase in local network activity, depolarization of the neuron (~25 mV), a marked increase in membrane potential variance (SD of Up = 2.5 mV), and the presence of higher-frequency (gamma, ~40 Hz) oscillations in the LFP. Periods of depolarization mediated by network activity provide “windows of opportunity” based upon increased neuronal gain and may be of long (example 1), medium (example 2), or short (example 3) duration. Thus, various frequency components of synaptic activity interact to initiate action potentials. Inset expands window 3, where the depolarizing half (~12.5 ms) of one full LFP oscillation cycle provides a short temporal window for the integration of both excitatory and inhibitory postsynaptic potentials (PSPs).
Models of Rapid Response Modulation
Modulation of the input/output function lies at the heart of rapid changes in cortical functional connectivity. Could rapid changes in membrane potential, conductance, and variance during different behavioral states underlie the well-known changes in neuronal responsiveness occurring in cortical neurons according to context and behavior? This question has been explored through several computational studies (Azouz, 2005; Chance et al., 2002; Compte et al., 2003b; El Boustani et al., 2007; Ho and Destexhe, 2000; Kuhn et al., 2004; Murphy and Miller, 2003; Prescott and De Koninck, 2003; Rudolph and Destexhe, 2003). These studies have revealed that there are several mechanisms for modulation of neuronal responsiveness, the mix of which can cause a multiplicative, or nearly multiplicative, increase in neuronal gain. In the absence of significant levels of membrane potential variance (also called “noise”), tonic depolarization increases neuronal responsiveness by shifting input-output curves along the input axis (Figure 1A; change input offset) (Cardin et al., 2008; Chance et al., 2002; Compte et al., 2003b; Ho and Destexhe, 2000; Shu et al., 2003a). A similar effect can be obtained by decreases in membrane conductance in the absence of membrane potential noise (Chance et al., 2002; Ho and Destexhe, 2000; Shu et al., 2003a). These lateral shifts in the input-output curve result in a large enhancement of the responsiveness to small inputs and a moderate or only small enhancement of larger inputs (dashed gray lines in Figure 1A). If the input range is restricted to only the initial portions of the input-output curve, then depolarization or decreases in conductance (in the absence of membrane potential variance) can result in what appears to be a change in gain (e.g., Figure 1A; portions where dashed gray overlaps with solid red or blue). It is thus critical to determine the input-output relationship over a wide range of subthreshold as well as saturating inputs. In real neurons in vivo, the membrane potential of neurons exhibits substantial rapid variations, during all behavioral states, owing to fluctuations in synaptic bombardment (Figure 2) (Steriade et al., 2001). The addition of membrane potential variance causes neurons to discharge in a probabilistic fashion across a wide range of synaptic barrage amplitudes (Ho and Destexhe, 2000; Shu et al., 2003a). By providing a variable level of depolarization, membrane variance can actually enhance the probability that subthreshold inputs become suprathreshold, thus enhancing the responsiveness to weak inputs (Chance et al., 2002; Ho and Destexhe, 2000; Shu et al., 2003a). Various combinations of changes in membrane potential, membrane conductance, and membrane potential variance can be utilized to result in several types of shifts in neuronal responsiveness, including changes in the slope of the input-output function (Ayaz and Chance, 2009; Chance et al., 2002; Ho and Destexhe, 2000; Shu et al., 2003a). Indeed, it has been suggested that increases in neuronal gain can be achieved through a simultaneous decrease in membrane conductance and variance, such as could occur from the concerted withdrawal of barrages of synaptic potentials, the composition of which is perfectly balanced so as to not depolarize or hyperpolarize the neuronal membrane potential (Chance et al., 2002). Such a model predicts that increases in neuronal gain are associated with decreases in local network activity.
A consequence of membrane potential variance is that the average frequency of firing in response to an input can follow a power law function [output = (input)x; Figure 3A] (Miller and Troyer, 2002). This power law relationship between membrane potential and firing rate is not merely a theoretical possibility; it is often observed in cortical neurons in vivo when the cells are synaptically driven with sensory stimulation (Anderson et al., 2000; Priebe and Ferster, 2008; Sanchez-Vives et al., 2000) (Figure 3B). Such a relationship between membrane potential and firing rate has broad and important implications for how any tonic changes in membrane potential (such as from a neuromodulatory input) affect the gain of these cells (e.g., Disney et al., 2007; Thurley et al., 2008). For instance, depolarization of cortical neurons by a few millivolts, as occurs with barrages of synaptic potentials, will result in only a small increase in activity at the lower end of the membrane potential-firing rate relationship but a much larger increase in the firing rate at the high end of this relationship (Figures 3A and 3B). The resulting larger increase in firing rate with higher levels of input (Figures 3A and 3B) can result in a percent enhancement that is quite similar (but not identical) at each point of the input-output curve (Figure 3C). In other words, over the range of voltages in which natural synaptic activity activates action potentials in cortical neurons, the presence of membrane potential variance induces a nonlinear relationship between membrane potential and firing rate. The presence of this power law behavior can cause simple depolarization to nonetheless appear as a multiplicative-like increase in gain (Murphy and Miller, 2003). These results suggest that multiplicative-like gain modulation to sensory stimuli could be obtained simply by depolarizing neurons, through the release of neuromodulators (McCormick, 1992), the alteration of intrinsic ionic conductances (Sanchez-Vives et al., 2000), or by barrages of synaptic activity (Haider et al., 2007; Ho and Destexhe, 2000; Shu et al., 2003a).
Figure 3. Modulation of Neuronal Gain through an Interaction of Membrane Potential and Intrinsic Nonlinearities in Input-Output Transformation of Cortical Neurons.
(A) In the absence of neuronal variance, neurons respond to depolarization with an abrupt increase in firing rate once threshold has been reached (black). However, in the presence of membrane potential variance, the average relationship between firing rate and membrane potential can exhibit a power law (red).
(B) Intracellular recordings of cortical neuronal responses to visual stimuli in vivo have revealed, on average, a power law relationship between membrane potential and firing rate.
(C) In the presence of a power law relationship between membrane potential and firing rate, changes in either excitatory or inhibitory background conductance that depolarize or hyperpolarize neurons (±2–5 mV), respectively, can result in multiplicative-like changes in the input-output relationship of cortical neurons.
(D) Depolarization induced through the intracellular injection of current results in a multiplicative-like change in the contrast response function curve of visual cortical neurons.
(E) Scaling the hyperpolarized curve from (D) with a constant gain factor reveals a multiplicative-like gain change. (F) Depolarization that occurs spontaneously owing to synaptic bombardment in vivo can also result in multiplicative-like increases in neuronal responsiveness of the contrast response function.
(B) Adapted by permission from Macmillan Publishers Ltd: Nature Neuroscience (Priebe et al., 2004), copyright 2004; (C) modified from Murphy and Miller (2003); (D) modified from Sanchez-Vives et al. (2000); (E) modified from Haider et al. (2007).
In summary, tonic changes in membrane potential in the presence of membrane potential variance—resulting from the high variability of synaptic barrages—can result in rapid, nonlinear changes in neuronal responsiveness and gain. Membrane potential variance causes neurons to respond in a probabilistic manner as opposed to all-or-none threshold elements, and increasing depolarization can have large effects on the average input-output relations of cortical neurons. As we will see, experimental interrogation of synaptic barrages in vivo during active cortical states strongly suggests that such a multiplicative-like enhancement of responsiveness can indeed occur by transient epochs of synaptic depolarization.
Local Network Activity Dominates the Membrane Potential of Cortical Neurons In Vivo
Cortical neurons receive the great majority of their synaptic inputs from neurons within the local network (i.e., <1 mm distant) (Binzegger et al., 2004). Consequently, the activity of the local network will have a dominating, but not exclusive, influence on the membrane potential of nearby cells. This is important since investigations of the effects of changes in membrane potential, membrane conductance, and membrane potential variance demonstrate that the primary determinant of the probability of action potential generation is the level of membrane potential depolarization (Shu et al., 2003a). The closer a neuron is to action potential threshold (usually around −53 mV for cortical pyramidal neurons) the more likely it is to fire upon stimulation, all else being equal. In the absence of any synaptic input, the membrane potential of a cortical neuron in vivo is markedly hyperpolarized (e.g., between −80 and −65 mV, depending on layer, cell type, and state of the animal) and dominated by K+ conductances (Pare et al., 1998). Under these conditions, the typical cortical neuron is −10–25 mV below action potential threshold. Depolarizing a typical cortical pyramidal cell that has an input resistance of 50 MΩ by 10 mV with a balanced barrage of excitatory and inhibitory synaptic potentials would result in approximately a 30% increase in membrane conductance (Shu et al., 2003a). Although this increase in conductance by itself would decrease neuronal responsiveness, the increase in excitability provided by the depolarization is six times greater and therefore swamps the conductance effect (Shu et al., 2003a). Of course, it should be kept in mind that increases in membrane conductance can themselves change the spatiotemporal integrative properties of cortical neurons (Bernander et al., 1991; Borg-Graham et al., 1998; Holt and Koch, 1997). In general, however, balanced barrages of excitatory and inhibitory synaptic potentials (discussed in detail later) have their effects on action potential discharge largely through determining the membrane potential level of the postsynaptic neuron.
How many neurons must discharge to cause a typical cortical pyramidal cell to fire? The average monosynaptic excitatory postsynaptic potential (EPSP) from one cortical neuron to another is on the order of 0.5 mV, far too small to elicit an action potential in a quiescent neuron (Markram et al., 1997; Thomson et al., 2002). A back-of-the-envelope calculation suggests that on average approximately 50–100 excitatory synaptic potentials (along with balanced inhibitory potentials) would need to be received every 10 ms to keep the typical cortical pyramidal cell depolarized by the 20 mV between resting membrane potential and firing threshold. Since cortical neurons receive inputs from approximately 3,000–10,000 other neurons (Binzegger et al., 2004; Larkman, 1991), this indicates that even a low average level of action potential activity (e.g., 0.1–1 Hz) in the presynaptic network could provide this maintained depolarization, depending also upon the level of activation of inhibitory networks. This average firing rate is well within the range of cortical neurons active in the waking state (Beloozerova et al., 2003; Shoham et al., 2006).
It is important to keep in mind that synaptic potentials arrive throughout the dendrites, soma, and initial segment of the axon and interact in a spatially and temporally complex manner, the details of which are beyond the scope of this review, although this important issue has received considerable attention (Larkum et al., 2004; London and Hausser, 2005; Magee, 2000; Major et al., 2008; Milojkovic et al., 2005; Murayama et al., 2009; Nevian et al., 2007; Rudolph and Destexhe, 2003; Williams, 2004). Since nearly all action potentials that are responsible for synaptic communication between cortical neurons are initiated in the axon initial segment (Kole et al., 2008; McCormick et al., 2007; Shu et al., 2007; Stuart et al., 1997; Yu et al., 2008), the fluctuations as they appear at the level of the soma and axon initial segment are of particular importance. Luckily, owing to its large size, the soma is by far the most common place to record the intracellular membrane potential fluctuations of cortical neurons. For our present purposes, we propose that the precise and rapid control of somatic membrane potential, conductance, and variance is achieved through control of concerted fluctuations in local network activity that can be engaged by local and distant cortical, as well as subcortical, neurons. Importantly, long-range connections are overwhelmingly excitatory, but synapse onto both local inhibitory and excitatory neurons (McGuire et al., 1991), which are themselves densely interconnected with one another. Experimental investigations into the influence of synaptic bombardment on neuronal responsiveness in local cortical networks have largely focused on “spontaneous” cortical activity, of which the slow oscillation is perhaps the best-characterized example.
The Cortical Slow Oscillation: A Model System to Investigate Neuronal Responsiveness and Excitatory-Inhibitory Interactions during Active Cortical States
How can we begin to examine the synaptic mechanisms of rapid changes in the state and sensory responsiveness of single cortical neurons and their extended interactions within an active cortical network? Studies at multiple levels and in many systems indicate that one key consequence of dense, local connectivity is that the cortex exhibits spontaneous, persistent activity in the absence of any external stimulation (for review, see Destexhe et al., 2003; Major and Tank, 2004). This spontaneous, or ongoing, activity is present during active behavior, quiet resting, sleep, anesthesia, and even in cortical slices in vitro. However, the spatial and temporal patterns of spontaneous activity in the cortex differ markedly across these different conditions.
Studies in anesthetized cat visual cortex indicate that both extended and local cortical regions exhibit dynamic activity correlations varying over tens to hundreds of milliseconds. Importantly, local regions that exhibit the strongest correlation of excitability correspond to regions of cortex that exhibit similar stimulus preferences, e.g., for the orientation of a drifting bar of light (Arieli et al., 1996; Kenet et al., 2003). If a network is stimulated appropriately and enters into an excitable state, temporally coincident responses may be transmitted throughout this transiently interconnected group of neurons, facilitating their interaction (Abeles et al., 1995; Fujisawa et al., 2008; Harris, 2005; Rutishauser and Douglas, 2008; Tiesinga et al., 2008). These studies indicate that local cortical networks have a strong tendency to transiently enter into stable states of enhanced excitability that dynamically interact with sensory stimulation. There is reason to believe, as we will discuss, that network activation in the waking state may exhibit at least some characteristics similar to those described during anesthesia (Abeles et al., 1995; Fujisawa et al., 2008; Poulet and Petersen, 2008; Steriade et al., 2001). What are the cellular mechanisms that allow local cortical networks to rapidly switch into and out of these semistable and excitable states?
In the cortex, perhaps the most well-studied pattern of recurrent network activity is that of the slow (<1 Hz) oscillation, which is characterized by a rhythmic alternation between activated and deactivated states (Figure 2). The cortical slow oscillation has been used to investigate a diverse array of cortical network dynamics, ranging from sensory processing (Haider et al., 2007; Hasenstaub et al., 2007; Lampl et al., 1999; Petersen et al., 2003; Sachdev et al., 2004), short-term synaptic plasticity (Crochet et al., 2005; Reig et al., 2006; Shu et al., 2006), communication between brain areas (Hahnet al., 2007), and the dynamics of local recurrent networks (Cossart et al., 2003; Destexhe et al., 2003; Haider et al., 2006; Luczak et al., 2007; Sanchez-Vives and McCormick, 2000). This experimental work has also inspired a series of computational studies (Compte et al., 2003b; Destexhe et al., 2007; El Boustani et al., 2007; Holcman and Tsodyks, 2006; Parga and Abbott, 2007; Rudolph et al., 2005).
Examination of the cortical slow oscillation in anesthetized and naturally sleeping animals has yielded considerable insight into the basic mechanisms of rapid changes in the state of cortical networks, stable propagation of these activity states, and the synchronization of activity across cortical regions. We propose that insights gained from these studies provide useful and testable predictions about the mechanisms underlying rapid changes in functional connectivity of cortical networks during behavior. The cortical slow oscillation was first described in studies of anesthetized rat auditory (Metherate and Ashe, 1993) and cat association cortex in vivo (Steriade et al., 1993b), where rhythmic oscillations of the local field potential (LFP), approximately once every second or two, were seen mirrored in the membrane potential of nearby neurons (e.g., Figure 2). This rhythmic cycle of depolarization and hyperpolarization survived thalamic lesions and transection of the corpus callosum (Steriade et al., 1993a) and even occurs in slices of many cortical regions (Cossart et al., 2003; Sanchez-Vives and McCormick, 2000; Shu et al., 2003a, 2003b), indicating that the core mechanisms of its generation exist in local cortical circuits. Similar epochs of depolarization and hyperpolarization were found in striatal and corticostriatal neurons of anesthetized rodents, where it results from synaptic bombardment originating in cortical networks (Cowan and Wilson, 1994; Stern et al., 1997). Importantly, this type of activity occurs not only in anesthesia but also naturally during slow wave sleep (SWS) (Destexhe et al., 1999; Steriade et al., 2001) and may even occur during quiet waking (Petersen et al., 2003; Poulet and Petersen, 2008). The activation and withdrawal of persistent activity during neocortical Up and Down states has been compared to pharmacologically induced network activity (Tahvildari et al., 2008), spontaneous network activity in organotypic cortical cultures (Blackwell et al., 2003; Johnson and Buonomano, 2007), and subthreshold depolarizing events in cortical dendrites (Major et al., 2008; Milojkovic et al., 2005). While the “Up/Down” terminology may be convenient for comparison of grossly similar cortical dynamics, the mechanisms of generation, time course, and consequences for neural responsiveness are dramatically different in these varying depolarizing-hyperpolarizing phenomena (for review, see Major and Tank, 2004) and different still from the pattern of activity observed in striatal neurons (Wilson and Kawaguchi, 1996). Our perspective is that the cortical Up state, or active state of the slow oscillation, as originally identified, is a synaptically generated, rhythmic oscillation of cortical origin that is seen simultaneously in the somatic, dendritic, and proximal axonal membrane potential of single neurons and in the local cortical network, as typically reflected in the local field potential (Haider et al., 2006; Kerr et al., 2005; Lampl et al., 1999; Sanchez-Vives and McCormick, 2000; Shu et al., 2006; Steriade et al., 1993b; Waters and Helmchen, 2006).
Examination of the slow oscillation reveals that the transitions between semistable states are rapid, occurring in approximately 50–100 ms (Figure 2). During the activated period, the membrane potential of an individual cortical neuron is characterized by depolarization of 15–25 mV, a modest increase in membrane conductance, and irregular action potential firing driven by irregularity in the depolarizing potentials that reach action potential threshold (Compte et al., 2003a, 2003b; Softky and Koch, 1993; van Vreeswijk and Sompolinsky, 1996). This firing irregularity is also an important hallmark of neuronal activity in awake, behaving animals (Compte et al., 2003a; Shadlen and Newsome, 1998). Many studies have demonstrated that activity in the local network generates the depolarized and moderately variable (SD of 2–3 mV) membrane potential of the Up state (Anderson et al., 2000; Destexhe et al., 2003; Haider et al., 2006; Petersen et al., 2003; Sanchez-Vives and McCormick, 2000; Shu et al., 2003b) through an interaction of inhibitory and excitatory synaptic potentials (Haider et al., 2006; Hasenstaub et al., 2005; Miura et al., 2007; Shu et al., 2003b; van Vreeswijk and Sompolinsky, 1996; Vogels and Abbott, 2009). Long-range connections play a key role in the communication, propagation, and synchronization of this locally generated activity (Timofeev et al., 2000; Volgushev et al., 2006).
Response Modulation in Active Cortical Networks: Excitatory-Inhibitory Interactions and Timing
The flow of synaptic activity through the cortex, which is highly nonlinear and only partially hierarchical, is dependent on several factors, including the amplitude of the particular synaptic event in question (i.e., the “signal”) in relation to the membrane potential and spike threshold, as well as the timing of the synaptic event in relation to other synaptic potentials (i.e., the “background”). As we have discussed, the nonlinear transformation of membrane potential depolarization to spike output will take place in each of the neurons in an ensemble, partially determining the ensuing pattern of synaptic activity and shaping how each neuron interacts with the evolving barrage of synaptic potentials. Neurons that maintain enhanced excitability will continue to interact and remain in the ensemble, while those neurons that are refractory (or actively inhibited) will transiently fall out of ensemble membership. Given these complex properties of network dynamics, it becomes critical to understand the mechanisms by which the amplitude-time course of the membrane potential of cortical neurons is controlled and how this interacts with synaptic barrages that might be considered “signals.” This key question has recently been addressed using the cortical slow oscillation as a model, since it is characterized by alternating periods of neuronal quiescence and recurrent network activity. This property of the slow oscillation allows the investigator to examine the influence of background, or spontaneous, synaptic barrages on, for example, responses to sensory evoked synaptic potentials constituting “signals.”
Intracellular recordings of synaptic activity in vivo during Up states of the slow oscillation, as well as during sensory-evoked responses, reveal that cortical networks operate through a remarkable balance of synaptic excitation and inhibition (Haider et al., 2006; Higley and Contreras, 2006; Marino et al., 2005; Shu et al., 2003b; Wehr and Zador, 2003). In general, as synaptic excitation to a cortical region increases, so does the level of inhibition, owing to the highly recurrent nature of intracortical excitatory and inhibitory networks (Binzegger et al., 2004; Douglas and Martin, 2007b; Haider et al., 2006; Kapfer et al., 2007; Murphy and Miller, 2009; Sanchez-Vives and McCormick, 2000; Shu et al., 2003b; Silberberg and Markram, 2007; van Vreeswijk and Sompolinsky, 1996; Vogels and Abbott, 2009). This general balancing of excitatory and inhibitory inputs to cortical neurons and networks must be generated in large part locally, since long-range inhibitory connections are rare in the neocortex. Intracellular analysis of sustained activity in cortical networks as seen during Up states reveals that while the general level of membrane potential is set by the excitatory-inhibitory balance and the intensity of synaptic barrages, the moment-to-moment variations of membrane potential are determined by rapid fluctuations in the timing of excitatory, and especially inhibitory, synaptic potentials. In other words, spike generation on a short timescale is determined by rapid departures from a precise excitatory-inhibitory balance, which lies on top of a stable depolarization that is mediated by a more prolonged excitatory-inhibitory balance (Gabernet et al., 2005; Hasenstaub et al., 2005; Higley and Contreras, 2006; Kapfer et al., 2007; Okun and Lampl, 2008; Pouille and Scanziani, 2001; Rudolph et al., 2007; Vogels and Abbott, 2009). This is important because there are generally two synaptic components underlying action potential timing in cortical neurons. The broader, less temporally precise component is generated through recurrent excitatory and inhibitory interactions that set the basal—yet variable—membrane potential of the neuron. These broad changes in excitability are visible preferentially at lower frequencies (<10 Hz). In addition to this component, rapid, higher-frequency (30–100 Hz) fluctuations in inhibitory and excitatory synaptic potentials determine the precise probability and timing of action potential generation, even to the millisecond level (Cardin et al., 2009; Hasenstaub et al., 2005; Higley and Contreras, 2006; Mainen and Sejnowski, 1995; Nowak et al., 1997; Pouille and Scanziani, 2001). Importantly, it is the higher-frequency components of membrane potential fluctuations that most strongly influence the precise timing of action potentials (Mainen and Sejnowski, 1995; Nowak et al., 1997) and have thus been proposed to play an important role in determining functional connectivity among populations of cortical neurons (Salinas and Sejnowski, 2001; Womelsdorf et al., 2007).
These results emphasize the interactions of different temporal bandwidths and “windows of opportunity” in cortical processing (Figure 2; see also Canolty et al., 2006; Sirota et al., 2008). Depolarizing a cortical neuron by 15–25 mV from true resting potential to firing threshold may require the summed efforts of prolonged (e.g., seconds or longer) depolarizations mediated by ongoing recurrent network activation (e.g., window 1, Figure 2), in addition to shorter-duration (e.g., tens to hundreds of ms) events that are brought about through temporary coactivation of a subpopulation of cortical neurons resulting in rapid changes in the excitatory/inhibitory balance (e.g., windows 2 and 3, Figure 2). One advantage to such a framework of temporarily increased recurrent excitation balanced with inhibition is that the network can maintain a dynamic range that is close to spike threshold, and thereby is capable of responding rapidly to small and fast fluctuations in ensemble dynamics (Kapfer et al., 2007; Murphy and Miller, 2009; Pouille and Scanziani, 2001; Rudolph and Destexhe, 2003; Silberberg and Markram, 2007; Swadlow, 2002; Vogels and Abbott, 2009; Wilent and Contreras, 2005). A concomitant function of this fast-responding, balanced regime is an ongoing check on runaway excitation that is present in pathological states, such as cortical seizure activity (McCormick and Contreras, 2001) and perhaps even during disordered information flow characteristic of higher cognitive dysfunctions (Aghajanian, 2009; Vogels and Abbott, 2009).
The chief advantage of this relatively balanced activity is its dynamic nature: momentary excesses of excitation, or rapid withdrawal of inhibition, may transiently depolarize the membrane potential and lead to enhanced spike generation. The time frame of these imbalances of excitation or inhibition may be as little as only a few milliseconds, as evidenced by regular-spiking (RS) pyramidal neuron firing leading fast-spiking (FS) inhibitory neuron activity by −4 ms during fast network activation; this temporal lag has also been confirmed by direct measurement of EPSPs and IPSPs in nearby pairs of pyramidal neurons (Hasenstaub et al., 2005; Okun and Lampl, 2008). Moreover, studies in behaving animals suggest that a time window equal to the period of the gamma oscillation (−10–30 ms) may be a critical constraint for the dynamic coupling of interacting neural populations (for review, see Fries et al., 2007; Harris, 2005). In support of this crucial temporal window, the membrane potential trajectory preceding sensory evoked spikes is often composed of a rapid hyperpolarization-depolarization sequence occurring within −20 ms (around 50 Hz), presumably mediated by a dynamic interaction of inhibitory and excitatory neurons and synaptic potentials (Hasenstaub et al., 2005; Nowak et al., 1997; Poulet and Petersen, 2008). An important consequence of a hyperpolarizing potential followed by a rapid depolarizing potential is a lower spike threshold and a more rapid action potential response (Azouz and Gray, 2003). Furthermore, this time window of 10–30 ms is also on the same order as the membrane integration time for cortical pyramidal cells in vivo during spontaneous activity, further constraining the summation of synaptic barrages (Leger et al., 2005). Therefore, through multiple mechanisms, this narrow temporal window dictates how effectively the network responds to the timing of synaptic potentials (Azouz, 2005; Singer and Gray, 1995). Accordingly, intracellular studies have revealed that facilitation of spiking responses during increased levels of network activity is accompanied by a heightened sensitivity to rapid depolarizations (indicative of synchronized synaptic barrages), due to a shorter membrane time constant and accumulation of sodium channel inactivation (Azouz and Gray, 1999; Henze and Buzsaki, 2001; Nowak et al., 1997). Together these observations suggest that rapid, balanced interactions between excitatory and inhibitory circuits in active local networks dictate neuronal sensitivity to synchronized synaptic inputs.
The excitatory-inhibitory interactions leading to such precise moments of enhanced excitability have also been examined in vitro, where spike-triggered averages of randomly fluctuating artificial excitatory and inhibitory conductance stimuli revealed that the withdrawal of inhibition may be at least as important as increased excitation for the production of action potentials (Hasenstaub et al., 2005; Piwkowska et al., 2008; Tiesinga et al., 2008). Analytical methods have also suggested that withdrawal of inhibition may be critical for production of spikes in vivo (Azouz and Gray, 2008; Rudolph et al., 2007). Further, when synaptic conductances are pharmacologically separated in vivo, it is observed that there is more variance in the inhibitory rather than the excitatory conductances, mirroring the observed increase in power at higher frequencies in IPSPs over EPSPs during network activation (Haider et al., 2006; Hasenstaub et al., 2005). These results strongly suggest that fast variations in membrane potential that define windows of neuronal excitability depend critically upon distinct subnetworks of inhibitory neurons and their interactions with locally and distantly projecting excitatory cells.
Of the wide variety of inhibitory interneurons in the cerebral cortex (Markram et al., 2004), the activity of fast-spiking (FS) interneurons, especially basket and chandelier cells, is of particular relevance to the control of rapid variations in membrane potential at the soma and proximal axon, since FS interneurons synapse predominantly onto the soma, axon initial segment, and proximal dendrites of cortical pyramidal cells (Buzsaki and Chrobak, 1995; Csicsvari et al., 2003; Penttonen et al., 1998; Peters, 1984). Not only are the synaptic terminals of these cells well-placed to control the spike output of cortical pyramidal neurons, but many of the physiological aspects of FS interneurons allow these cells to communicate activity across a broad range of temporal frequencies. FS interneurons generate short-duration action potentials and are capable of discharging at high frequencies with little spike frequency adaptation (McCormick et al., 1985; Nowak et al., 2003). The responses of their membranes to the injection of broad-band fluctuations show less decrement of higher frequencies in comparison to other cell types (Destexhe et al., 2001; Hasenstaub et al., 2005), a fact that results in part from the shorter membrane time constant of FS interneurons (Cruikshank et al., 2007). FS interneurons are better able to maintain synaptic transmission at higher frequencies than RS pyramidal cells (Galarreta and Hestrin, 1998; Varela et al., 1999), and they receive kinetically faster EPSPs from presynaptic excitatory cells (Thomson et al., 2002). FS interneurons also form direct electrical synapses with one another, enhancing their synchronization (Connors and Long, 2004). FS interneurons in vivo discharge with a wide range of frequencies, including synchronization to gamma (30–80 Hz) oscillations (Cardin et al., 2007; Csicsvari et al., 1999; Hasenstaub et al., 2005; Penttonen et al., 1998), and are characterized by a marked sensitivity to the activation of afferent inputs (Azouz et al., 1997; Cruikshank et al., 2007; Hirsch et al., 2003; Jones et al., 2000; Nowak et al., 2007; Swadlow et al., 1998). Finally, direct and temporally precise activation of FS neurons through genetically encoded light-activated channels results in the specific enhancement of higher-frequency oscillations in cortical networks (Cardin et al., 2009). Together, these results indicate that the cortical FS interneuron network is responsible for conveying broad-band frequency fluctuations in postsynaptic targets, thereby controlling the probability and timing of action potential generation in pyramidal neurons that contribute to both local network interactions as well as more distant cortico-cortical communication.
All of these results together suggest that rapid modulation of functional connectivity across cortical areas is critically regulated by local inhibitory subnetworks responsible for precise spike timing in nearby pyramidal neurons and thus proper control of information flow. The cortex contains numerous subtypes of interneurons, in addition to the FS subtype, that collectively exhibit a rich repertoire of local network excitatory and inhibitory interactions, the details of which are only beginning to be revealed (Fuentealba et al., 2008; Kapfer et al., 2007; Kawaguchi, 1997; Klausberger et al., 2003; Markram et al., 2004; Silberberg and Markram, 2007). Similarly, subclasses of cortical pyramidal cell can be distinguished based upon their anatomical connections and morphologies (DeFelipe et al., 2002), intrinsic electro-physiological properties (Connors and Gutnick, 1990; Douglas and Martin, 2007a; McCormick et al., 1985; Nowak et al., 2003), and responses to neurotransmitters (Gil et al., 1997; McCormick, 1992). As with interneurons, these properties are nonrandomly distributed, yielding strong correlations between all of these measures (Nelson et al., 2006). Of particular relevance to our discussion is the finding that connections between cortical neurons are also highly nonrandom, with preferred pathways and subcircuits appearing to be the rule rather than the exception (Petreanu et al., 2009; Sawatari and Callaway, 2000; Song et al., 2005).
The distribution of single excitatory postsynaptic potential amplitudes between cortical neurons is highly skewed, with a small but significant fraction exhibiting large values (e.g., several mV; Feldmeyer et al., 1999; Markram et al., 1997). During any moment in time, the pathways that exhibit large amplitudes may be considered preferred, while those eliciting small-amplitude responses (or failures) are nonpreferred, with a more moderate range of synaptic strengths in between these two extremes. The occurrence of bidirectional connectivity among nearby pyramidal neurons is substantially higher than chance, as is the probability that a third neuron forms a functionally connected “triplet” with these two interconnected neurons (Song et al., 2005). Moreover, the strength of synaptic connections between neurons within the input layer 4 may be quite specific and restricted to only those neurons that share the same feed-forward inputs from the thalamus (Feldmeyer et al., 1999). The feed-forward transfer of information from input layer 4 neurons to superficial layer neurons is also highly specific; interconnected neurons in layer 2/3 also share inputs from common pools of layer 4 neurons. However, these pools of feed-forward inputs are not shared if the neurons in layers 2/3 are not connected to one another (Feldmeyer et al., 1999; Shepherd and Svoboda, 2005; Yoshimura et al., 2005). Thus, nearby pyramidal neurons form highly specific connections that establish unique, but flexible, cortical subnetworks.
Of particular importance to our discussion is the finding that, regardless of the connectivity relationships among pairs of neurons, superficial layers receive equipotent global excitatory synaptic inputs from deep layers and equipotent global inhibitory input from within a layer (Yoshimura et al., 2005). This raises the intriguing possibility that nearby and spatially overlapping neuronal populations that are preferentially interconnected may be modulated cohesively by more global excitatory and inhibitory background activity. Moreover, the amplitude of postsynaptic potentials between cortical pyramidal neurons may be facilitated by depolarization of the somatic membrane potential (Shu et al., 2006), adding an additional “analog” mechanism of enhanced interactions among functionally coactive subnetworks of pyramidal neurons. All of these studies together suggest that the functional connectivity among neurons in preferred subnetworks could be rapidly modulated by synaptic barrages resulting from low levels of sustained activity across the larger neural population, as we have described. What is the evidence that ongoing changes in global excitatory and inhibitory background activity levels may rapidly modulate responsiveness to specific “signals,” such as sensory evoked synaptic potentials?
Rapid Modulation of Sensory Responsiveness in Recurrent Networks In Vivo
It is essential to efficient behavior that the responsiveness of cortical neurons and networks be rapidly modulated according to changes in context or shifts in behavioral demands. In the visual cortex, several studies have examined how ongoing changes in spontaneous activity (as measured, for example, by the EEG) affect visual response properties (e.g., Haider et al., 2007; Livingstone and Hubel, 1981; Worgotter et al., 1998). Perhaps the simplest and most instructive example for our discussion is a study in which the sensory response during the lack of network activity (during the Down state of the slow oscillation) was compared with sensory responses elicited when the cortical network was strongly activated (during the Up state) (Haider et al., 2007). These experiments clearly showed that the spiking response to short duration (50 ms) visual stimulation of the receptive field was enhanced, more than two-fold, when primary visual cortical networks were active versus silent. Importantly, this enhancement during the Up state was continuously modulated by ongoing variations in network activity levels. Comparing visual responses elicited during different levels of the activated (Up) state revealed that the input-output curve underwent a multiplicative-like increase in gain (of up to 60%) with depolarization of the recorded neuron induced by increases in local network activity (Figure 3F). Responses to the intracellular injection of synaptic conductance-like stimuli in these same neurons also revealed response enhancement, indicating that the increase in responsiveness was due to the neuronal membrane potential level and not purely the result of alterations in presynaptic activity (Haider et al., 2007; Shu et al., 2003a). In fact, the dominant factor determining the enhanced cortical response in this case was the level of membrane potential depolarization, not the magnitude of the sensory-evoked synaptic potential, since direct depolarization of visual cortical neurons also caused a multiplicative-like increase in neuronal gain (Figure 3D) (Sanchez-Vives et al., 2000). Could such simple mechanisms underlie response enhancement as observed in behaving animals?
Perhaps the most well-known example of cortical response enhancement due to cognitive state is found in tasks engaging attention. Attentional signals have been proposed to arise from higher cortical areas (“top-down”) that facilitate the interactions between neurons in lower cortical areas that encode object features that are to be detected (Hahnloser et al., 2002; Miller and Cohen, 2001; Reynolds and Chelazzi, 2004). Attentional modulation of neuronal responsiveness is a powerful example of interareal gain control and has been particularly well studied in the visual system (Desimone and Duncan, 1995; Fries et al., 2001; Lee and Maunsell, 2009; Martinez-Trujillo and Treue, 2004; Maunsell and Treue, 2006; McAdams and Reid, 2005; Reynolds and Heeger, 2009; Reynolds et al., 2000). Spatial attention can rapidly increase neuronal response amplitude by up to 40% or more, with results that are consistent with both a shift along the input axis (e.g., contrast gain enhancement [Reynolds et al., 2000]) as well as multiplicative response gain (Martinez-Trujillo and Treue, 2004; Williford and Maunsell, 2006; see also Lee and Maunsell, 2009; Reynolds and Heeger, 2009). Although intracellular examination of the synaptic mechanisms producing these alterations is not yet possible in behaving animals, in vitro recording, computational models, and intracellular recording studies in anesthetized animals in vivo all suggest that response facilitation may occur through simple depolarization of membrane potential via balanced excitatory and inhibitory synaptic bombardment.
Can small amounts of depolarization have a behaviorally relevant effect? The firing rate of cortical neurons is rather sensitive to membrane potential, increasing by an average of three to seven spikes/s per mV of depolarization (depending on the position along the input-output curve, Figure 1A) (Carandini and Ferster, 2000; Haider et al., 2007; Sanchez-Vives et al., 2000). These results suggest that the ongoing variations in membrane potential that occur in the waking state, which cover a range of −10 mV, remarkably similar to the range of the Up state (Steriade et al., 2001), may have a very strong, multiplicative-like effect on neuronal responsiveness. Indeed, studies of behaving primates occasionally show increases in spontaneous activity of a few spikes/s upon engagement of selective attention, even in the absence of visual stimuli (Luck et al., 1997; Reynolds et al., 2000; Sundberg et al., 2009). We suspect that attention may result in a slight depolarization of cortical neurons in the attended sensory dimension (which may be spatial location, form, color, motion, etc.), a mechanism that should also be accompanied by an increase in background activity levels. Since depolarization of ≤1 mV may cause a significant increase in neuronal responsiveness, in both excitatory and inhibitory neurons (e.g., three to seven spikes/s; Carandini and Ferster, 2000; Contreras and Palmer, 2003), we suspect that gain modulation in subnetworks of excitatory and inhibitory neurons engaged during behavior may be achieved by moderate average changes in the membrane potential, ranging from submillivolt up to several millivolts. These small, rapid changes in membrane potential are distinct from those mediated by classical Up and Down states, although they may use similar network mechanisms.
How is membrane potential rapidly modulated in individual neurons along with changing background activity levels? All of our observations thus far suggest a basic mechanism for sensory response facilitation: a balanced mixture of excitatory and inhibitory synaptic bombardment tonically depolarizes target neurons. Importantly, as long as the reversal potential of this mixture of excitatory and inhibitory barrages is more positive than the resting membrane potential, the neuron will rapidly depolarize. This depolarization will be counteracted by the activation of intrinsic K+ conductances and facilitated by the activation of the persistent Na+ current (Bean, 2007; Llinas, 1988; Waters and Helmchen, 2006). This net synaptic depolarization in the presence of a noisy baseline can yield a nonlinear, multiplicative- like increase in neuronal gain (Murphy and Miller, 2003). This enhanced activity is subsequently disseminated within a coactivated ensemble of neurons, both local and distant (Figure 1B). The increased responsiveness of these coactivated neurons may result in facilitated interactions between the members, resulting in a competitive advantage over other groups of neurons. Thus, moderate amounts of depolarization in subgroups of local cortical neurons may have a powerful, network-wide effect upon responsiveness and provide the main cellular mechanism for rapidly altering functional connectivity in cortical networks. The facilitating interactions may then allow the activity of coactivated neurons to temporarily increase their discharge in a “pop-out” effect employing “winner-take-all” or “attractor” dynamics. Such dynamics are likely to underlie diverse behavioral phenomena such as binocular rivalry, contextual modulation, binary decision making, and working memory (Albright and Stoner, 2002; Ardid et al., 2007; Barraclough et al., 2004; Gilbert and Sigman, 2007; Hahnloser et al., 2002; Logothetis, 1998; Luna et al., 2005; Rutishauser and Douglas, 2008; Vogels et al., 2005; Wang, 2008; Wong et al., 2007). Since the activity of neuronal populations is constantly changing and influencing other populations, a continual and dynamic flow of synaptic barrages provides the context upon which neuronal signals interact. The moment-to-moment evolution of synaptic activity not only links groups of neurons and cortical territories together but also links the past to the present and future of cortical network behavior (e.g., Harris, 2005).
Rapid Modulation of Membrane Potential and Background Activity during Waking
In the preceding sections, we have detailed how specific properties of balanced excitatory and inhibitory synaptic barrages may result in transient depolarizations that interact with nonlinear spike transformation to modulate the gain and functional connectivity of cortical networks. What is the evidence that the mechanisms revealed in anesthetized animals and in slices in vitro are present in awake animals? Intracellular recordings in waking rodents, cats, and monkeys indicate that neuronal activity in the awake cortex exhibits an activated baseline state, similar to that of the Up state of slow wave sleep (Chen and Fetz, 2005; Crochet and Petersen, 2006; Lee et al., 2006; Steriade et al., 2001). Indeed, the transition from slow wave sleep to waking is associated with a loss of Down states, giving the impression that the waking state is a prolonged Up-like state that merely lacks rhythmic hyperpolarization or deactivation (Steriade et al., 2001). These findings suggest that understanding the cellular mechanisms of the Up state of slow wave sleep and anesthesia may yield clues about the basic mechanisms of the baseline waking state (Destexhe et al., 2007).
Interwoven with this baseline activation, there are rapid modulations of neuronal responsiveness and activity, in relation to behavioral demands and cortical processing (Brecht et al., 2004b; Ferezou et al., 2006; Fries et al., 2007; Pesaran et al., 2002; Poulet and Petersen, 2008; Singer and Gray, 1995). The baseline membrane potential of the waking animal exhibits a steady depolarization such that it is typically within a few millivolts of firing threshold, but variations of up to several millivolts rapidly occur around this baseline level (Chen and Fetz, 2005; Crochet and Petersen, 2006; Lee et al., 2006; Steriade et al., 2001). There are further important variations on this theme of sustained activation or depolarization during waking. First, the waking cortex exhibits a broad range of states that correlate with the general level of arousal (e.g., drowsiness, quiet waking, alert, attentive, active behavior, etc. [e.g., Bezdudnaya et al., 2006]) as well as the specifics of the behavioral task at hand. Second, the level of depolarization and synaptic activity is likely to vary dramatically between different cell types and layers, since extracellular recordings in awake animals reveal large differences in spontaneous and evoked discharge rates between excitatory and FS inhibitory cells, as well as in cells across cortical lamina (Beloozerova et al., 2003; Brecht et al., 2004b; de Kock et al., 2007; Houweling and Brecht, 2008; Mitchell et al., 2007).
As mentioned, a key difference between slow wave sleep and active waking is that the synchronized and prolonged hyperpolarizing epochs similar to Down states appear to be relatively rare, especially during active behavior. In addition, while the transitions between Up and Down states of slow wave sleep and anesthesia exhibit broad propagation and synchrony, the occurrence and synchronization of faster oscillations of membrane potential and action potential activity of the attentive and waking brain are much more restricted to select populations of neurons (Destexhe et al., 1999; Fujisawa et al., 2008; Poulet and Petersen, 2008; Tsujimoto et al., 2008).
In the inattentive or quiet resting state, the situation may be different. Recent whole-cell recordings and voltage-sensitive dye imaging of somatosensory cortex in quietly resting rodents indicate that large regions of cortex exhibit spatiotemporally correlated waves of hyperpolarization and depolarization at 1–5 Hz, in some ways similar to Up and Down states (Crochet and Petersen, 2006; Poulet and Petersen, 2008). These large, slow oscillations of membrane potential are highly synchronized between neighboring neurons and thus generate slow oscillations in the local field potential (Poulet and Petersen, 2008). During active behavior (i.e., vibrissal whisking), the amplitude of these waves is dramatically reduced and is replaced with membrane potential dynamics that more closely resemble those previously observed in awake primates and cats (Crochet and Petersen, 2006; Ferezou et al., 2007; Poulet and Petersen, 2008). Interestingly, action potentials in individual cortical neurons during active whisking of the vibrissa are each preceded by a large (5–10 mV) fast depolarizing, presumably synaptic, event. In contrast to the slow fluctuations of the quiet waking state, this fast synaptic event during active behavior does not occur throughout the local network (Poulet and Petersen, 2008). This is expected if the action potential activity of individual cells is driven by unique combinations of relatively sparse, but synchronized, discharge within the large numbers of presynaptic cortical neurons that influence the recorded cell (DeWeese and Zador, 2006; Houweling and Brecht, 2008). We hypothesize that each action potential is initiated by the interaction of multiple synaptic components: one which collectively sets the general level of membrane potential properties (particularly the level of depolarization) and another which occurs in a well-defined subnetwork of cortical neurons, the synchronized activity of which determines the precise timing of the action potential.
How likely is it that the maintained increase in neuronal firing occurring during waking has the same basis as the maintained increase in activity associated with the Up state of the slow oscillation? These two periods of activity are similar in that they both are associated with a highly variable pattern of action potential generation (Compte et al., 2003a; Hasenstaub et al., 2005; Shadlen and Newsome, 1998), which is indicative of underlying variation in excitatory and inhibitory synaptic interactions (Mainen and Sejnowski, 1995; McCormick et al., 2007; Shadlen and Newsome, 1998; Vogels and Abbott, 2009). In addition, both changes in persistent activity in waking animals and transitions into and out of Up states can be very rapid, within 50–100 ms (Figure 2). Finally, cortical Up states are typically associated with high-frequency LFP oscillations (Figure 2) (Buzsaki and Draguhn, 2004; Destexhe et al., 2007; Hasenstaub et al., 2005) as is often seen during increases in cortical activity in behaving animals (Csicsvari et al., 2003; Fries et al., 2001, 2007; Pesaran et al., 2008; Singer and Gray, 1995). In contrast to persistent cortical activity in the waking animal, however, cortical Up states occur nearly simultaneously throughout all or nearly all neurons in the local network (Contreras and Steriade, 1995; Haider et al., 2006; Hasenstaub et al., 2005; Lampl et al., 1999; Volgushev et al., 2006), while persistent activity in the waking state can be much sparser, with neighboring neurons capable of behaving relatively independently (Genovesio et al., 2005). In particular, intracellular recordings in awake animals suggests that single spikes in a given neuron are preceded by relatively large-amplitude synaptic potentials that are not shared or visible in the membrane potential of nearby neurons (Chen and Fetz, 2005; Poulet and Petersen, 2008). Similarly, the simultaneously recorded activity across large populations of neurons may be highly independent and exhibit significant trial-to-trial variability (Greenberg et al., 2008; Kerr et al., 2007; Ohki et al., 2005). If nearby neurons receive relatively unique constellations of strong connections amidst a sea of weaker ones, it becomes possible that highly specific and sparse transmission of sensory responses occurs even if background activity levels are shared (Olshausen and Field, 2004). In this manner, small differences (<1 to a few millivolts) in synaptic potentials, riding on top of a depolarized baseline membrane potential, would produce nonlinear increases in spike output, as we have already detailed, and facilitate the temporary interaction of specific pathways. We suggest that although similar cellular mechanisms may be involved in both awake and anesthetized or sleeping cortical dynamics, network interactions in awake behaving animals are less synchronized as compared to sleeping or anesthetized conditions, restricting interactions to select subnetworks of neurons that vary with the behavioral task at hand. We presume that this parcellation of cortical networks in behaving animals depends upon the rapid interaction of cortico-cortical excitatory and local inhibitory subnetworks, utilizing similar though not identical mechanisms as those observed in anesthetized and sleeping animals.
Cortical Network Dynamics during Behavior: Neural Variability Reflects Changes in Functional Connectivity
The mechanisms that enable local cortical networks to quickly change their responsiveness through recurrent interactions have strong implications for rapid cortical dynamics in awake, behaving animals. As we have seen, recurrent networks in the cerebral cortex may control the amplitude, variability, and timing of action potential responses, so as to provide appropriate control over the spatiotemporal flow of neuronal activity elicited by the rapidly changing demands of behavior. These issues have been addressed in vivo with a variety of preparations, most notable of which are recent investigations of trial-to-trial response variability and contextual modulation of response amplitude and timing.
Repeated presentation of a sensory stimulus, or performance of a mental process or task, usually involves significant trial-to-trial variability (Shadlen and Newsome, 1998). Although portions of this variability arise from the inability to precisely control experimental variables (such as eye movements), a large part of it derives from spontaneous fluctuations in the activity and state of the cortical network (Arieli et al., 1996; Fox et al., 2007; Gur et al., 1997). A case in point: spontaneous variations in firing of motor cortical neurons during a delay period spanning stimulus delivery and ensuing motor command (i.e., when the movement was being planned) are highly correlated with the subsequent variability in velocity of the instructed movement (Churchland et al., 2006a). These firing rate variations were moderate (tens of spikes per second) and therefore may be reflective of small to moderate (<2–4 mV) changes in membrane potential. Indeed, if depolarization of neurons was responsible for increases in neuronal responsiveness, it may also explain the decrease in reaction time that is associated with trials in which the patterns of spikes (within the spike train) exhibited less variability (Churchland et al., 2006b). Sustained depolarization should cause spikes to occur earlier and more frequently (since the membrane potential is closer to threshold) and should also decrease long interspike intervals. These effects of depolarization should enhance neuronal interactions and decrease behavioral reaction times. Such results support our conjecture that trial-to-trial variability is indicative of alterations in functional connectivity, as determined by the interplay of ongoing patterns of synaptic activity with the anatomical connections of the cortex, and illustrate that changes in background activity reflect changes in behavioral performance.
While it is not yet routinely possible to directly examine membrane potential dynamics underlying variations in functional connectivity in awake, behaving animals, it is possible to examine changes in functional connectivity by performing multiple single-unit recordings. Alterations in functional connectivity that are driven by behavioral context have been recently demonstrated in the medial temporal area (MT) of the monkey visual system (Cohen and Newsome, 2008). Pairs of neurons in this motion-sensitive visual area changed the degree of their correlated activity during the display of noncoherent “noisy” stimuli depending on whether the animal was attempting to detect motion in the direction shared by the receptive field properties of the two neurons, or in an orthogonal, nonshared direction (Cohen and Newsome, 2008). This change in correlated activity that depended upon behavioral context indicates that single cells were able to rapidly and flexibly participate in different ensembles of interacting neurons, even in the presence of identical visual stimuli. Modulation of neuronal interactions by internal activity states has also been demonstrated in simultaneous ensemble recordings from >100 rat prefrontal cortical neurons while animals ran a maze for a food reward. These experiments showed that putative monosynaptic influences appeared in spike cross-correlation histograms transiently and only during specific portions of the task (Fujisawa et al., 2008) (Figures 4A and 4B). Subsequently, diagrams of functionally interconnected networks of cells revealed large, distributed functional neuronal ensembles in the medial prefrontal cortex, in which the participating members change according to the behavioral performance of the animal and the spike history of the neuron and network (Figures 4C and 4D). These recordings also support the idea that the cortex operates through a relatively sparse code in which individual neurons discharge relatively rarely (average firing rate of <1 Hz), but with transient and marked increases in firing occurring in response to activation of particular patterns of network activity tied to unique behavioral states (Olshausen and Field, 2004; Shoham et al., 2006). Such rapid alterations in prefrontal network dynamics could readily communicate with specific motor cortical ensembles to control the time of action (Narayanan and Laubach, 2006).
Figure 4. State-Dependent Modifications of Functional Connectivity during Behavior.
(A) Short-latency (putative monosynaptic) neuronal interactions between simultaneously recorded putative pyramidal (red) and inhibitory (blue) cells in rat prefrontal cortex during the performance of a maze task, as judged from extracellular recordings and cross-correlation spike histograms.
(B) For this pair, the strength of interaction varied as a function of spatial position in the maze, with the strongest interactions apparent near the decision point of the maze (middle).
(C) Local network diagram constructed from ensembles of simultaneously recorded pyramidal and putative inhibitory neurons reveal similar short-latency enhancement of monosynaptic interactions that are spatially dependent and whose strength varies significantly with behavioral demands. Interactions in red (1, 2) are significantly enhanced on rightward turns during maze running.
(D) Same ensemble of neurons upon leftward turns during maze running. Some functional connections that are spatially modulated are present in both directions of running (2), while others appear (3) or disappear (1) during leftward turns, indicating rapid changes in functional connectivity during ongoing behavior. Adapted by permission from Macmillan Publishers Ltd: Nature Neuroscience (Fujisawa et al., 2008), copyright 2008.
Although the cellular mechanisms underlying transient periods of functional connectivity cannot be examined with extracellular recordings, several possibilities exist, including synaptic facilitation and depression, or network interactions involving the large number of interposed neurons that are not recorded. For example, the facilitation of a monosynaptic excitatory interaction between two neurons may be achieved through the rapid depolarization of both the pre- and postsynaptic cells by a third neuron (or neurons), allowing the transient appearance of this monosynaptic connection in the extracellularly recorded spike-rate histograms during restricted portions of behavior. Keep in mind that this depolarization could result from increases in excitation, withdrawal of inhibition, or some balanced mixture between the two. A mechanism relying upon depolarization of interacting or interposed neuronal groups to rapidly facilitate functional connections, in highly specific task-dependent ways, implies that the patterns of depolarization in individual cortical neurons selectively regulate how information flows through the cortical network. This routing of information by transient increases in net depolarizing synaptic bombardment, or withdrawal of net hyperpolarizing synaptic activity, will occur in nearby and often spatially overlapping neuronal populations. Changes in synaptic bombardment could modulate overlapping circuits differentially owing to differences in the timing of synaptic potentials received by these networks, differences in the amplitude of synaptic interconnections, or differences in intrinsic properties of the constituent neurons, as we have discussed. Of course, these possibilities are not mutually exclusive and will require well-formulated hypotheses and experiments to elucidate the underlying mechanisms in behaving animals.
Future Directions for Examination of Rapid Cortical Dynamics
The functional analysis of neuronal circuits has always depended upon the application of some variation of three basic techniques: record, stimulate, or lesion. Current investigations into the dynamics of cortical networks are no exception, although recent advances in all three of these approaches have greatly increased the finesse of the experimenter. Ideally, to fully investigate cortical network dynamics, an investigator would have detailed information on the connectivity pattern and activity state of all the cells involved, as well as the ability to rapidly manipulate and monitor any of the constituent cells during natural behavior. The densely interconnected and ever-changing activity of intact cortical networks makes this ideal very far from practical reach now, and maybe forever. However, the application of current technologies, as well as those that are just being developed, promises to yield information that, while not all encompassing, is certainly toward this ideal.
Monitoring the activity of multiple interconnected cortical neurons is now possible through either arrays of extracellular recording electrodes (for review, see Buzsaki, 2004) or the bulk application of Ca2+-sensitive dyes (Garaschuk et al., 2006; Kerr et al., 2005; Ohki et al., 2005; Sato et al., 2007). Combining the latter with mice in which particular subpopulations of neurons are genetically labeled with fluorescent proteins may yield valuable information on the dynamic interactions of these identified cell types with other subpopulations of cortical neurons or during sensory stimulation (Sohya et al., 2007). This approach is being developed to study the dynamics of neural activity in vivo, using two-photon microscopy (Svoboda and Yasuda, 2006), and could be readily applied to active cortical slices in vitro (Sanchez-Vives and McCormick, 2000). One serious limitation to this technique is the relatively poor time resolution, primarily owing to the slow kinetics of neuronal Ca2+ levels (Helmchen et al., 1996). Unfortunately, many of the most interesting and relevant cortical dynamics occur at a rate that is more rapid than can be currently resolved using internal Ca2+ levels as a reporter. These limitations can be overcome with the use of voltage-sensitive dyes, but when applied extracellularly these suffer from nonspecific staining of all (including nonneuronal) cortical tissue and therefore are unable to reveal single-cell dynamics (Grinvald and Hildesheim, 2004; Homma et al., 2009). While combined extracellular or intracellular recording and imaging may yield precise temporal and/or membrane potential data, this will necessarily be limited to a small number of cells and requires a suitable preparation and significant effort and expertise on the part of the experimenter. Nonetheless, the combination of imaging and traditional electrophysiological recording methods will certainly be advantageous for the monitoring of cortical network dynamics. Important questions to be answered include: What is the network basis of large, fast synaptic events that give rise to action potentials? Do action potentials arise from the activation of a unique combination of sparse yet reliable neurons in restricted subnetworks, or do spikes mainly arise from distributed and heterogeneous patterns of activity? If there are repeating patterns, do they occupy succinct spatial and temporal bandwidths? How do these two different components interact to give rise to sensory-evoked action potentials?
On the side of stimulation and lesion techniques, it is now possible, using optogenetic techniques, to selectively depolarize or hyperpolarize the membrane potential of selected subgroups of neurons with the application of light (Boyden et al., 2005; Zemelman et al., 2002; Zhang et al., 2007). Channelrhodopsin and halorhodopsin are two light-sensitive and genetically encodable proteins that depolarize or hyperpolarize the membrane potential and can be delivered to subpopulations of neurons through the use of cell-specific promoters, retroviral delivery, or in utero electroporation (Cardin et al., 2009; Gradinaru et al., 2007; Huber et al., 2008; Nagel et al., 2003; Petreanu et al., 2007). This methodology has already been used to optically stimulate neural populations that drive perception and behavior (Douglass et al., 2008; Huber et al., 2008) and has also been used to change global brain state (Adamantidis et al., 2007). It is likely that the expression of these light-sensitive channels will be under increasingly more precise genetic control (e.g., the Cre-lox system), allowing investigators to make use of the ever increasing variety of mice that express Cre in specific subpopulations of neurons (Cardin et al., 2009; Luo et al., 2008). The resulting expression of light-sensitive channels/transporters in subpopulations of neurons will allow investigators to control the membrane potential of these cells specifically and noninvasively. This technique could be used to examine the influence of changes in membrane potential of a particular subclass of neurons (e.g., FS interneurons) on the operation of the network (Cardin et al., 2009). The targeting of light-sensitive channels/transporters to particular locations in neurons (e.g., axon initial segment, apical and basal dendrites) would also be useful for examining the role of each of these substructures in cortical network dynamics (Gradinaru et al., 2007). One general question that could be addressed is: Does subthreshold manipulation of membrane potential, conductance, and variance in subnetworks and/or substructures of pyramidal neurons confer a competitive advantage such that these cells exhibit enhanced network interactions, e.g., during bistable sensory perception or binary decision making? As all techniques have their advantages and limitations, significant difficulties to these optogenetic strategies remain, particularly in the titration of expression level needed for subthreshold or suprathreshold activation, the cell-type specificity of channel expression, as well as the toxicity or compensatory changes induced by specific levels of protein expression or ionic disruption. Careful researchers will always keep in mind that any lesion or stimulation technique suffers from problems of interpretation due to, for example, rapid compensatory mechanisms.
As always, constrained and testable hypotheses on the mechanisms of cortical dynamics are of utmost value to the experimenter. One key hypothesis put forth in our discussion is that behaviorally relevant changes in neuronal excitability should be associated with changes in membrane potential, conductance, and variance. As we have emphasized throughout this review, the interaction of known (e.g., sensory) “signals” with changes in background activity (“noise”) are particularly important for the rapid modulation of neuronal responsiveness. For example, increases in neuronal excitability associated with shifts in attention may be mediated by either depolarization of cortical neurons through changes in ongoing barrages of synaptic activity (Haider et al., 2007; Murphy and Miller, 2003) or by decreases in membrane potential variance and conductance owing to decreases in ongoing synaptic barrages (Chance et al., 2002). Both of these hypotheses are testable with intracellular and/or whole-cell recordings in awake behaving animals, a technique that is thankfully starting to move out of the shadows as a party trick and into the limelight as a serious tool for the neurophysiologist (Brecht et al., 2004a; Chen and Fetz, 2005; Houweling and Brecht, 2008; Lee et al., 2006; Petersen et al., 2003; Poulet and Petersen, 2008; Timofeev et al., 2001).
Finally, our understanding of feed-forward (i.e., thalamic) activation of sensory cortical networks is relatively well-understood compared to our understanding of the mechanisms by which long-range and feed-back connections activate cortical circuits. This is of crucial importance to our understanding of rapid cortical dynamics, for it is the distributed activity traversing long-range connections across multiple cortical areas that underlies the unity and diversity of behavior. We suspect that long-range synaptic connections have the same specificity and “preferred” functionality as observed in local cortical subnetworks, and these properties may allow, for example, specific subnetworks of neurons to perform local versus long-range computations (Le Be et al., 2007; Petreanu et al., 2007). In combination with the above-mentioned techniques, it may become possible to selectively perturb and reroute cortical activity during behavior and functionally manipulate higher-level perception, e.g., altering motion discrimination by manipulating neuronal signals in primate MT or altering the memory of specific visual cues by perturbing persistent activity in prefrontal cortex. Given the pace of technological development and experimental application in just the last 30 years since the first single-channel patch-clamp recordings (Neher and Sakmann, 1976; Sakmann, 2006), this short list of dream experiments will likely come to reality in the not-so-distant future.
Conclusion
A fundamental task of cortical network operation is the dynamic and rapid modulation of neuronal responsiveness according to the ever-changing demands of behavior. Anatomical constraints dictate that the majority of a single neuron’s activity is determined by fluctuations in local network activity levels, which necessarily engage the unique recurrent properties of excitatory and inhibitory subnetworks. We have outlined how the challenge of rapid flexibility faced by the cortex could be solved by a simple and general mechanism: modulation of neuronal membrane potential through concerted synaptic bombardment in specific excitatory and inhibitory subnetworks of functionally connected neurons. This bombardment causes a noisy and elevated level of depolarization that can enhance responsiveness rapidly and in a multiplicative manner to a variety of inputs. Precise control of membrane potential fluctuations via local network activity may serve as the main mechanism to dynamically link one cortical area to another, one cortical neuron to another, and even substructures of individual neurons (e.g., dendritic branches) together. Therefore, synaptic barrages operate in a holistic manner: functionally associating and dissociating information across vast cortical territory, while simultaneously modulating interactions both between and within individual cortical neurons. This fundamental operating principle of the cortex utilizes the interaction of a highly recurrent local cortical network architecture with intrinsic nonlinearities to rapidly and dynamically alter neuronal responsive to incoming inputs. These enhanced local network interactions in turn facilitate communication with distant cortical neurons through long-range connections. By selectively and temporally modulating the ongoing patterns of activity in distal cortical regions, shared windows of excitability— determined by the biophysical rules of recurrent networks—serve to transiently link one cortical area to another, facilitating network computation and synchronous arrival of information in downstream targets. Our understanding of the rapid control of synaptic barrages in local cortical networks has gained considerably from the study of activated cortical states in a diversity of preparations, from anesthetized, sleeping, and behaving animals, to cortical slices in vitro and models in silico. A continued dialog between these and other rapidly emerging physiological and computational approaches will be necessary to unravel the full details of local and large-scale network interactions of the cerebral cortex.
ACKNOWLEDGMENTS
We thank past and present members of the McCormick lab, James Mazer, Jon Touryan, Mark Laubach, and the anonymous reviewers for critical and helpful comments. This work was supported by the NIH, NINDS, NEI, and the Kavli Institute for Neuroscience.
REFERENCES
- Abeles M, Bergman H, Gat I, Meilijson I, Seidemann E, Tishby N, Vaadia E. Cortical activity flips among quasi-stationary states. Proc. Natl. Acad. Sci. USA. 1995;92:8616–8620. doi: 10.1073/pnas.92.19.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian GK. Modeling “psychosis” in vitro by inducing disordered neuronal network activity in cortical brain slices. Psychopharmacology (Berl) 2009 doi: 10.1007/s00213-009-1484-9. in press. Published online February 25, 2009. 10.1007/s00213-009-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright TD, Stoner GR. Contextual influences on visual processing. Annu. Rev. Neurosci. 2002;25:339–379. doi: 10.1146/annurev.neuro.25.112701.142900. [DOI] [PubMed] [Google Scholar]
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu. Rev. Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Lampl I, Gillespie DC, Ferster D. The contribution of noise to contrast invariance of orientation tuning in cat visual cortex. Science. 2000;290:1968–1972. doi: 10.1126/science.290.5498.1968. [DOI] [PubMed] [Google Scholar]
- Ardid S, Wang XJ, Compte A. An integrated microcircuit model of attentional processing in the neocortex. J. Neurosci. 2007;27:8486–8495. doi: 10.1523/JNEUROSCI.1145-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- Ayaz A, Chance FS. Gain modulation of neuronal responses by subtractive and divisive mechanisms of inhibition. J. Neurophysiol. 2009;101:958–968. doi: 10.1152/jn.90547.2008. [DOI] [PubMed] [Google Scholar]
- Azouz R. Dynamic spatiotemporal synaptic integration in cortical neurons: neuronal gain, revisited. J. Neurophysiol. 2005;94:2785–2796. doi: 10.1152/jn.00542.2005. [DOI] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Cellular mechanisms contributing to response variability of cortical neurons in vivo. J. Neurosci. 1999;19:2209–2223. doi: 10.1523/JNEUROSCI.19-06-02209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Adaptive coincidence detection and dynamic gain control in visual cortical neurons in vivo. Neuron. 2003;37:513–523. doi: 10.1016/s0896-6273(02)01186-8. [DOI] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Stimulus-selective spiking is driven by the relative timing of synchronous excitation and disinhibition in cat striate neurons in vivo. Eur. J. Neurosci. 2008;28:1286–1300. doi: 10.1111/j.1460-9568.2008.06434.x. [DOI] [PubMed] [Google Scholar]
- Azouz R, Gray CM, Nowak LG, McCormick DA. Physiological properties of inhibitory interneurons in cat striate cortex. Cereb. Cortex. 1997;7:534–545. doi: 10.1093/cercor/7.6.534. [DOI] [PubMed] [Google Scholar]
- Barraclough DJ, Conroy ML, Lee D. Prefrontal cortex and decision making in a mixed-strategy game. Nat. Neurosci. 2004;7:404–410. doi: 10.1038/nn1209. [DOI] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG, Swadlow HA. Activity of different classes of neurons of the motor cortex during locomotion. J. Neurosci. 2003;23:1087–1097. doi: 10.1523/JNEUROSCI.23-03-01087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernander O, Douglas RJ, Martin KA, Koch C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc. Natl. Acad. Sci. USA. 1991;88:11569–11573. doi: 10.1073/pnas.88.24.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdudnaya T, Cano M, Bereshpolova Y, Stoelzel CR, Alonso JM, Swadlow HA. Thalamic burst mode and inattention in the awake LGNd. Neuron. 2006;49:421–432. doi: 10.1016/j.neuron.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Binzegger T, Douglas RJ, Martin KA. A quantitative map of the circuit of cat primary visual cortex. J. Neurosci. 2004;24:8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell KT, Czubayko U, Plenz D. Quantitative estimate of synaptic inputs to striatal neurons during up and down states in vitro. J. Neurosci. 2003;23:9123–9132. doi: 10.1523/JNEUROSCI.23-27-09123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg-Graham LJ, Monier C, Fregnac Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature. 1998;393:369–373. doi: 10.1038/30735. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Brecht M, Fee MS, Garaschuk O, Helmchen F, Margrie TW, Svoboda K, Osten P. Novel approaches to monitor and manipulate single neurons in vivo. J. Neurosci. 2004a;24:9223–9227. doi: 10.1523/JNEUROSCI.3344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M, Schneider M, Sakmann B, Margrie TW. Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature. 2004b;427:704–710. doi: 10.1038/nature02266. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr. Opin. Neurobiol. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu. Rev. Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Carandini M, Ferster D. Membrane potential and firing rate in cat primary visual cortex. J. Neurosci. 2000;20:470–484. doi: 10.1523/JNEUROSCI.20-01-00470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Palmer LA, Contreras D. Stimulus feature selectivity in excitatory and inhibitory neurons in primary visual cortex. J. Neurosci. 2007;27:10333–10344. doi: 10.1523/JNEUROSCI.1692-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Palmer LA, Contreras D. Cellular mechanisms underlying stimulus-dependent gain modulation in primary visual cortex neurons in vivo. Neuron. 2008;59:150–160. doi: 10.1016/j.neuron.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai L-H, Moore CI. Activation of fast spiking interneurons induces gamma oscillations and shapes sensory transmission. Nature. 2009 doi: 10.1038/nature08002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance FS, Abbott LF, Reyes AD. Gain modulation from background synaptic input. Neuron. 2002;35:773–782. doi: 10.1016/s0896-6273(02)00820-6. [DOI] [PubMed] [Google Scholar]
- Chen D, Fetz EE. Characteristic membrane potential trajectories in primate sensorimotor cortex neurons recorded in vivo. J. Neurophysiol. 2005;94:2713–2725. doi: 10.1152/jn.00024.2005. [DOI] [PubMed] [Google Scholar]
- Churchland MM, Afshar A, Shenoy KV. A central source of movement variability. Neuron. 2006a;52:1085–1096. doi: 10.1016/j.neuron.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Ryu SI, Santhanam G, Shenoy KV. Neural variability in premotor cortex provides a signature of motor preparation. J. Neurosci. 2006b;26:3697–3712. doi: 10.1523/JNEUROSCI.3762-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Newsome WT. Context-dependent changes in functional circuitry in visual area MT. Neuron. 2008;60:162–173. doi: 10.1016/j.neuron.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A, Constantinidis C, Tegner J, Raghavachari S, Chafee MV, Goldman-Rakic PS, Wang XJ. Temporally irregular mnemonic persistent activity in prefrontal neurons of monkeys during a delayed response task. J. Neurophysiol. 2003a;90:3441–3454. doi: 10.1152/jn.00949.2002. [DOI] [PubMed] [Google Scholar]
- Compte A, Sanchez-Vives MV, McCormick DA, Wang XJ. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J. Neurophysiol. 2003b;89:2707–2725. doi: 10.1152/jn.00845.2002. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu. Rev. Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J. Neurosci. 1995;15:604–622. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Palmer L. Response to contrast of electrophysiologically defined cell classes in primary visual cortex. J. Neurosci. 2003;23:6936–6945. doi: 10.1523/JNEUROSCI.23-17-06936.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Aronov D, Yuste R. Attractor dynamics of network UP states in the neocortex. Nature. 2003;423:283–288. doi: 10.1038/nature01614. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J. Neurophysiol. 1994;71:17–32. doi: 10.1152/jn.1994.71.1.17. [DOI] [PubMed] [Google Scholar]
- Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat. Neurosci. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- Crochet S, Chauvette S, Boucetta S, Timofeev I. Modulation of synaptic transmission in neocortex by network activities. Eur. J. Neurosci. 2005;21:1030–1044. doi: 10.1111/j.1460-9568.2005.03932.x. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat. Neurosci. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving Rat. J. Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol. Rev. 2006;86:1033–1048. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- de Kock CP, Bruno RM, Spors H, Sakmann B. Layer- and cell-type-specific suprathreshold stimulus representation in rat primary somatosensory cortex. J. Physiol. 2007;581:139–154. doi: 10.1113/jphysiol.2006.124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Alonso-Nanclares L, Arellano JI. Microstructure of the neocortex: comparative aspects. J. Neurocytol. 2002;31:299–316. doi: 10.1023/a:1024130211265. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J. Neurosci. 1999;19:4595–4608. doi: 10.1523/JNEUROSCI.19-11-04595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Fellous JM, Sejnowski TJ. Fluctuating synaptic conductances recreate in vivo-like activity in neocortical neurons. Neuroscience. 2001;107:13–24. doi: 10.1016/s0306-4522(01)00344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Pare D. The high-conductance state of neocortical neurons in vivo. Nat. Rev. Neurosci. 2003;4:739–751. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Hughes SW, Rudolph M, Crunelli V. Are corticothalamic ‘up’ states fragments of wakefulness? Trends Neurosci. 2007;30:334–342. doi: 10.1016/j.tins.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWeese MR, Zador AM. Non-Gaussian membrane potential dynamics imply sparse, synchronous activity in auditory cortex. J. Neurosci. 2006;26:12206–12218. doi: 10.1523/JNEUROSCI.2813-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ. Gain modulation by nicotine in macaque v1. Neuron. 2007;56:701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Mapping the matrix: the ways of neocortex. Neuron. 2007a;56:226–238. doi: 10.1016/j.neuron.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Recurrent neuronal circuits in the neocortex. Curr. Biol. 2007b;17:R496–R500. doi: 10.1016/j.cub.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Douglass AD, Kraves S, Deisseroth K, Schier AF, Engert F. Escape behavior elicited by single, channelrhodopsin-2-evoked spikes in zebrafish somatosensory neurons. Curr. Biol. 2008;18:1133–1137. doi: 10.1016/j.cub.2008.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Boustani S, Pospischil M, Rudolph-Lilith M, Destexhe A. Activated cortical states: experiments, analyses and models. J. Physiol. (Paris) 2007;101:99–109. doi: 10.1016/j.jphysparis.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Egger V, Lubke J, Sakmann B. Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single ‘barrel’ of developing rat somatosensory cortex. J. Physiol. 1999;521:169–190. doi: 10.1111/j.1469-7793.1999.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou I, Bolea S, Petersen CC. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron. 2006;50:617–629. doi: 10.1016/j.neuron.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B, Petersen CC. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–923. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fries P, Nikolic D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Begum R, Capogna M, Jinno S, Marton LF, Csicsvari J, Thomson A, Somogyi P, Klausberger T. Ivy cells: a population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron. 2008;57:917–929. doi: 10.1016/j.neuron.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa S, Amarasingham A, Harrison MT, Buzsaki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat. Neurosci. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Frequency-dependent synaptic depression and the balance of excitation and inhibition in the neocortex. Nat. Neurosci. 1998;1:587–594. doi: 10.1038/2822. [DOI] [PubMed] [Google Scholar]
- Garaschuk O, Milos RI, Konnerth A. Targeted bulk-loading of fluorescent indicators for two-photon brain imaging in vivo. Nat. Protocols. 2006;1:380–386. doi: 10.1038/nprot.2006.58. [DOI] [PubMed] [Google Scholar]
- Genovesio A, Brasted PJ, Mitz AR, Wise SP. Prefrontal cortex activity related to abstract response strategies. Neuron. 2005;47:307–320. doi: 10.1016/j.neuron.2005.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M. Brain states: top-down influences in sensory processing. Neuron. 2007;54:677–696. doi: 10.1016/j.neuron.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, Deisseroth K. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J. Neurosci. 2007;27:14231–14238. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DS, Houweling AR, Kerr JN. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat. Neurosci. 2008;11:749–751. doi: 10.1038/nn.2140. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Hildesheim R. VSDI: a new era in functional imaging of cortical dynamics. Nat. Rev. Neurosci. 2004;5:874–885. doi: 10.1038/nrn1536. [DOI] [PubMed] [Google Scholar]
- Gur M, Beylin A, Snodderly DM. Response variability of neurons in primary visual cortex (V1) of alert monkeys. J. Neurosci. 1997;17:2914–2920. doi: 10.1523/JNEUROSCI.17-08-02914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn TT, Sakmann B, Mehta MR. Differential responses of hippocampal subfields to cortical up-down states. Proc. Natl. Acad. Sci. USA. 2007;104:5169–5174. doi: 10.1073/pnas.0700222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnloser RH, Douglas RJ, Hepp K. Attentional recruitment of inter-areal recurrent networks for selective gain control. Neural Comput. 2002;14:1669–1689. doi: 10.1162/08997660260028665. [DOI] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J. Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, Yu Y, McCormick DA. Enhancement of visual responsiveness by spontaneous local network activity in vivo. J. Neurophysiol. 2007;97:4186–4202. doi: 10.1152/jn.01114.2006. [DOI] [PubMed] [Google Scholar]
- Harris KD. Neural signatures of cell assembly organization. Nat. Rev. Neurosci. 2005;6:399–407. doi: 10.1038/nrn1669. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 2005;47:423–435. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Sachdev RN, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. J. Neurosci. 2007;27:9607–9622. doi: 10.1523/JNEUROSCI.2184-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior: A Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- Helmchen F, Imoto K, Sakmann B. Ca2+ buffering and action potential-evoked Ca2+ signaling in dendrites of pyramidal neurons. Biophys. J. 1996;70:1069–1081. doi: 10.1016/S0006-3495(96)79653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Buzsaki G. Action potential threshold of hippocampal pyramidal cells in vivo is increased by recent spiking activity. Neuroscience. 2001;105:121–130. doi: 10.1016/s0306-4522(01)00167-1. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Contreras D. Balanced excitation and inhibition determine spike timing during frequency adaptation. J. Neurosci. 2006;26:448–457. doi: 10.1523/JNEUROSCI.3506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Martinez LM, Pillai C, Alonso JM, Wang Q, Sommer FT. Functionally distinct inhibitory neurons at the first stage of visual cortical processing. Nat. Neurosci. 2003;6:1300–1308. doi: 10.1038/nn1152. [DOI] [PubMed] [Google Scholar]
- Ho N, Destexhe A. Synaptic background activity enhances the responsiveness of neocortical pyramidal neurons. J. Neurophysiol. 2000;84:1488–1496. doi: 10.1152/jn.2000.84.3.1488. [DOI] [PubMed] [Google Scholar]
- Holcman D, Tsodyks M. The emergence of Up and Down states in cortical networks. PLoS Comput. Biol. 2006;2:e23. doi: 10.1371/journal.pcbi.0020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt GR, Koch C. Shunting inhibition does not have a divisive effect on firing rates. Neural Comput. 1997;9:1001–1013. doi: 10.1162/neco.1997.9.5.1001. [DOI] [PubMed] [Google Scholar]
- Homma R, Baker BJ, Jin L, Garaschuk O, Konnerth A, Cohen LB, Bleau CX, Canepari M, Djurisic M, Zecevic D. Wide-field and two-photon imaging of brain activity with voltage- and calcium-sensitive dyes. Methods Mol. Biol. 2009;489:43–79. doi: 10.1007/978-1-59745-543-5_3. [DOI] [PubMed] [Google Scholar]
- Houweling AR, Brecht M. Behavioural report of single neuron stimulation in somatosensory cortex. Nature. 2008;451:65–68. doi: 10.1038/nature06447. [DOI] [PubMed] [Google Scholar]
- Huber D, Petreanu L, Ghitani N, Ranade S, Hromadka T, Mainen Z, Svoboda K. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HA, Buonomano DV. Development and plasticity of spontaneous activity and Up states in cortical organotypic slices. J. Neurosci. 2007;27:5915–5925. doi: 10.1523/JNEUROSCI.0447-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MS, MacDonald KD, Choi B, Dudek FE, Barth DS. Intracellular correlates of fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J. Neurophysiol. 2000;84:1505–1518. doi: 10.1152/jn.2000.84.3.1505. [DOI] [PubMed] [Google Scholar]
- Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat. Neurosci. 2007;10:743–753. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Selective cholinergic modulation of cortical GABAergic cell subtypes. J. Neurophysiol. 1997;78:1743–1747. doi: 10.1152/jn.1997.78.3.1743. [DOI] [PubMed] [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature. 2003;425:954–956. doi: 10.1038/nature02078. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat. Rev. Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JN, Greenberg D, Helmchen F. Imaging input and output of neocortical networks in vivo. Proc. Natl. Acad. Sci. USA. 2005;102:14063–14068. doi: 10.1073/pnas.0506029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JN, de Kock CP, Greenberg DS, Bruno RM, Sakmann B, Helmchen F. Spatial organization of neuronal population responses in layer 2/3 of rat barrel cortex. J. Neurosci. 2007;27:13316–13328. doi: 10.1523/JNEUROSCI.2210-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat. Neurosci. 2008;11:178–186. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- Kuhn A, Aertsen A, Rotter S. Neuronal integration of synaptic input in the fluctuation-driven regime. J. Neurosci. 2004;24:2345–2356. doi: 10.1523/JNEUROSCI.3349-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampl I, Reichova I, Ferster D. Synchronous membrane potential fluctuations in neurons of the cat visual cortex. Neuron. 1999;22:361–374. doi: 10.1016/s0896-6273(00)81096-x. [DOI] [PubMed] [Google Scholar]
- Larkman AU. Dendritic morphology of pyramidal neurones of the visual cortex of the rat: III. Spine distributions. J. Comp. Neurol. 1991;306:332–343. doi: 10.1002/cne.903060209. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Senn W, Luscher HR. Top-down dendritic input increases the gain of layer 5 pyramidal neurons. Cereb. Cortex. 2004;14:1059–1070. doi: 10.1093/cercor/bhh065. [DOI] [PubMed] [Google Scholar]
- Le Be JV, Silberberg G, Wang Y, Markram H. Morphological, electrophysiological, and synaptic properties of corticocallosal pyramidal cells in the neonatal rat neocortex. Cereb. Cortex. 2007;17:2204–2213. doi: 10.1093/cercor/bhl127. [DOI] [PubMed] [Google Scholar]
- Lee J, Maunsell JH. A normalization model of attentional modulation of single unit responses. PLoS ONE. 2009;4:e4651. doi: 10.1371/journal.pone.0004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, Manns ID, Sakmann B, Brecht M. Whole-cell recordings in freely moving rats. Neuron. 2006;51:399–407. doi: 10.1016/j.neuron.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Leger JF, Stern EA, Aertsen A, Heck D. Synaptic integration in rat frontal cortex shaped by network activity. J. Neurophysiol. 2005;93:281–293. doi: 10.1152/jn.00067.2003. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981;291:554–561. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. Single units and conscious vision. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1998;353:1801–1818. doi: 10.1098/rstb.1998.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London M, Hausser M. Dendritic computation. Annu. Rev. Neurosci. 2005;28:503–532. doi: 10.1146/annurev.neuro.28.061604.135703. [DOI] [PubMed] [Google Scholar]
- Lorente de No R. Cerebral Cortex: Architecture, intracortical connections, motor projections. In: Fulton JF, editor. Physiology of the Nervous System. New York: Oxford University Press; 1938. [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J. Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Luczak A, Bartho P, Marguet SL, Buzsaki G, Harris KD. Sequential structure of neocortical spontaneous activity in vivo. Proc. Natl. Acad. Sci. USA. 2007;104:347–352. doi: 10.1073/pnas.0605643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna R, Hernandez A, Brody CD, Romo R. Neural codes for perceptual discrimination in primary somatosensory cortex. Nat. Neurosci. 2005;8:1210–1219. doi: 10.1038/nn1513. [DOI] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic integration of excitatory synaptic input. Nat. Rev. Neurosci. 2000;1:181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. Plasticity of dendritic function. Curr. Opin. Neurobiol. 2005;15:334–342. doi: 10.1016/j.conb.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Reliability of spike timing in neocortical neurons. Science. 1995;268:1503–1506. doi: 10.1126/science.7770778. [DOI] [PubMed] [Google Scholar]
- Major G, Tank D. Persistent neural activity: prevalence and mechanisms. Curr. Opin. Neurobiol. 2004;14:675–684. doi: 10.1016/j.conb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Major G, Polsky A, Denk W, Schiller J, Tank DW. Spatiotemporally graded NMDA spike/plateau potentials in basal dendrites of neocortical pyramidal neurons. J. Neurophysiol. 2008;99:2584–2601. doi: 10.1152/jn.00011.2008. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Marino J, Schummers J, Lyon DC, Schwabe L, Beck O, Wiesing P, Obermayer K, Sur M. Invariant computations in local cortical networks with balanced excitation and inhibition. Nat. Neurosci. 2005;8:194–201. doi: 10.1038/nn1391. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurones in the developing rat neocortex. J. Physiol. 1997;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo JC, Treue S. Feature-based attention increases the selectivity of population responses in primate visual cortex. Curr. Biol. 2004;14:744–751. doi: 10.1016/j.cub.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Treue S. Feature-based attention in visual cortex. Trends Neurosci. 2006;29:317–322. doi: 10.1016/j.tins.2006.04.001. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J. Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Reid RC. Attention modulates the responses of simple cells in monkey primary visual cortex. J. Neurosci. 2005;25:11023–11033. doi: 10.1523/JNEUROSCI.2904-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog. Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu. Rev. Physiol. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J. Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Shu Y, Yu Y. Neurophysiology: Hodgkin and Huxley model-still standing? Nature. 2007;445:E1–E2. doi: 10.1038/nature05523. [DOI] [PubMed] [Google Scholar]
- McGuire BA, Gilbert CD, Rivlin PK, Wiesel TN. Targets of horizontal connections in macaque primary visual cortex. J. Comp. Neurol. 1991;305:370–392. doi: 10.1002/cne.903050303. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Ionic flux contributions to neocortical slow waves and nucleus basalis-mediated activation: whole-cell recordings in vivo. J. Neurosci. 1993;13:5312–5323. doi: 10.1523/JNEUROSCI.13-12-05312.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller KD, Troyer TW. Neural noise can explain expansive, power-law nonlinearities in neural response functions. J. Neurophysiol. 2002;87:653–659. doi: 10.1152/jn.00425.2001. [DOI] [PubMed] [Google Scholar]
- Milojkovic BA, Radojicic MS, Antic SD. A strict correlation between dendritic and somatic plateau depolarizations in the rat prefrontal cortex pyramidal neurons. J. Neurosci. 2005;25:3940–3951. doi: 10.1523/JNEUROSCI.5314-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Miura K, Tsubo Y, Okada M, Fukai T. Balanced excitatory and inhibitory inputs to cortical neurons decouple firing irregularity from rate modulations. J. Neurosci. 2007;27:13802–13812. doi: 10.1523/JNEUROSCI.2452-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama M, Perez-Garci E, Nevian T, Bock T, Senn W, Larkum ME. Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature. 2009;457:1137–1141. doi: 10.1038/nature07663. [DOI] [PubMed] [Google Scholar]
- Murphy BK, Miller KD. Multiplicative gain changes are induced by excitation or inhibition alone. J. Neurosci. 2003;23:10040–10051. doi: 10.1523/JNEUROSCI.23-31-10040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BK, Miller KD. Balanced amplification: a new mechanism of selective amplification of neural activity patterns. Neuron. 2009;61:635–648. doi: 10.1016/j.neuron.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Olliq D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976;260:799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Sugino K, Hempel CM. The problem of neuronal cell types: a physiological genomics approach. Trends Neurosci. 2006;29:339–345. doi: 10.1016/j.tins.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Nevian T, Larkum ME, Polsky A, Schiller J. Properties of basal dendrites of layer 5 pyramidal neurons: a direct patch-clamp recording study. Nat. Neurosci. 2007;10:206–214. doi: 10.1038/nn1826. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Sanchez-Vives MV, McCormick DA. Influence of low and high frequency inputs on spike timing in visual cortical neurons. Cereb. Cortex. 1997;7:487–501. doi: 10.1093/cercor/7.6.487. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Azouz R, Sanchez-Vives MV, Gray CM, McCormick DA. Electrophysiological classes of cat primary visual cortical neurons in vivo as revealed by quantitative analyses. J. Neurophysiol. 2003;89:1541–1566. doi: 10.1152/jn.00580.2002. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Sanchez-Vives MV, McCormick DA. Lack of orientation and direction selectivity in a subgroup of fast-spiking inhibitory interneurons: Cellular and synaptic mechanisms and comparison with other electrophysiological cell types. Cereb. Cortex. 2007;18:1058–1078. doi: 10.1093/cercor/bhm137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki K, Chung S, Ch’ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- Okun M, Lampl I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat. Neurosci. 2008;11:535–537. doi: 10.1038/nn.2105. [DOI] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ. Sparse coding of sensory inputs. Curr. Opin. Neurobiol. 2004;14:481–487. doi: 10.1016/j.conb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Pare D, Shink E, Gaudreau H, Destexhe A, Lang EJ. Impact of spontaneous synaptic activity on the resting properties of cat neocortical pyramidal neurons In vivo. J. Neurophysiol. 1998;79:1450–1460. doi: 10.1152/jn.1998.79.3.1450. [DOI] [PubMed] [Google Scholar]
- Parga N, Abbott LF. Network model of spontaneous activity exhibiting synchronous transitions between up and down States. Front Neurosci. 2007;1:57–66. doi: 10.3389/neuro.01.1.1.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttonen M, Kamondi A, Acsady L, Buzsaki G. Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur. J. Neurosci. 1998;10:718–728. doi: 10.1046/j.1460-9568.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat. Neurosci. 2002;5:805–811. doi: 10.1038/nn890. [DOI] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Free choice activates a decision circuit between frontal and parietal cortex. Nature. 2008;453:406–409. doi: 10.1038/nature06849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. Chandelier Cells. In: Peters A, Jones EG, editors. In Cerebral Cortex. Cellular Components of the Cerebral Cortex. New York: Plenum Press; 1984. pp. 361–379. [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc. Natl. Acad. Sci. USA. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwkowska Z, Pospischil M, Brette R, Sliwa J, Rudolph-Lilith M, Bal T, Destexhe A. Characterizing synaptic conductance fluctuations in cortical neurons and their influence on spike generation. J. Neurosci. Methods. 2008;169:302–322. doi: 10.1016/j.jneumeth.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y. Gain control of firing rate by shunting inhibition: roles of synaptic noise and dendritic saturation. Proc. Natl. Acad. Sci. USA. 2003;100:2076–2081. doi: 10.1073/pnas.0337591100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D. Inhibition, spike threshold, and stimulus selectivity in primary visual cortex. Neuron. 2008;57:482–497. doi: 10.1016/j.neuron.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Priebe NJ, Mechler F, Carandini M, Ferster D. The contribution of spike threshold to the dichotomy of cortical simple and complex cells. Nat. Neurosci. 2004;7:1113–1122. doi: 10.1038/nn1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig R, Gallego R, Nowak LG, Sanchez-Vives MV. Impact of cortical network activity on short-term synaptic depression. Cereb. Cortex. 2006;16:688–695. doi: 10.1093/cercor/bhj014. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu. Rev. Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61:168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26:703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- Rudolph M, Destexhe A. A fast-conducting, stochastic integrative mode for neocortical neurons in vivo. J. Neurosci. 2003;23:2466–2476. doi: 10.1523/JNEUROSCI.23-06-02466.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph M, Pelletier JG, Pare D, Destexhe A. Characterization of synaptic conductances and integrative properties during electrically induced EEG-activated states in neocortical neurons in vivo. J. Neurophysiol. 2005;94:2805–2821. doi: 10.1152/jn.01313.2004. [DOI] [PubMed] [Google Scholar]
- Rudolph M, Pospischil M, Timofeev I, Destexhe A. Inhibition determines membrane potential dynamics and controls action potential generation in awake and sleeping cat cortex. J. Neurosci. 2007;27:5280–5290. doi: 10.1523/JNEUROSCI.4652-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U, Douglas RJ. State-dependent computation using coupled recurrent networks. Neural Comput. 2008 doi: 10.1162/neco.2008.03-08-734. in press. Published online February 11, 2009. 10.1162/neco.2008.03-08-734. [DOI] [PubMed] [Google Scholar]
- Sachdev RN, Ebner FF, Wilson CJ. Effect of subthreshold up and down states on the whisker-evoked response in somatosensory cortex. J. Neurophysiol. 2004;92:3511–3521. doi: 10.1152/jn.00347.2004. [DOI] [PubMed] [Google Scholar]
- Sakmann B. Patch pipettes are more useful than initially thought: simultaneous pre- and postsynaptic recording from mammalian CNS synapses in vitro and in vivo. Pflugers Arch. 2006;453:249–259. doi: 10.1007/s00424-006-0172-4. [DOI] [PubMed] [Google Scholar]
- Salinas E, Abbott LF. A model of multiplicative neural responses in parietal cortex. Proc. Natl. Acad. Sci. USA. 1996;93:11956–11961. doi: 10.1073/pnas.93.21.11956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat. Rev. Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat. Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Nowak LG, McCormick DA. Membrane mechanisms underlying contrast adaptation in cat area 17 in vivo. J. Neurosci. 2000;20:4267–4285. doi: 10.1523/JNEUROSCI.20-11-04267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TR, Gray NW, Mainen ZF, Svoboda K. The functional microarchitecture of the mouse barrel cortex. PLoS Biol. 2007;5:e189. doi: 10.1371/journal.pbio.0050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawatari A, Callaway EM. Diversity and cell type specificity of local excitatory connections to neurons in layer 3B of monkey primary visual cortex. Neuron. 2000;25:459–471. doi: 10.1016/s0896-6273(00)80908-3. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J. Neurosci. 1998;18:3870–3896. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Svoboda K. Laminar and columnar organization of ascending excitatory projections to layer 2/3 pyramidal neurons in rat barrel cortex. J. Neurosci. 2005;25:5670–5679. doi: 10.1523/JNEUROSCI.1173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham S, O’Connor DH, Segev R. How silent is the brain: is there a “dark matter” problem in neuroscience? J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2006;192:777–784. doi: 10.1007/s00359-006-0117-6. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Badoual M, Bal T, McCormick DA. Barrages of synaptic activity control the gain and sensitivity of cortical neurons. J. Neurosci. 2003a;23:10388–10401. doi: 10.1523/JNEUROSCI.23-32-10388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003b;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature. 2006;441:761–765. doi: 10.1038/nature04720. [DOI] [PubMed] [Google Scholar]
- Shu Y, Duque A, Yu Y, Haider B, McCormick DA. Properties of action-potential initiation in neocortical pyramidal cells: evidence from whole cell axon recordings. J. Neurophysiol. 2007;97:746–760. doi: 10.1152/jn.00922.2006. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu. Rev. Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsaki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom PJ, Rancz EA, Roth A, Hausser M. Dendritic excitability and synaptic plasticity. Physiol. Rev. 2008;88:769–840. doi: 10.1152/physrev.00016.2007. [DOI] [PubMed] [Google Scholar]
- Softky WR, Koch C. The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J. Neurosci. 1993;13:334–350. doi: 10.1523/JNEUROSCI.13-01-00334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohya K, Kameyama K, Yanagawa Y, Obata K, Tsumoto T. GABAergic neurons are less selective to stimulus orientation than excitatory neurons in layer II/III of visual cortex, as revealed by in vivo functional Ca2+ imaging in transgenic mice. J. Neurosci. 2007;27:2145–2149. doi: 10.1523/JNEUROSCI.4641-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Sjostrom PJ, Reigl M, Nelson S, Chklovskii DB. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 2005;3:e68. doi: 10.1371/journal.pbio.0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J. Neurosci. 1993a;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J. Neurosci. 1993b;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J. Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Stern EA, Kincaid AE, Wilson CJ. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J. Neurophysiol. 1997;77:1697–1715. doi: 10.1152/jn.1997.77.4.1697. [DOI] [PubMed] [Google Scholar]
- Stuart G, Schiller J, Sakmann B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J. Physiol. 1997;505:617–632. doi: 10.1111/j.1469-7793.1997.617ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg KA, Mitchell JF, Reynolds JH. Spatial attention modulates center-surround interactions in macaque visual area v4. Neuron. 2009;61:952–963. doi: 10.1016/j.neuron.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K, Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. 2006;50:823–839. doi: 10.1016/j.neuron.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Thalamocortical control of feed-forward inhibition in awake somatosensory ‘barrel’ cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:1717–1727. doi: 10.1098/rstb.2002.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA, Beloozerova IN, Sirota MG. Sharp, local synchrony among putative feed-forward inhibitory interneurons of rabbit somatosensory cortex. J. Neurophysiol. 1998;79:567–582. doi: 10.1152/jn.1998.79.2.567. [DOI] [PubMed] [Google Scholar]
- Tahvildari B, Alonso AA, Bourque CW. Ionic basis of ON and OFF persistent activity in layer III lateral entorhinal cortical principal neurons. J. Neurophysiol. 2008;99:2006–2011. doi: 10.1152/jn.00911.2007. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP, Mercer A, Morris OT. Target and temporal pattern selection at neocortical synapses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:1781–1791. doi: 10.1098/rstb.2002.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurley K, Senn W, Luscher HR. Dopamine increases the gain of the input-output response of rat prefrontal pyramidal neurons. J. Neurophysiol. 2008;99:2985–2997. doi: 10.1152/jn.01098.2007. [DOI] [PubMed] [Google Scholar]
- Tiesinga P, Fellous JM, Sejnowski TJ. Regulation of spike timing in visual cortical circuits. Nat. Rev. Neurosci. 2008;9:97–107. doi: 10.1038/nrn2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb. Cortex. 2000;10:1185–1199. doi: 10.1093/cercor/10.12.1185. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study. Proc. Natl. Acad. Sci. USA. 2001;98:1924–1929. doi: 10.1073/pnas.041430398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S, Martinez Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Genovesio A, Wise SP. Transient neuronal correlations underlying goal selection and maintenance in prefrontal cortex. Cereb. Cortex. 2008;18:2748–2761. doi: 10.1093/cercor/bhn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vreeswijk C, Sompolinsky H. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science. 1996;274:1724–1726. doi: 10.1126/science.274.5293.1724. [DOI] [PubMed] [Google Scholar]
- Varela JA, Song S, Turrigiano GG, Nelson SB. Differential depression at excitatory and inhibitory synapses in visual cortex. J. Neurosci. 1999;19:4293–4304. doi: 10.1523/JNEUROSCI.19-11-04293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels TP, Abbott LF. Gating multiple signlas through detailed balance of excitation and inhibition in spiking networks. Nat. Neurosci. 2009;12:483–491. doi: 10.1038/nn.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels TP, Rajan K, Abbott LF. Neural network dynamics. Annu. Rev. Neurosci. 2005;28:357–376. doi: 10.1146/annurev.neuro.28.061604.135637. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Chauvette S, Mukovski M, Timofeev I. Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave oscillations. J. Neurosci. 2006;26:5665–5672. doi: 10.1523/JNEUROSCI.0279-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Decision making in recurrent neuronal circuits. Neuron. 2008;60:215–234. doi: 10.1016/j.neuron.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Helmchen F. Background synaptic activity is sparse in neocortex. J. Neurosci. 2006;26:8267–8277. doi: 10.1523/JNEUROSCI.2152-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Wilent WB, Contreras D. Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nat. Neurosci. 2005;8:1364–1370. doi: 10.1038/nn1545. [DOI] [PubMed] [Google Scholar]
- Williams SR. Spatial compartmentalization and functional impact of conductance in pyramidal neurons. Nat. Neurosci. 2004;7:961–967. doi: 10.1038/nn1305. [DOI] [PubMed] [Google Scholar]
- Williford T, Maunsell JH. Effects of spatial attention on contrast response functions in macaque area V4. J. Neurophysiol. 2006;96:40–54. doi: 10.1152/jn.01207.2005. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Kawaguchi Y. The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J. Neurosci. 1996;16:2397–2410. doi: 10.1523/JNEUROSCI.16-07-02397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- Wong KF, Huk AC, Shadlen MN, Wang XJ. Neural circuit dynamics underlying accumulation of time-varying evidence during perceptual decision making. Front. Comput. Neurosci. 2007;1:6. doi: 10.3389/neuro.10.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worgotter F, Suder K, Zhao Y, Kerscher N, Eysel UT, Funke K. State-dependent receptive-field restructuring in the visual cortex. Nature. 1998;396:165–168. doi: 10.1038/24157. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Dantzker JL, Callaway EM. Excitatory cortical neurons form fine-scale functional networks. Nature. 2005;433:868–873. doi: 10.1038/nature03252. [DOI] [PubMed] [Google Scholar]
- Yu Y, Shu Y, McCormick DA. Cortical action potential back-propagation explains spike threshold variability and rapid-onset kinetics. J. Neurosci. 2008;28:7260–7272. doi: 10.1523/JNEUROSCI.1613-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemelman BV, Lee GA, Miesenbock G. Selective photostimulation of genetically chARGed neurons. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat. Rev. Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]