Abstract
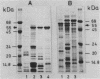
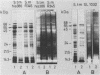
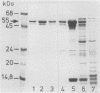
Surface proteins of different Salmonella R mutants were labeled selectively by treating live bacteria with cycloheptaamylose-dansylchloride. The labeled proteins were extracted from the cells with 6 M urea and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. From the urea extract a 55-kilodalton protein common to numerous Salmonella strains could be isolated by ion-exchange chromatography and gel filtration free of lipopolysaccharide. Immunization of rabbits with isolated protein led to the formation of specific antibodies. Such antiprotein antisera could be employed in Western blots for the specific identification of the 55-kilodalton protein in bacterial extracts containing mixtures of different Salmonella proteins. The importance of this antigen is emphasized by antisera against acetone-killed Salmonella bacteria, showing a preferential interaction with the 55-kilodalton protein in Western blots. Active immunization of mice with the 55-kilodalton protein afforded significant protection against experimental infection with S. typhimurium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerman C. R., Eisenstein T. K. Comparative efficacy and toxicity of a ribosomal vaccine, acetone-killed cells, lipopolysaccharide, and a live cell vaccine prepared from Salmonella typhhimurium. Infect Immun. 1978 Feb;19(2):575–582. doi: 10.1128/iai.19.2.575-582.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar N., Müller W., Schlecht S. Proteins from Salmonella R-mutants mediating protection against Salmonella typhimurium infection in mice I. Preparation of proteins free from lipopolysaccharide using various chromatographic methods. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Oct;253(1):88–101. [PubMed] [Google Scholar]
- Bhatnagar N., Schlecht S. Proteins from Salmonella R-mutants mediating protection against Salmonella typhimurium infection in mice. III. Separation and purification of soluble proteins and their use as vaccines and as precipitating antigens. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Dec;260(4):448–458. doi: 10.1016/s0176-6724(85)80066-3. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Calderón I., Lobos S. R., Rojas H. A., Palomino C., Rodríguez L. H., Mora G. C. Antibodies to porin antigens of Salmonella typhi induced during typhoid infection in humans. Infect Immun. 1986 Apr;52(1):209–212. doi: 10.1128/iai.52.1.209-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi G. C., Sharma V. K. Cell-mediated immunoprotection in calves immunized with rough Salmonella dublin. Br Vet J. 1981 Jul-Aug;137(4):421–430. [PubMed] [Google Scholar]
- Gmeiner J., Martin H. H. Phospholipid and lipopolysaccharide in Proteus mirabilis and its stable protoplast L-form. Difference in content and fatty acid composition. Eur J Biochem. 1976 Aug 16;67(2):487–494. doi: 10.1111/j.1432-1033.1976.tb10714.x. [DOI] [PubMed] [Google Scholar]
- Heckels J. E. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977 Apr;99(2):333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- Kenny K., Herzberg M. Antibody response and protection induced by immunization with smooth and rough strains in experimental salmonellosis. J Bacteriol. 1968 Feb;95(2):406–417. doi: 10.1128/jb.95.2.406-417.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Iinuma F., Tsuji A. Fluorescent labeling of proteins and a plasma membrane using cycloheptaamylose-dansyl chloride complex. Anal Biochem. 1974 Oct;61(2):632–637. doi: 10.1016/0003-2697(74)90431-x. [DOI] [PubMed] [Google Scholar]
- Legrum B., Fasold H., Passow H. Enhancement of anion equilibrium exchange by dansylation of the red blood cell membrane. Hoppe Seylers Z Physiol Chem. 1980 Oct;361(10):1573–1590. doi: 10.1515/bchm2.1980.361.2.1573. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lyman M. B., Stocker B. A., Roantree R. J. Evaluation of the immune response directed against the Salmonella antigenic factors O4,5 and O9. Infect Immun. 1979 Dec;26(3):956–965. doi: 10.1128/iai.26.3.956-965.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Identification of protective cell surface proteins in ribosomal fractions from Salmonella typhimurium. Infect Immun. 1979 Jun;24(3):808–816. doi: 10.1128/iai.24.3.808-816.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Bhatnagar N., Schlecht S. Osmotic shock fluids from salmonella R-mutants. Chemical composition and protective capacity against S. typhimurium infection in mice. Zentralbl Bakteriol Mikrobiol Hyg A. 1982 Jul;252(3):347–354. [PubMed] [Google Scholar]
- Müller W., Schlecht S., Westphal O. Schutzversuche gegen S. typhimurium-Infektion an MAusen. Chemische Zusammensetzung und biologische Wirksamkeit verschiedener Extrakte aus Salmonella-R Mutanten. Zentralbl Bakteriol A. 1980;248(1):64–80. [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- Schindler P. R., Teuber M. Ultrastructural study of Salmonella typhimurium treated with membrane-active agents: specific reaction dansylchloride with cell envelope components. J Bacteriol. 1978 Jul;135(1):198–206. doi: 10.1128/jb.135.1.198-206.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht S. Aktive Immunisierung gegen experimentelle Salmonellose in der Maus Die Wirksamkeit von Salmonella-R-Mutanten unterschiedlicher Herkunft gegenüber Infektionen mit verschiedenen Salmonella-Spezies. Zentralbl Bakteriol A. 1981 Aug;249(3):362–372. [PubMed] [Google Scholar]
- Schlecht S., Bhatnagar N. Proteins from Salmonella R-mutants mediating protection against Salmonella typhimurium infection in mice. II. Protection tests performed with proteins free from lipopolysaccharide. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 May;259(3):367–377. doi: 10.1016/s0176-6724(85)80039-0. [DOI] [PubMed] [Google Scholar]
- Schlecht S. Einfluss der Züchtungstemperatur auf die Produktion von Biomasse und Lipopolysaccharid sowie auf die chemische Zusammensetzung und Antigenität der Lipopolysaccharide von S-Formen und R-Mutanten des Genus Salmonella. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Apr;261(2):147–160. [PubMed] [Google Scholar]
- Schlecht S. Schutzimpfung mit Salmonella-R-Mutanten gegen S. typhi murium-Infektion im Mäuseversuch. Zentralbl Bakteriol Orig A. 1974;227(1-4):250–256. [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Okajima Y., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 2. Physical properties of the functional oligomeric aggregates. Eur J Biochem. 1979 Apr;95(3):441–448. doi: 10.1111/j.1432-1033.1979.tb12983.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang R. S., Lui S. W., Chau P. Y. Antigenic analysis of Barber's protein antigen prepared from Salmonella typhi and demonstration of protein-specific antibodies in sera of typhoid patients. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Apr;254(2):244–252. [PubMed] [Google Scholar]
- Ushiba D. Two types of immunity in experimental typhoid; "cellular immunity" and "humoral immunity". Keio J Med. 1965 Jun;14(2):45–61. doi: 10.2302/kjm.14.45. [DOI] [PubMed] [Google Scholar]
- Valtonen V. V. Mouse virulence of Salmonella strains: the effect of different smooth-type O side-chains. J Gen Microbiol. 1970 Dec;64(3):255–268. doi: 10.1099/00221287-64-3-255. [DOI] [PubMed] [Google Scholar]