Abstract
The successful differentiation of human embryonic stem cells (hESCs) to fibrochondrocyte-like cells and characterization of these differentiated cells is a critical step toward tissue engineering of musculoskeletal fibrocartilages (e.g., knee meniscus, temporomandibular joint disc, and intervertebral disc). In this study, growth factors and primary cell cocultures were applied to hESC embryoid bodies (EBs) for 3 weeks and evaluated for their effect on the synthesis of critical fibrocartilage matrix components: glycosaminoglycans (GAG) and collagens (types I, II, and VI). Changes in surface markers (CD105, CD44, SSEA, PDGFRα) after the differentiation treatments were also analyzed. The study was conducted in three phases: (1) examination of growth factors (TGF-β3, BMP-2, BMP-4, BMP-6, PDGF-BB, sonic hedgehog protein); (2) comparison of two cocultures (primary chondrocytes or fibrochondrocytes); and (3) the combination of the most effective growth factor and coculture regimen. TGF-β3 with BMP-4 yielded EBs positive for collagens I, II, and VI, with up to 6.7- and 4.8-fold increases in GAG and collagen, respectively. Analysis of cell surface markers showed a significant increase in CD44 with the TGF-β3 + BMP-4 treatment compared to the controls. Coculture with fibrochondrocytes resulted in up to a 9.8-fold increase in collagen II production. The combination of the growth factors BMP-4 + TGF-β3 with the fibrochondrocyte coculture led to an increase in cell proliferation and GAG production compared to either treatment alone. This study determined two powerful treatments for inducing fibrocartilaginous differentiation of hESCs and provides a foundation for using flow cytometry to purify these differentiated cells.
Introduction
Human embryonic stem cells (hESCs) are an emerging cell source for fibrocartilage tissue engineering [1]. These cells are exciting for tissue engineering applications as they have unlimited proliferative capacity and are multipotent [2,3]. Moreover, in the future, there is great potential for using these cells in patient-specific therapies, either through hESC banks or through somatic cell nuclear transfer [4,5]. Creating functional fibrocartilages, such as the temporomandibular joint disc, intervertebral disc, and the knee meniscus, requires that these multipotent cells be differentiated down a fibrocartilaginous lineage. Determining efficient and efficacious methodologies for differentiation is a critical goal for the field.
One way to recreate the cues needed for differentiation is through modifying the growth medium, and such cues can be taken from fibrocartilage, as well as cartilage-related literature. Differentiation down a cartilaginous lineage, as well as other lineages, can occur spontaneously through culture without specific differentiation treatments [6]. To make this differentiation more efficient and enhance cartilaginous matrix production, many groups are employing a “chondrogenic” base medium consisting of ITS+ [insulin, transferrin, selenium, and bovine serum albumin (BSA)], dexamethasone, ascorbic acid, and pyruvate [7–10]. Using the immense research with adult progenitor cells and primary cells as a guide, recent work has largely focused on supplementing this chondrogenic medium with growth factors from the transforming growth factor-β (TGF-β) superfamily. Levenberg et al. [11] used TGF-β1 to create cartilaginous matrix in hESC-seeded scaffolds and Toh et al. [12] found that supplementation with bone morphogenic protein-2 (BMP-2) increased glycosaminoglycans (GAG) and collagen II staining in hESC embryoid body (EB) outgrowths. In contrast, one study showed that chondrogenic medium alone outperformed two different differentiation regimens using sequential dosing of TGF-β3, TGF-β1, IGF-1, and BMP-2 [13]. Studies of hESC-derived cells and human embryonic germ cells have shown benefits with TGF-β growth factors (β1 or β3), while combinations with BMP-2 were not beneficial [14,15]. A larger variety of growth factors have been tried with mouse embryonic stem cells (mESCs), but the results have been mixed with respect to the most effective combination and dosing of TGF-βs and BMPs [16–19]. Other growth factor combinations including TGF-β3 + PDGF-BB and TGF-β1 + BMP-4 have also shown promise in mESC studies toward enhanced collagen II and GAG production [20]. Additional growth factors that have shown significant capacity to differentiate adult progenitors include BMP-6 and sonic hedgehog (SHH) protein [21–25]. As can be seen from the array of data, there is, as of yet, no gold standard for the differentiation of embryonic stem cells with growth factor combinations.
Another methodology for recapitulating the microenvironment milieu of development is coculture with primary cells. One study examined hESCs grown on inserts over “feeder layers” of first passage nasal chondrocytes [26]. The cells were cocultured for 4 weeks before implantation in a nude mouse in PDLLA foam scaffolds. The cocultured implants were found to have increased GAG and collagen, as well as a greater collagen II/I ratio over controls. Similar cocultures with varying degrees of contact between primary cells (intervertebral disc) and adult progenitor cells have also been used toward fibrocartilage applications [27,28]. One challenge with this approach is that it requires appropriate primary cells. However, the advantage is that the primary cells are more capable of recreating the complex biochemical signaling environment that may be needed for differentiation.
In this study, a wide range of differentiation conditions are studied with the objective of differentiating hESCs to cells that produce fibrocartilaginous extracellular matrix (ECM). Differentiation to “fibrochondrocytes” per se is not reasonable because specific markers are yet to be identified for a fibrochondrocyte. However, a functional definition of fibrocartilaginous differentiation can be applicable for tissue engineering purposes where the goal is functional restoration. Fibrocartilage is composed of varying ECM based on the region of the tissue. For example, the inner third of the meniscus contains collagen II while the outer portion is predominantly collagen I, with smaller amounts of type VI [29–32]. To engineer such a complex and heterogeneous tissue, it will be necessary to differentiate hESCs to cells capable of producing these types of matrix in varying ratios. This is a three-phase study examining differentiation through the use of (1) growth factors, (2) coculture conditions, and (3) the combination of the most effective growth factor and coculture regimens. In the first phase, we initially compare control chondrogenic medium to medium supplemented with TGF-β3, chosen over TGF-β1 because of its enhanced potency [33], to determine whether TGF-β3 treatment is beneficial compared to chondrogenic medium alone. Next, TGF-β3 treatment is combined with varying concentrations of PDGF-BB, SHH, BMP-2, BMP-4, and BMP-6 to further increase fibrocartilaginous matrix production. Specific hypotheses include the following: (1) TGF-β3 enhances fibrocartilaginous matrix production; (2) the performance of TGF-β3 is enhanced by combinations with other factors, including BMPs, PDGF-BB, and SHH; (3) combinations with BMPs specifically increase GAG production; and (4) combination specifically with BMP-4 in addition increases collagen production. In the second phase of the study, we compare hESC cocultures with primary articular chondrocytes and meniscal fibrochondrocytes. We hypothesize that cocultures lead to increased specific collagen production. Finally, the most beneficial growth factor and coculture methodology, as determined by fibrocartilaginous protein production, are studied in combination. In this final phase, the hypothesis is that the combination outperforms either treatment alone.
“Spin embryoid bodies” [34,35] of H9 hESCs are utilized to allow for uniform differentiation and analysis at the protein level to determine differentiation toward a fibrocartilaginous lineage. Looking toward future work with the differentiated cells, we also analyze an array of cell surface markers to identify markers predictive of protein production. Specifically, (1) CD44, the hyaluronan receptor and an important mesodermal marker; (2) CD105, the TGF-β3 receptor; (3) platelet-derived growth factor receptor α (PDGFRα), a mesodermal marker; and (4) SSEA-4, marker of undifferentiation in hESCs [3,20,36–38].
Materials and Methods
hESC culture
H9 hESCs (abbreviated “H9”; Wicell, Madison, WI, USA) were cultured according to the manufacturer's instructions on irradiated CF-1 mouse embryonic fibroblasts (MEFs) (Charles River Laboratory, Wilmington, MA, USA). Colonies were passaged using 0.1% type IV collagenase (Invitrogen, Carlsbad, CA, USA) every 4–6 days. Colonies were passaged on to Matrigel (BD Biosciences, San Jose, CA, USA) coated plates for the final passage before EB formation. While on Matrigel, the colonies were given MEF-conditioned medium supplemented with basic fibroblast growth factor (bFGF; Invitrogen).
Spin EB formation
Spin EBs [34,35] were prepared by lifting cells off the Matrigel with 4 min of 0.05% trypsin with EDTA (Invitrogen) application. Trypsin was stopped with hESC medium, the cells were counted and seeded at 1 × 105 cells/100 μL of bFGF-supplemented conditioned medium into low-adherence V-bottom 96-well plates (Sarstedt, Newton, NC, USA). The plates were centrifuged for 5 min at 950g. The plates were then allowed to incubate for 48 h. Spin EBs were lifted out of the wells with gentle pipetting and resuspended in appropriate medium for differentiation in six well plates, at approximately 20–30 EBs/well. Culture plates were coated in 2% agarose to prevent EBs from adhering to the well bottom.
Differentiation treatments
The base chondrogenic medium contained 1% fetal bovine serum (FBS) (Gemini, West Sacramento, CA, USA), DMEM with 4.5 g/L glucose and l-glutamine (Invitrogen), 1% nonessential amino acids, 0.4 mM proline, 50 μg/mL l-ascorbate-2 phosphate (Sigma, St. Louis, MO, USA), 100 μg/mL sodium pyruvate (Sigma), 1% ITS+ (BD Biosciences), and 100 nM dexamethasone. Growth factor treatments included TGF-β3 (Peprotech, Rocky Hill, NJ, USA), PDGF-BB (Peprotech), rhSHH-N (R&D Systems, Minneapolis, MN, USA), BMP-4 (Peprotech), BMP-2 (Peprotech), and BMP-6 (Promokine, Heidelberg, Germany). Low (L) and high (H) concentrations for each growth factor and the applied combinations are described in Table 1. All growth factors were diluted in accordance with the instructions from the manufacturer.
Table 1.
Differentiation Treatments
| Control | Chondrogenic base medium |
|---|---|
| T3 | 10 ng/mL TGF-β3 |
| PL | T3 + 20 ng/mL PDGF-BB |
| PH | T3 + 100 ng/mL PDGF-BB |
| SL | T3 + 200 ng/mL SHH |
| SH | T3 + 1000 ng/mL SHH |
| 2L | T3 + 20 ng/mL BMP-2 |
| 2H | T3 + 100 ng/mL BMP-2 |
| 4L | T3 + 20 ng/mL BMP-4 |
| 4H | T3 + 100 ng/mL BMP-4 |
| 6L | T3 + 20 ng/mL BMP-6 |
| 6H | T3 + 100 ng/mL BMP-6 |
| A | Articular chondrocyte coculture |
| M | Meniscal fibrochondrocyte coculture |
| 4HM | 4H + M |
Growth factor treatments and primary cell cocultures have been studied extensively in the past with mESCs and adult stem cells for chondrogenic differentiation. We approached the study of differentiation treatments in three phases: (1) examination of growth factors (TGF-β3, BMP-2, BMP-4, BMP-6, PDGF-BB, sonic hedgehog protein); (2) comparison of two cocultures (hESCs with primary chondrocytes or fibrochondrocytes in the presence of TGF-β3); and (3) combination of the most effective growth factor and coculture regimen selected from phases 1 and 2, respectively.
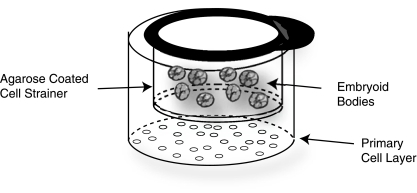
The primary cells for feeder layers, meniscal fibrochondrocytes and articular chondrocytes, were harvested from the inner two-thirds of the medial meniscus and the distal femora, respectively, of approximately 1-week-old male calves (Research 87, Boston, MA, USA) <36 h after slaughter. Tissue was minced and digested overnight with 0.2% collagenase II (Worthington, Lakewood, NJ, USA) in 10% FBS culture medium. The FBS culture medium is DMEM with 4.5 g/L glucose and l-glutamine (Gibco, Grand Island, NY, USA), 10% FBS (Gemini), 1% fungizone, 1% penicillin/streptomycin, 1% nonessential amino acids, 0.4 mM proline, 10 mM HEPES, and 50 μg/mL l-ascorbic acid (Sigma). To obtain sufficient cells for the experiment, cells from three animals were frozen at −80°C and later pooled to create a mixed animal primary cell population. Cocultures were prepared by thawing cells into a T-75 tissue culture–treated plastic flask (BD Biosciences) for 48 h; cells were then collected by trypsinization and irradiated with 6,000 rads. Cells were seeded at 5 × 105 cells per well of a 6-well plate. To separate EBs from the feeder layers, nylon mesh cell strainers were coated along the bottom and sides with 2% agarose (Sigma). EBs were then placed within the strainers. The agarose coating created a barrier between the EBs and the feeder layer while allowing diffusion of media and proteins (Fig. 1). Control treatment EBs were grown on agarose-coated wells or agarose-coated cell strainers.
FIG. 1.
Coculture set-up: Nylon cell strainers were coated with 2% agarose and placed in wells seeded with irradiated primary cells. Embryoid bodies (EBs) were placed within the agarose-coated strainer. This set-up creates a cell-cell barrier between the EBs and the irradiated primary cells while allowing the diffusion of media and proteins.
For all treatments, half medium changes were made every 3 days. New feeder layers were prepared each week. EBs were cultured a total of 3 weeks before analysis.
Immunohistochemistry and histology
Samples were frozen and sectioned at 12 μm. Immunohistochemical analysis was performed by fixing sections in chilled acetone, rehydrating, treating with 3% H2O2 in methanol, and blocking with horse serum. The following primary antibodies were diluted in PBS and applied for 1 h: 1:100 rabbit anti-human collagen VI pAb (US Biological, Swampscott, MA, USA), 1:750 rabbit anti-human collagen II pAB (Cedarlane, Burlington, NC, USA), and 1:650 mouse anti-human collagen I (Chondrex, Redmond, WA, USA). Visualization using a secondary biotinylated antibody, the ABC reagent, and DAB was performed using the Vectastain kit (Vector Laboratories, Burlingame, CA, USA), and counterstaining was done with Harris's hematoxylin. Sections of articular cartilage and meniscal fibrocartilage were run as positive controls, while samples were stained without application of the primary antibody as negative controls. To determine whether undesired differentiation had occurred, Von Kossa and oil red-O stains were performed for evidence of calcification and adipose tissue, respectively. In addtion, both Alcian Blue and Safranin-O were employed as qualitative methods for demonstrating chondrogenesis.
Quantitative biochemistry
Samples were lyophilized for 48 h and digested in 125 μg/mL papain (Sigma) for 18 h at 60°C. Cell number was determined using Picogreen Cell Proliferation Assay Kit (Molecular Probes, Eugene, OR, USA). A hydroxyproline assay was performed to gauge total collagen using bovine collagen standards (Biocolor, Newtonabbey, Northern Ireland). After finding positive Alcian Blue and Safranin-O staining, we then proceeded to quantify the GAG and other matrix production through a sulfated GAG assay using the Blyscan GAG Assay Kit (Biocolor). For each assay, 5–10 samples were analyzed. Data are reported as total matrix produced normalized to that produced by control or TGF-β3, as well as total matrix divided by the number of cells in the EB to give marix production per cell.
Samples for enzyme-linked immunosorbent assay (ELISA) were digested in papain at 4°C for 4 days and then a 1/10 volume of elastase (Sigma) solution in 10× TBS buffer was added to achieve a concentration of 0.1 mg/mL elastase. Samples were allowed to digest an additional 48 h. Between each incubation step in the ELISA, plates were washed using PBS with 0.05% Tween-20. For the collagen I ELISA, plates were incubated overnight at 4°C with 1:400 mouse anti-human capture mAb (US Biological), blocked with 2% BSA, samples and standards were added, then exposed to rabbit anti-human pAb (US Biological). Finally, goat anti-rabbit pAb was added, and the color developed in TMB as a liquid peroxidase substrate. The collagen II ELISA was performed using the Chondrex (Redmond, WA, USA) capture mAb, the Chondrex-biotinylated mAb, and streptavidin peroxidase was used with TMB to develop the color. Absorbance was read at 450 nm in a Genios plate reader (Tecan, San Jose, CA, USA). For the ELISAs, 4–8 samples were analyzed.
Flow cytometry
The best performing differentiation treatments from each phase were selected for flow cytometry analysis. EBs were analyzed 48 h after formation (“undifferentiated”) and after 3 weeks of differentiation treatment. Three samples were prepared per treatment per primary antibody. EBs were digested to obtain a single cell suspension, by applying trypsin for 1 h, followed by 0.2% type II collagenase (Worthington). The collagenase digestion was terminated when undigested material was no longer visible (1–1.25 h). Cells were blocked with goat serum, and the following primary antibodies were applied at 10 μg/mL for 30 min: mouse IgG isotype control (Invitrogen), mouse anti-human PDGFRα (R&D Systems), mouse anti-human CD44 (Sigma), or mouse anti-human CD105 (Invitrogen). In addition, mouse anti-human SSEA-4 (Developmental Studies Hybridoma Bank) was applied at 0.6 μg/mL. Cells were washed in PBS, and then the Alexa Fluor 488 goat anti-mouse FITC (Molecular Probes, Carlsbad, CA, USA) was applied for 30 min. Samples were fixed in 0.5% paraformaldehyde, and stored at 4°C until analysis. Samples were run on a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed for forward scatter and the percentage of cells labeled at a fluorescence level exceeding a 95% threshold on the isotype control.
Statistics
All data were compiled as mean ± standard deviation and a one-factor ANOVA was used to examine means from the quantitative biochemistry and flow cytometry data. If analysis showed a significant difference, a Tukey's post hoc or Student's t-test analysis was performed. A significance level of p < 0.05 was used in all statistical tests performed. The R2 values were calculated using linear regression of the data sets under evaluation [39].
Results
Growth factor differentiation
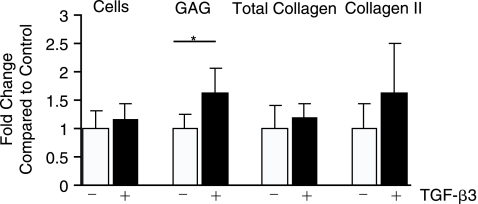
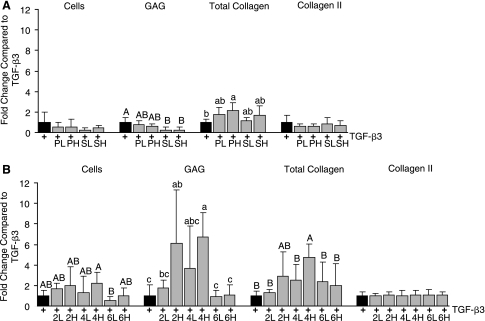
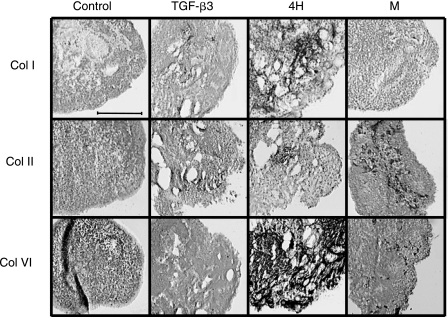
TGF-β3 treatment resulted in a significant 60% increase in GAG production over control (Fig. 2). There was no significant difference between the control and the TGF-β3-treated group in total collagen or collagen II quantity. Immunohistochemistry indicated very faint and nonuniform staining for collagen I, although no collagen I was detected by ELISA. As TGF-β3 showed benefit toward fibrocartilaginous differentiation, combinations of this growth factor were tested with varying concentrations of (1) PDGF-BB or SHH and (2) BMP-2, BMP-4, or BMP-6. In the first experiment, high and low concentrations of PDGF-BB or SHH were combined with TGF-β3, and all results were normalized to the TGF-β3-treated group. Immunohistochemistry showed the presence of collagen VI in all groups and collagen II was present in those treated with SHH. Quantitative biochemistry showed that there was no significant change in cell number per EB or collagen II production for combination with either PDGF-BB or SHH (Fig. 3A). In contrast, SHH treatment actually decreased GAG production compared to TGF-β3 alone. PH showed a 115% increase in total collagen production, and SHH showed a trend toward increasing collagen production. When normalized per cell, PH did not show improvement in collagen or GAG over TGF-β3 alone; only SH showed an increase in collagen per cell compared to TGF-β3. A comparison of BMPs combined with TGF-β3 netted greater improvements in matrix production. Immunohistochemistry demonstrated positive staining for collagen II and VI in all BMP-treated groups; however, collagen I was only present in 4H, 6L, and 6H (Fig. 4). Only 4H showed an increase in cell number over TGF-β3 (Fig. 3B). Both 2H and 4H showed increased GAG production, 6.1- and 6.7-fold increases, respectively. Only 4H showed a significant 4.8-fold increase in collagen over TGF-β3 alone. There was no increase in collagen II with any treatment. Normalization per cell showed similar trends to the per sample data, with the exception of 6L showing significantly greater collagen per cell compared to TGF-β3 alone. No collagen I was detectable by ELISA for these experiments; however, immunohistochemistry showed intense staining in the 4H group, and pale staining in the 6H group (Fig. 4). Von Kossa and oil red-O staining for extraneous differentiation were negative (data not shown).
FIG. 2.
TGF-β3 treatment resulted in increased GAG synthesis over control (chondrogenic medium with no growth factor addition), while collagen II content trended higher. Taking an optimization approach, follow-up studies examined combinations with TGF-β3. *p < 0.05.
FIG. 3.
(A)Combinations of PDGF with TGF-β3 at the high dose showed increased total collagen, but showed no other benefits over TGF-β3 alone. SHH was shown to decrease GAG synthesis. (B) Combinations of TGF-β3 with BMP-2 or BMP-4 proved to be powerful stimuli, as both collagen and GAG synthesis were increased over TGF-β3 alone. Experimental groups not connected by the same letter, within each type of analysis (cells, GAG, total collagen, collagen II) are statistically different (p < 0.05).
FIG. 4.
(A)Immunohistochemistry for collagens I (Col I), II (Col II), and VI (Col VI). Control-treated EBs showed minimal collagen II staining, while TGF-β3 resulted in staining for both Col I and II. Combining BMP-4 with TGF-β3 treatment resulted in positive staining for all of the critical fibrocartilage collagens. The meniscal fibrochondrocyte cocultures showed intense Col II staining. Scale bar is 200 μM.
Coculture mediated differentiation
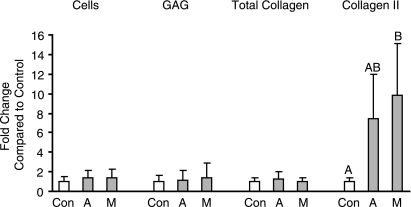
In a preliminary study, it was found that a primary cell feeder layer density of 5.0 × 105 cells/well showed more effect on matrix production than 2.5 × 105 cells/well (data not shown); therefore, the greater feeder density was utilized in the follow-up experiment. Culture of EBs in an agarose-coated cell strainer in the absence of a feeder was also compared to EBs grown in agarose-coated wells; no significant differences were found between the groups for matrix production or cell proliferation (data not shown). Use of articular cartilage and meniscal fibrocartilage-derived feeder layers only significantly increased specific collagen production (Fig. 5). While collagen I was undetectable in all groups either through ELISA or immunohistochemistry, collagen type II content was 7- and 9-fold that of the control in the chondrocyte and fibrochondrocyte cocultures, respectively. These changes were similar when normalized to cell number. Von Kossa and oil red-O staining for extraneous differentiation were negative (data not shown).
FIG. 5.
(A)Coculturing hESCs with either chondrocytes or fibrochondrocytes resulted in dramatic changes in collagen II content compared to chondrogenic medium alone (control). Groups not connected by the same letter are statistically different (p < 0.05).
Combination of growth factor and coculture conditions
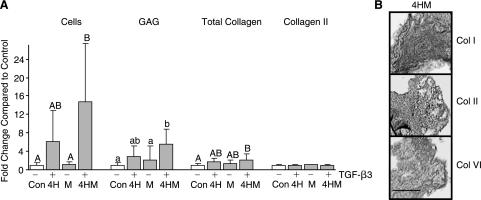
The combination treatment of BMP-4 + TGF-β3 and fibrochondrocyte coculture resulted in GAG and collagen content 7- and 2-fold, respectively, that of the control, as well as enhanced cell proliferation over control (Fig. 6A). However, there was no significant difference compared to the BMP-4 + TGF-β3 treatment alone. Collagen I, II, and VI were present as shown by immunohistochemistry (Fig. 6B). When normalized to the cell content, no significant differences were found. Von Kossa and oil red-O staining for extraneous differentiation were negative (data not shown).
FIG. 6.
(A)Combination of BMP-4 + TGF-β3 and the meniscal fibrochondrocyte coculture resulted in significant increases in cell number, GAG, and total collagen compared to control. Groups not connected by the same letter are statistically different (p < 0.05). (B) Immunohistochemistry for collagen I (Col I), II, and VI. The combination treatment showed all three fibrocartilaginous collagens. Scale bar is 200 μM.
Flow cytometry
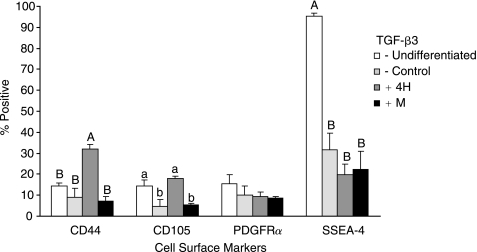
Undifferentiated EBs and EBs treated with control medium, BMP-4 + TGF-β3, and a fibrochondrocyte coculture were analyzed for the presence of four cell surface markers (Fig. 7). The selected differentiation treatments were the highest protein producers determined in each phase. Only the growth factor combination significantly increased CD44, 32.2 ± 1.9% positive. Both undifferentiated cells and growth factor combination-treated cells showed increased CD105, at 14.3 ± 3.2% and 17.9 ± 1.0% positive, respectively. All of the treatment groups showed significant drops in SSEA-4 compared to undifferentiated cells. The forward scatter, given as dimensionless measures of mean fluorescence intensity, offers a gauge of cell size (from largest to smallest): for the BMP-4 + TGF-β3 groups it was 693 ± 39, 578 ± 2 for the undifferentiated cells, 490 ± 14 for fibrochondrocyte cocultured cells, and 466 ± 17 for control-treated cells (see Supplementary Fig. 1, available online at http://www.liebertpub.com/rej). The combination treatment produced cells with similar levels of surface markers and size distribution to those resulting from the BMP-4 + TGF-β3 treatment (data not shown).
FIG. 7.
(A)Flow cytometry for cell surface markers. All treatments significantly decreased SSEA-4, a sensitive marker for undifferentiation. Only BMP-4 + TGF-β3 led to increased CD44 and CD105, a marker or mesodermal differentiation and the TGF-β3 receptor, respectively. These markers may be used in future studies to isolate differentiated cells with capacity for differentiation to fibrochondrocyte-like cells. Experimental groups not connected by the same letter within each marker analysis are statistically different (p < 0.05).
Discussion
Fibrocartilage tissue engineering with hESCs is in its infancy; this study is the first to examine a large variety of growth factor combinations and cocultures specifically toward fibrocartilage differentiation. Earlier work has identified an appropriate hESC line and differentiation time [1] and in this study, a three-phase approach was taken to refine the differentiation process. First, growth factor treatments were compared and TGF-β3 was found to enhance GAG synthesis, while its combination with BMP-4 was found to increase GAG, total collagen, and collagen type I production. In the second phase, this study found that coculture with fibrochondrocytes successfully induced greater collagen II production over control or articular chondrocyte cocultures. Combinations of TGF-β3 with PDGF-BB or SHH were found to be less fruitful. In the third phase, combining the best performing growth factor combination with the coculture resulted in a trend of increased GAG and collagen synthesis over either treatment alone. Flow cytometry analysis showed that the treatments differentially affected the cell surface geography and cell size, demonstrating a powerful link between protein production and cell size and surface markers. Overall, we have identified a treatment, BMP-4 + TGF-β3, that results in enhanced synthesis of important fibrocartilaginous proteins, while using a coculture with fibrochondrocytes markedly increases collagen II production. Moreover, increased CD44, maintenance of CD105 levels, as well as marked differences in cell size indicate that future work purifying these cells may be possible.
The utility of TGF-β3 in enhancing GAG synthesis correlates well to several previous studies of hESCs, mESCs, and other similar cell lines [11,15,16,20,40]. The lack of enhanced collagen II production or GAG synthesis when TGF-β3 was combined with PDGF-BB or SHH was surprising, given evidence with mesenchymal stem cells (MSCs) and mESCs to support these combinations [20,21]. SHH acts upstream of TGF-β signaling and is known to increase the expression of TGF-β isoforms [41,42]. One hypothesis for the lack of response is that the TGF-β3 concentration used here was already at a saturation level. Future work may study whether SHH could be more effective in the presence of BMPs, as downstream expression of BMP receptors has been shown [43].
Three different BMPs were examined in this study since each has shown utility in different systems; for example, BMP-2 combined with TGF-β3 outperformed similar combinations with BMP-4 and BMP-6 in a study of MSCs [44]; BMP-6 appears to be particularly effective with adipose-derived progenitor cells compared to BMP-2, BMP-4, and BMP-7 [23,24], while BMP-4 has outperformed BMP-2 in mESC chondrogenesis [20]. The large increases in fibrocartilaginous matrix synthesis seen in this study with BMP-2 or BMP-4 combined with TGF-β3 contrasts with recent work with hESC-derived cells [15,16] but are supported by work with mESCs [18,20]. Additional studies with adult progenitors, both bone marrow and adipose derived, also show positive and even synergistic results in combining a TGF-β growth factor with a BMP [23–25,45]. The increases in GAG and collagen seen in this study may have been due to direct effects of the BMPs, BMP-induced increases in TGF-β receptors [24], or a combination of these two effects. Overall, combinations of TGF-β3 and BMP-4 merits greater study as the increases in matrix, 6.7-fold in GAG and 4.8-fold in collagen compared to TGF-β3 alone, were significant. Although this study examined a dose range covering most previously studied concentrations of BMPs, 20–100 ng/mL, a dose-response was still observed; thus, a saturation concentration may still need to be identified.
An alternative to growth factor–mediated differentiation is the use of cocultures to provide the biochemical stimuli for differentiation. In using primary cells, there are the added challenges of an appropriate source and varying potency of the primary cells. Despite these considerations, cocultures may provide a powerful stimulus, one that may even be responsive to the signals given off by the differentiating cells; i.e., there is opportunity for cross-talk between the two cell populations. It was hypothesized in this study that the cocultures would differentially regulate specific collagen production as the cells themselves have very different collagen synthesis profiles, and, interestingly, both cell types appeared to increase collagen II production with little effect on collagen I. The 7- to 10-fold increase in collagen II production is an important advance in fibrocartilaginous differentiation. Earlier work with nasal chondrocytes cocultured with the hESC H1 line showed large increases in GAG production and collagen II staining [26]. Future work will further examine appropriate “dosing” of the coculture, including issues such as cell density, how often new feeder layers should be prepared, and whether feeder layers should be treated with growth factors or other stimuli. In addition, recent studies using chondrocyte-conditioned medium to differentiate MSCs [28,46] or the mixing of MSCs and primary cells [28,47,48] also show promise. Future work comparing conditioned medium, direct mixing of the cells, and the set-up used in this study in which the two cell populations were separated by a protein-permeable barrier will help further elucidate the role of cross-talk between the progenitor cells and primary cells. Moreover, in this study, a cross-species coculture was utilized because of convenience in cell procurement; future studies must look toward comparing different sources of primary cells for cocultures in terms of their practicality and especially the elimination of source-to-source variation in the level of stimulus provided.
One of the prominent challenges in using a coculture is that there may be variation in the differentiating capacity of the primary cells used in the cocultures, especially if taken from different donors. This variation may explain the results of the combination growth factor–coculture study (phase 3) versus the coculture alone study (phase 2). Although coculture cells were pooled from multiple animals for each phase, there may have been variation in the differentiating capacity of these two pooled populations of cells. This variation was evidenced by the large increases in collagen II measured in phase 2 while these increases in collagen II quantity were not reproduced in phase 3. In contrast, the increases in collagen and GAG, expected with the use of the growth factors, were observed in both phases. The coculture clearly played a role in terms of mitogenic stimulation, as the combination treatment resulted in a large increase in cell number. Future work utilizing cell lines may enhance the consistency of this differentiation tool. However, coculture with patient-derived cells may be more appropriate for future therapeutic use. In such a case, examination and assessment of the differentiating capacity of cocultures will be critical as cocultures can clearly serve as powerful differentiation stimuli.
The flow cytometry component of this study was undertaken as a first evaluation of an important tool for later studies: purifying populations of differentiated cells. Flow cytometry analysis may also be a powerful tool to identify a marker indicative of fibrocartilaginous matrix production, which could be used to screen large numbers of differentiation strategies rather than laborious and expensive protein-level analyses. First, we used SSEA-4 expression as a gauge of whether the cells had retained their multipotency. As expected, there was a 70–80% drop in this marker during the differentiation period studied. We then examined three other cell surface markers that have been linked to chondrogenesis: PDGFRα, CD44, and CD105. Although PDGFRα+ mESCs have shown significant increases in chondrogenesis [20], none of the treatments markedly changed PDGFRα expression in this study. A particularly promising potential marker for cartilaginous differentiation is CD44; this marker appears early in chondrogenesis and is important in the formation of chondrocyte pericellular matrix [49–51]. This marker has also been correlated to increased chondrogenicity in adult chondrocyte populations [38,52]. Of the markers examined, CD44 was the only one that significantly increased over the undifferentiated and control treatment populations. We can speculate that increased presence of this hyaluronan receptor correlates with the enhanced GAG production seen in the EBs created with the BMP-4 + TGF-β3 treatment (R2 = 0.78). Furthermore, since increased GAG was not seen with the fibrochondrocyte coculture, it is consistent that CD44 was not increased. It was hypothesized that CD105 would increase with TGF-β3 treatment and be a positive indicator of fibrochondrogenesis. Comparisons between the BMP-4 + TGF-β3 group and the control/coculture groups, which were not treated with TGF-β3, support this hypothesis. The lack of difference between the undifferentiated cells and the BMP4 + TGF-β3 group suggests that the treatment led to the maintenance of this cell surface maker, while in the other groups, the marker was lost during culture. Thus, CD105 could be used as a differentiation indicator only after the cells have been purified of cells expressing undifferentiation markers. The presence of CD105 has been used before differentiation to isolate a chondrogenic population; by maintaining the presence of this surface marker, the BMP4 + TGF-β3 treatment may have had increased efficacy in fibrocartilage differentiation [37]. These data lay a foundation for using flow cytometry to purify particularly promising populations for chondrogenesis. For example, treated cells could first be purified to remove all SSEA-4+ cells, and then sorted to obtain a CD44+ and CD105+ population. As this study cannot show to what degree these populations overlap, such an examination will be important to more concretely establish these markers for chondrogenesis.
This work shows significant gains in fibrocartilage matrix synthesis in hESC EBs comparing 15 differentiation strategies. The combination of BMP-4 + TGF-β3 was effective in stimulating production of collagens I, II, and VI, and markedly increasing total collagen and GAG production. It was also demonstrated that BMP-4 + TGF-β3 differentiated hESCs posses a greater forward scatter (gauge of cell size) and the highest CD44 presence compared to the other groups; both of these changes may serve as important markers for purifying these cells. These refined methods for differentiation can now be applied in a tissue engineering strategy to create hESC-derived fibrocartilage.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge an unrestricted fund from Rice University and NIAMS R01 AR47839 for funding this work. We would also like to thank the Hertz Foundation for its support of G.M.H. We also thank Prof. Thomas Zwaka of Baylor College of Medicine for helpful discussions. The monoclonal antibody for SSEA-4, developed by Davor Solter, was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA, USA.
References
- 1.Hoben GM. Koay EJ. Athanasiou KA (In press) Fibrochondrogenesis in two embryonic stem cell lines: Effects of differentiation timelines. Stem Cells. 2008;26(2):422–30. doi: 10.1634/stemcells.2007-0641. [DOI] [PubMed] [Google Scholar]
- 2.Rao BM. Zandstra PW. Culture development for human embryonic stem cell propagation: molecular aspects and challenges. Curr Opin Biotechnol. 2005;16:568–576. doi: 10.1016/j.copbio.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Thomson JA. Itskovitz-Eldor J. Shapiro SS. Waknitz MA. Swiergiel JJ. Marshall VS. Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 4.Jaenisch R. Human cloning—the science and ethics of nuclear transplantation. N Engl J Med. 2004;351:2787–2791. doi: 10.1056/NEJMp048304. [DOI] [PubMed] [Google Scholar]
- 5.Civin CI. Rao MS. How many human embryonic stem cell lines are sufficient? A U.S. perspective. Stem Cells. 2006;24:800–803. doi: 10.1634/stemcells.2006-0084. [DOI] [PubMed] [Google Scholar]
- 6.Khoo ML. McQuade LR. Smith MS. Lees JG. Sidhu KS. Tuch BE. Growth and differentiation of embryoid bodies derived from human embryonic stem cells: effect of glucose and basic fibroblast growth factor. Biol Reprod. 2005;73:1147–1156. doi: 10.1095/biolreprod.104.036673. [DOI] [PubMed] [Google Scholar]
- 7.Johnstone B. Hering TM. Caplan AI. Goldberg VM. Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 8.Mackay AM. Beck SC. Murphy JM. Barry FP. Chichester CO. Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 9.Yoo JU. Barthel TS. Nishimura K. Solchaga L. Caplan AI. Goldberg VM. Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka H. Murphy CL. Murphy C. Kimura M. Kawai S. Polak JM. Chondrogenic differentiation of murine embryonic stem cells: effects of culture conditions and dexamethasone. J Cell Biochem. 2004;93:454–462. doi: 10.1002/jcb.20171. [DOI] [PubMed] [Google Scholar]
- 11.Levenberg S. Huang NF. Lavik E. Rogers A. Itskovitz-Eldor J. Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toh WS. Yang Z. Liu H. Heng BC. Lee EH. Cao T. Effects of culture conditions and bone morphogenetic protein 2 on extent of chondrogenesis from human embryonic stem cells. Stem Cells. 2007;25:950–960. doi: 10.1634/stemcells.2006-0326. [DOI] [PubMed] [Google Scholar]
- 13.Koay EJ. Hoben GM. Athanasiou KA. Tissue engineering with chondrogenically-differentiated human embryonic stem cells. Stem Cells. 2007;25(9):2183–2190. doi: 10.1634/stemcells.2007-0105. [DOI] [PubMed] [Google Scholar]
- 14.Kim MS. Hwang NS. Lee J. Leong K. Shamblott MJ. Gearhart J. Elisseeff J. Musculoskeletal differentiation of cells derived from human embryonic germ cells. Stem Cells. 2005;23:113–123. doi: 10.1634/stemcells.2004-0110. [DOI] [PubMed] [Google Scholar]
- 15.Hwang NS. Varghese S. Zhang Z. Elisseeff J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006;12:2695–2706. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- 16.Hwang NS. Kim MS. Sampattavanich S. Baek JH. Zhang Z. Elisseeff J. Effects of three-dimensional culture and growth factors on the chondrogenic differentiation of murine embryonic stem cells. Stem Cells. 2006;24:284–291. doi: 10.1634/stemcells.2005-0024. [DOI] [PubMed] [Google Scholar]
- 17.zur Nieden NI. Kempka G. Rancourt DE. Ahr HJ. Induction of chondro-, osteo- and adipogenesis in embryonic stem cells by bone morphogenetic protein-2: effect of cofactors on differentiating lineages. BMC Dev Biol. 2005;5:1. doi: 10.1186/1471-213X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegert C. Kramer J. Hargus G. Müller J. Guan K. Wobus AM. Müller PK. Rohwedel J. Differentiation plasticity of chondrocytes derived from mouse embryonic stem cells. J Cell Sci. 2002;115:4617–4628. doi: 10.1242/jcs.00171. [DOI] [PubMed] [Google Scholar]
- 19.Kramer J. Hegert C. Guan K. Wobus AM. Müller PK. Rohwedel J. Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mech Dev. 2000;92:193–205. doi: 10.1016/s0925-4773(99)00339-1. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama N. Duryea D. Manoukian R. Chow G. Han CY. Macroscopic cartilage formation with embryonic stem-cell-derived mesodermal progenitor cells. J Cell Sci. 2003;116:2015–2028. doi: 10.1242/jcs.00417. [DOI] [PubMed] [Google Scholar]
- 21.Warzecha J. Gottig S. Bruning C. Lindhorst E. Arabmothlagh M. Kurth A. Sonic hedgehog protein promotes proliferation and chondrogenic differentiation of bone marrow-derived mesenchymal stem cells in vitro. J Orthop Sci. 2006;11:491–496. doi: 10.1007/s00776-006-1058-1. [DOI] [PubMed] [Google Scholar]
- 22.Enomoto-Iwamoto M. Nakamura T. Aikawa T. Higuchi Y. Yuasa T. Yamaguchi A. Nohno T. Noji S. Matsuya T. Kurisu K. Koyama E. Pacifici M. Iwamoto M. Hedgehog proteins stimulate chondrogenic cell differentiation and cartilage formation. J Bone Miner Res. 2000;15:1659–1668. doi: 10.1359/jbmr.2000.15.9.1659. [DOI] [PubMed] [Google Scholar]
- 23.Estes BT. Wu AW. Guilak F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006;54:1222–1232. doi: 10.1002/art.21779. [DOI] [PubMed] [Google Scholar]
- 24.Hennig T. Lorenz H. Thiel A. Goetzke K. Dickhut A. Geiger F. Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682–691. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 25.Indrawattana N. Chen G. Tadokoro M. Shann LH. Ohgushi H. Tateishi T. Tanaka J. Bunyaratvej A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320:914–919. doi: 10.1016/j.bbrc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 26.Vats A. Bielby RC. Tolley N. Dickinson SC. Boccaccini AR. Hollander AP. Bishop AE. Polak JM. Chondrogenic differentiation of human embryonic stem cells: the effect of the micro-environment. Tissue Eng. 2006;12:1687–1697. doi: 10.1089/ten.2006.12.1687. [DOI] [PubMed] [Google Scholar]
- 27.Richardson SM. Walker RV. Parker S. Rhodes NP. Hunt JA. Freemont AJ. Hoyland JA. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells. 2006;24:707–716. doi: 10.1634/stemcells.2005-0205. [DOI] [PubMed] [Google Scholar]
- 28.Le Visage C. Kim SW. Tateno K. Sieber AN. Kostuik JP. Leong KW. Interaction of human mesenchymal stem cells with disc cells: changes in extracellular matrix biosynthesis. Spine. 2006;31:2036–2042. doi: 10.1097/01.brs.0000231442.05245.87. [DOI] [PubMed] [Google Scholar]
- 29.Vanderploeg EJ. Wilson CG. Levenston ME. Immunolocalization of type VI collagen in the bovine meniscus; Paper presented at the 52nd Annual Meeting of the Orthopaedic Research Society; Chicago, IL. 2006. [Google Scholar]
- 30.Wildey GM. McDevitt CA. Matrix protein mRNA levels in canine meniscus cells in vitro. Arch Biochem Biophys. 1998;353:10–15. doi: 10.1006/abbi.1998.0647. [DOI] [PubMed] [Google Scholar]
- 31.Carvalho HF. Felisbino SL. Keene DR. Vogel KG. Identification, content, and distribution of type VI collagen in bovine tendons. Cell Tissue Res. 2006;325:315–324. doi: 10.1007/s00441-006-0161-0. [DOI] [PubMed] [Google Scholar]
- 32.Quarto R. Campanile G. Cancedda R. Dozin B. Modulation of commitment, proliferation, and differentiation of chondrogenic cells in defined culture medium. Endocrinology. 1997;138:4966–4976. doi: 10.1210/endo.138.11.5522. [DOI] [PubMed] [Google Scholar]
- 33.Graycar JL. Miller DA. Arrick BA. Lyons RM. Moses HL. Derynck R. Human transforming growth factor-beta 3: recombinant expression, purification, and biological activities in comparison with transforming growth factors-beta 1 and -beta 2. Mol Endocrinol. 1989;3:1977–1986. doi: 10.1210/mend-3-12-1977. [DOI] [PubMed] [Google Scholar]
- 34.Ng ES. Davis RP. Azzola L. Stanley EG. Elefanty AG. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106:1601–1603. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- 35.Burridge PW. Anderson D. Priddle H. Barbadillo Muñoz MD. Chamberlain S. Allegrucci C. Young LE. Denning C. Improved human embryonic stem cell embryoid body homogeneity and cardiomyocyte differentiation from a novel V-96 plate aggregation system highlights interline variability. Stem Cells. 2007;25:929–938. doi: 10.1634/stemcells.2006-0598. [DOI] [PubMed] [Google Scholar]
- 36.Ataliotis P. Platelet-derived growth factor A modulates limb chondrogenesis both in vivo and in vitro. Mech Dev. 2000;94:13–24. doi: 10.1016/s0925-4773(00)00321-x. [DOI] [PubMed] [Google Scholar]
- 37.Majumdar MK. Banks V. Peluso DP. Morris EA. Isolation, characterization, and chondrogenic potential of human bone marrow-derived multipotential stromal cells. J Cell Physiol. 2000;185:98–106. doi: 10.1002/1097-4652(200010)185:1<98::AID-JCP9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Barbero A. Grogan SP. Mainil-Varlet P. Martin I. Expansion on specific substrates regulates the phenotype and differentiation capacity of human articular chondrocytes. J Cell Biochem. 2006;98:1140–1149. doi: 10.1002/jcb.20754. [DOI] [PubMed] [Google Scholar]
- 39.Rosner B. Fundamentals of Biostatistics. Brooks/Cole; Pacific Grove, CA: 2000. [Google Scholar]
- 40.Kawaguchi J. Mee PJ. Smith AG. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone. 2005;36:758–769. doi: 10.1016/j.bone.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez J. Sohn P. Zeng X. Doetschman T. Robbins DJ. Serra R. TGFbeta2 mediates the effects of hedgehog on hypertrophic differentiation and PTHrP expression. Development. 2002;129:1913–1924. doi: 10.1242/dev.129.8.1913. [DOI] [PubMed] [Google Scholar]
- 42.Heberlein U. Wolff T. Rubin GM. The TGF beta homolog dpp and the segment polarity gene hedgehog are required for propagation of a morphogenetic wave in the Drosophila retina. Cell. 1993;75:913–926. doi: 10.1016/0092-8674(93)90535-x. [DOI] [PubMed] [Google Scholar]
- 43.Murtaugh LC. Chyung JH. Lassar AB. Sonic hedgehog promotes somitic chondrogenesis by altering the cellular response to BMP signaling. Genes Dev. 1999;13:225–237. doi: 10.1101/gad.13.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekiya I. Larson BL. Vuoristo JT. Reger RL. Prockop DJ. Comparison of effect of BMP-2, −4, and −6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320:269–276. doi: 10.1007/s00441-004-1075-3. [DOI] [PubMed] [Google Scholar]
- 45.Sekiya I. Vuoristo JT. Larson BL. Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci USA. 2002;99:4397–4402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang NS. Varghese S. Puleo C. Zhang Z. Elisseeff JH. Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol. 2007;212:281–284. doi: 10.1002/jcp.21052. [DOI] [PubMed] [Google Scholar]
- 47.Richmon JD. Sage AB. Shelton E. Schumacher B. Sah RL. Watson D. Effect of growth factors on cell proliferation, matrix deposition, and morphology of human nasal septal chondrocytes cultured in monolayer. Laryngoscope. 2005;115:1553–1560. doi: 10.1097/01.MLG.0000175541.31131.A5. [DOI] [PubMed] [Google Scholar]
- 48.Pound JC. Green DW. Chaudhuri JB. Mann S. Roach HI. Oreffo ROC. Strategies to promote chondrogenesis and osteogenesis from human bone marrow cells and articular chondrocytes encapsulated in polysaccharide templates. Tissue Eng. 2006;12:2789–2799. doi: 10.1089/ten.2006.12.2789. [DOI] [PubMed] [Google Scholar]
- 49.Rousche KT. Knudson CB. Temporal expression of CD44 during embryonic chick limb development and modulation of its expression with retinoic acid. Matrix Biol. 2002;21:53–62. doi: 10.1016/s0945-053x(01)00189-5. [DOI] [PubMed] [Google Scholar]
- 50.Knudson CB. Nofal GA. Pamintuan L. Aguiar DJ. The chondrocyte pericellular matrix: a model for hyaluronan-mediated cell-matrix interactions. Biochem Soc Trans. 1999;27:142–147. doi: 10.1042/bst0270142. [DOI] [PubMed] [Google Scholar]
- 51.Knudson CB. Hyaluronan and CD44: strategic players for cell-matrix interactions during chondrogenesis and matrix assembly. Birth Defects Res C Embryo Today. 2003;69:174–196. doi: 10.1002/bdrc.10013. [DOI] [PubMed] [Google Scholar]
- 52.Grogan SP. Barbero A. Diaz-Romero J. Cleton-Jansen A. Soeder S. Identification of markers to characterize and sort human articular chondrocytes with enhanced in vitro chondrogenic capacity. Arthritis Rheum. 2007;56:586–595. doi: 10.1002/art.22408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.