Abstract
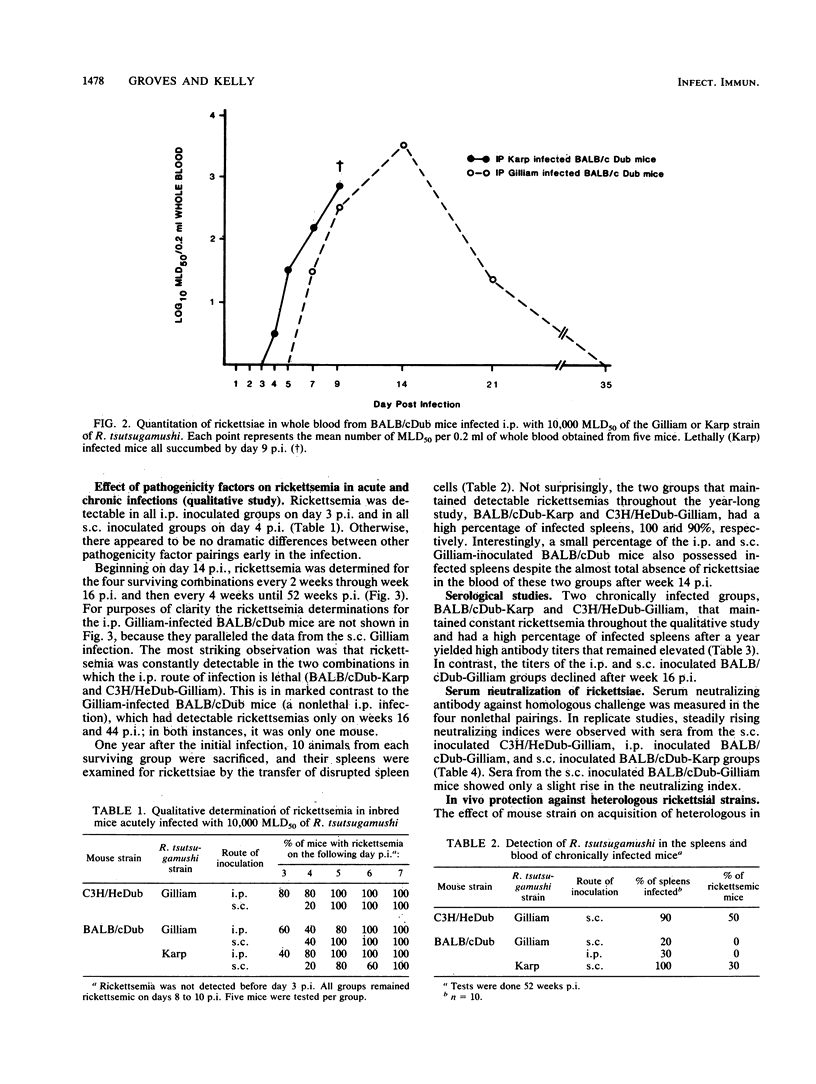
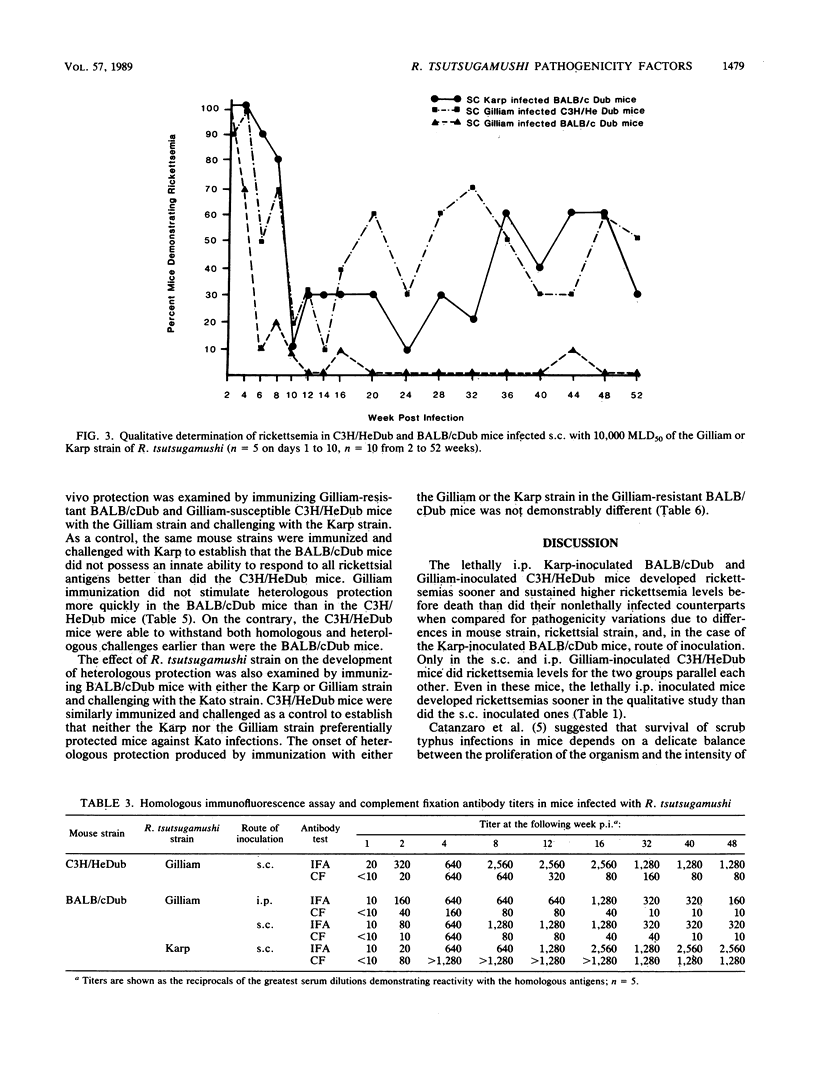
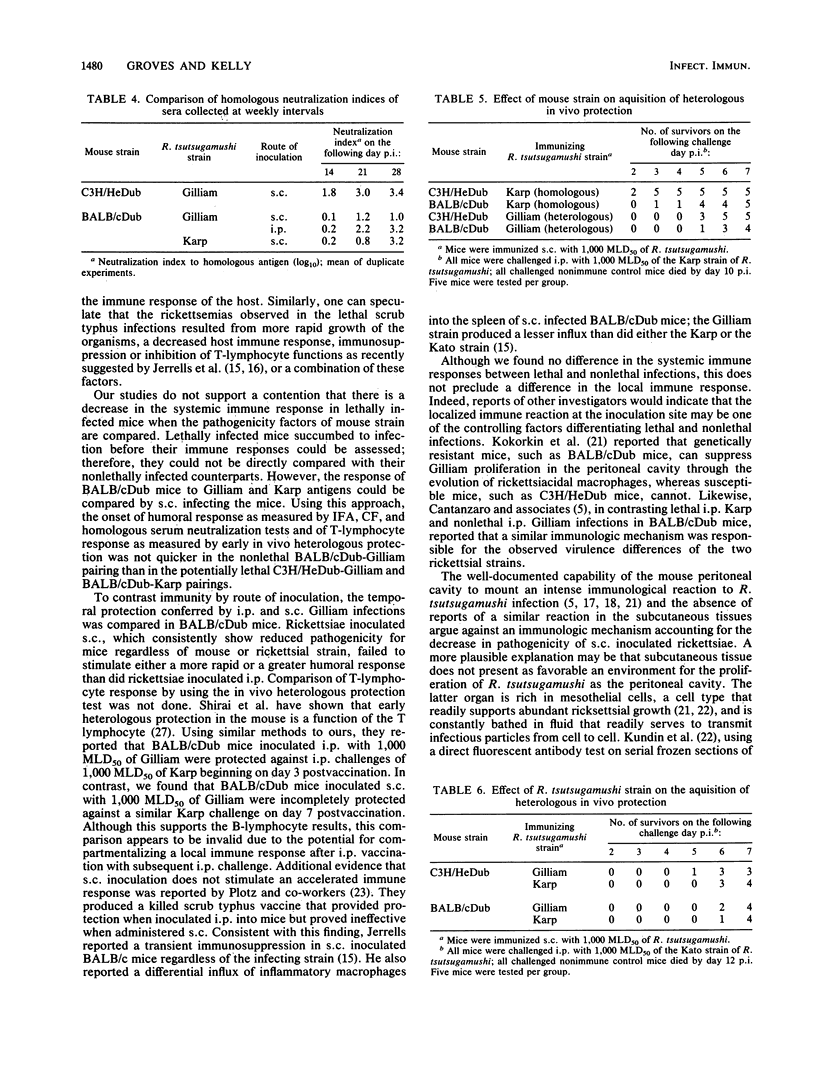
Pathogenicity of Rickettsia tsutsugamushi for laboratory mice is known to be influenced by at least three factors: (i) route of inoculation, (ii) antigenic strain, and (iii) natural resistance of the host. By using Karp, Gilliam, and Kato strains of R. tsutsugamushi, we examined the effect of these three pathogenicity factors on the kinetics of infection and the development of immunity in BALB/cDub and C3H/HeDub mice. The appearance of rickettsemia in the pathogenic infections generally preceded infections of reduced pathogenicity by 1 to 2 days in both magnitude and time of onset. Mice infected by the subcutaneous route with normally pathogenic rickettsiae, i.e., Gilliam-infected C3H/HeDub mice and Karp-infected BALB/cDub mice, consistently maintained a detectable rickettsemia over a 1-year period. Rickettsiae were recovered from the spleens of 95% (19 of 20) of these mice 52 weeks postinfection. In contrast, mice with infections of reduced pathogenicity, i.e., BALB/cDub mice infected by intraperitoneal and subcutaneous inoculation with Gilliam, did not have detectable rickettsemia from week 20 through week 52 postinfection except for a single mouse on week 44 postinfection. Rickettsiae were detected in the spleens of only 40% (8 of 20) of these mice after 1 year. In both Gilliam-infected mouse strains, protection against heterologous challenge with Karp or Kato rickettsial strains was incomplete up to 7 days postimmunization. Infections of reduced pathogenicity did not result from an enhanced systemic immune response by the host. The onset of the humoral response was not different for the pathogenic and reduced-pathogenicity infections. Pathogenicity differences seemed to result from the more rapid growth of the rickettsiae in the pathogenic infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOZEMAN F. M., ELISBERG B. L. Serological diagnosis of scrub typhus by indirect immunofluorescence. Proc Soc Exp Biol Med. 1963 Mar;112:568–573. doi: 10.3181/00379727-112-28107. [DOI] [PubMed] [Google Scholar]
- Catanzaro P. J., Shirai A., Hilderbrandt P. K., Osterman J. V. Host defenses in experimental scrub typhus: histopathological correlates. Infect Immun. 1976 Mar;13(3):861–875. doi: 10.1128/iai.13.3.861-875.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg G. H., Jr, Osterman J. V. Experimental scrub typhus immunogens: gamma-irradiated and formalinized rickettsiae. Infect Immun. 1977 Jan;15(1):124–131. doi: 10.1128/iai.15.1.124-131.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing E. P., Jr, Takeuchi A., Shirai A., Osterman J. V. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect Immun. 1978 Mar;19(3):1068–1075. doi: 10.1128/iai.19.3.1068-1075.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves M. G., Osterman J. V. Host defenses in experimental scrub typhus: genetics of natural resistance to infection. Infect Immun. 1978 Feb;19(2):583–588. doi: 10.1128/iai.19.2.583-588.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves M. G., Rosenstreich D. L., Taylor B. A., Osterman J. V. Host defenses in experimental scrub typhus: mapping the gene that controls natural resistance in mice. J Immunol. 1980 Sep;125(3):1395–1399. [PubMed] [Google Scholar]
- JACKSON E. B., SMADEL J. E. Immunization against scrub typhus. II. Preparation of lyophilized living vaccine. Am J Hyg. 1951 May;53(3):326–331. doi: 10.1093/oxfordjournals.aje.a119457. [DOI] [PubMed] [Google Scholar]
- Jerrells T. R. Immunosuppression associated with the development of chronic infections with Rickettsia tsutsugamushi: adherent suppressor cell activity and macrophage activation. Infect Immun. 1985 Oct;50(1):175–182. doi: 10.1128/iai.50.1.175-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Host defenses in experimental scrub typhus: inflammatory response of congenic C3H mice differing at the Ric gene. Infect Immun. 1981 Mar;31(3):1014–1022. doi: 10.1128/iai.31.3.1014-1022.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Role of macrophages in innate and acquired host resistance to experimental scrub typhus infection of inbred mice. Infect Immun. 1982 Sep;37(3):1066–1073. doi: 10.1128/iai.37.3.1066-1073.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Palmer B. A., Osterman J. V. Gamma-irradiated scrub typhus immunogens: development of cell-mediated immunity after vaccination of inbred mice. Infect Immun. 1983 Jan;39(1):262–269. doi: 10.1128/iai.39.1.262-269.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNDIN W. D., LIU C., HARMON P., RODINA P. PATHOGENESIS OF SCRUB TYPHUS INFECTION (RICKETTSIA TSUTSUGAMUSHI) AS STUDIED BY IMMUNOFLUORESCENCE. J Immunol. 1964 Nov;93:772–781. [PubMed] [Google Scholar]
- Kelly D. J., Rees J. C. Effect of sublethal gamma radiation on host defenses in experimental scrub typhus. Infect Immun. 1986 Jun;52(3):718–724. doi: 10.1128/iai.52.3.718-724.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokorin I. N., Kyet C. D., Kekcheeva N. G., Miskarova E. D. Cytological investigation of Rickettsia tsutsugamushi infection of mice with different allotypic susceptibility to the agent. Acta Virol. 1976 Apr;20(2):147–151. [PubMed] [Google Scholar]
- SHISHIDO A., OHTAWARA M., HIKITA M., KITAOKA M. The nature of immunity against scrub typhus in mice. II. The cross-protection test with mice for identification and differentiation of several strains of Rickettsia orientalis newly isolated in Japan. Jpn J Med Sci Biol. 1959 Dec;12:391–404. doi: 10.7883/yoken1952.12.391. [DOI] [PubMed] [Google Scholar]
- SMADEL J. E., JACKSON E. B., LEY H. L., Jr Terramycin as a rickettsiostatic agent and its usefulness in patients with scrub typhus. Ann N Y Acad Sci. 1950 Sep;53(2):375–384. doi: 10.1111/j.1749-6632.1950.tb42172.x. [DOI] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Eisenberg G. H., Jr, Osterman J. V. Host defenses in experimental scrub typhus: effect of chloramphenicol. Infect Immun. 1977 Nov;18(2):324–329. doi: 10.1128/iai.18.2.324-329.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai A., Chan T. C., Gan E., Huxsoll D. L. Persistence and reactivation of Rickettsia tsutsugamushi infections in laboratory mice. Jpn J Med Sci Biol. 1979 Jun;32(3):179–184. doi: 10.7883/yoken1952.32.179. [DOI] [PubMed] [Google Scholar]
- Shirai A., Wisseman C. L., Jr Serologic classification of scrub typhus isolates from Pakistan. Am J Trop Med Hyg. 1975 Jan;24(1):145–153. doi: 10.4269/ajtmh.1975.24.145. [DOI] [PubMed] [Google Scholar]


