Abstract
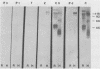
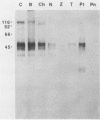
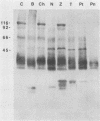
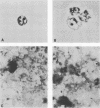
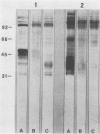
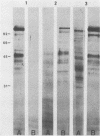
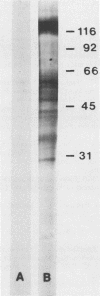
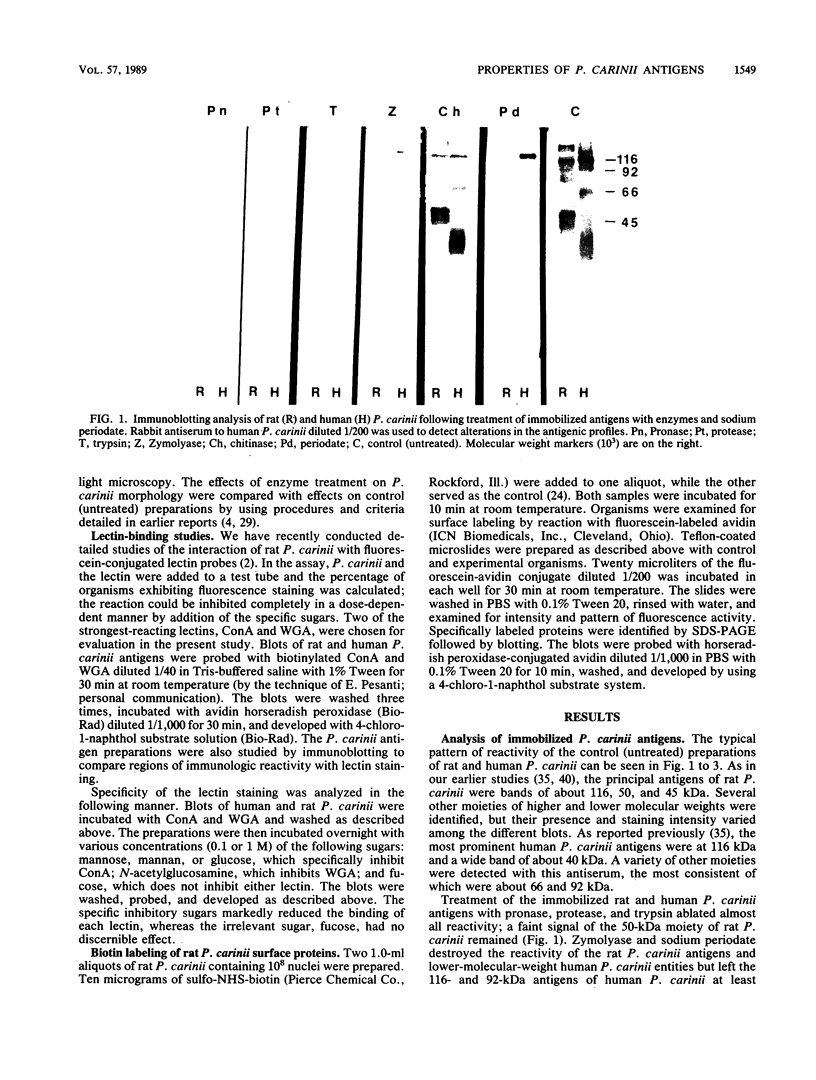
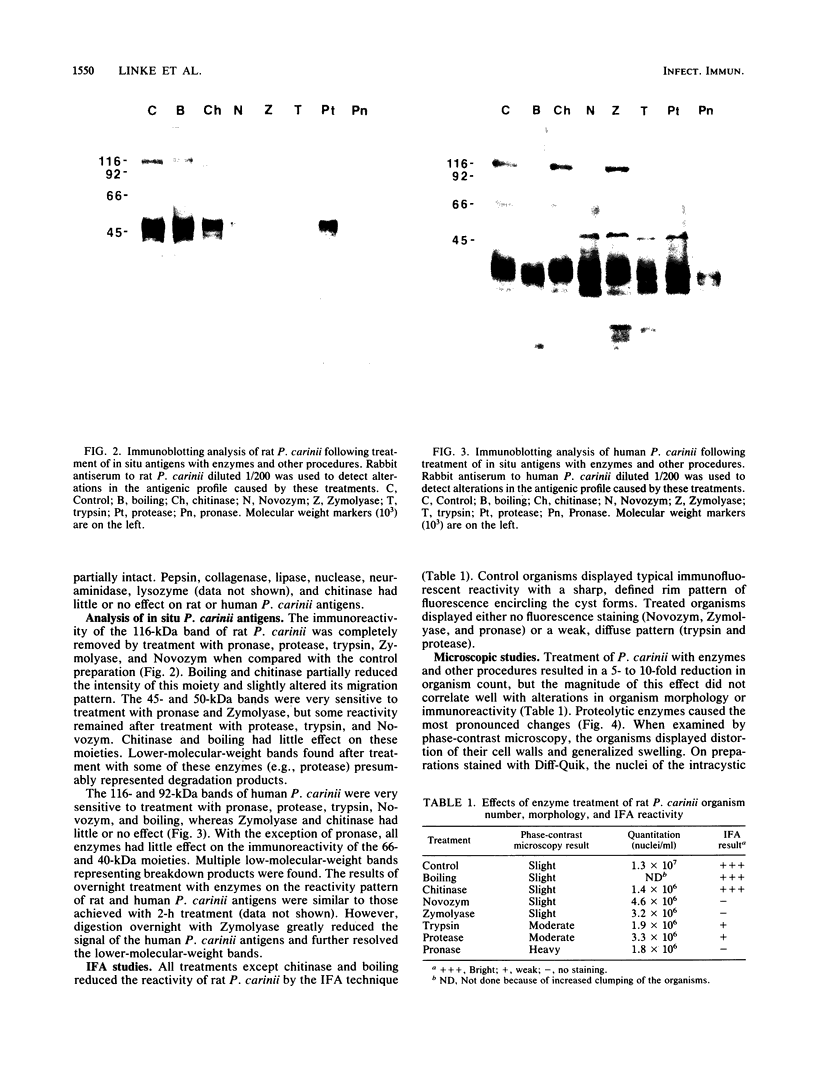
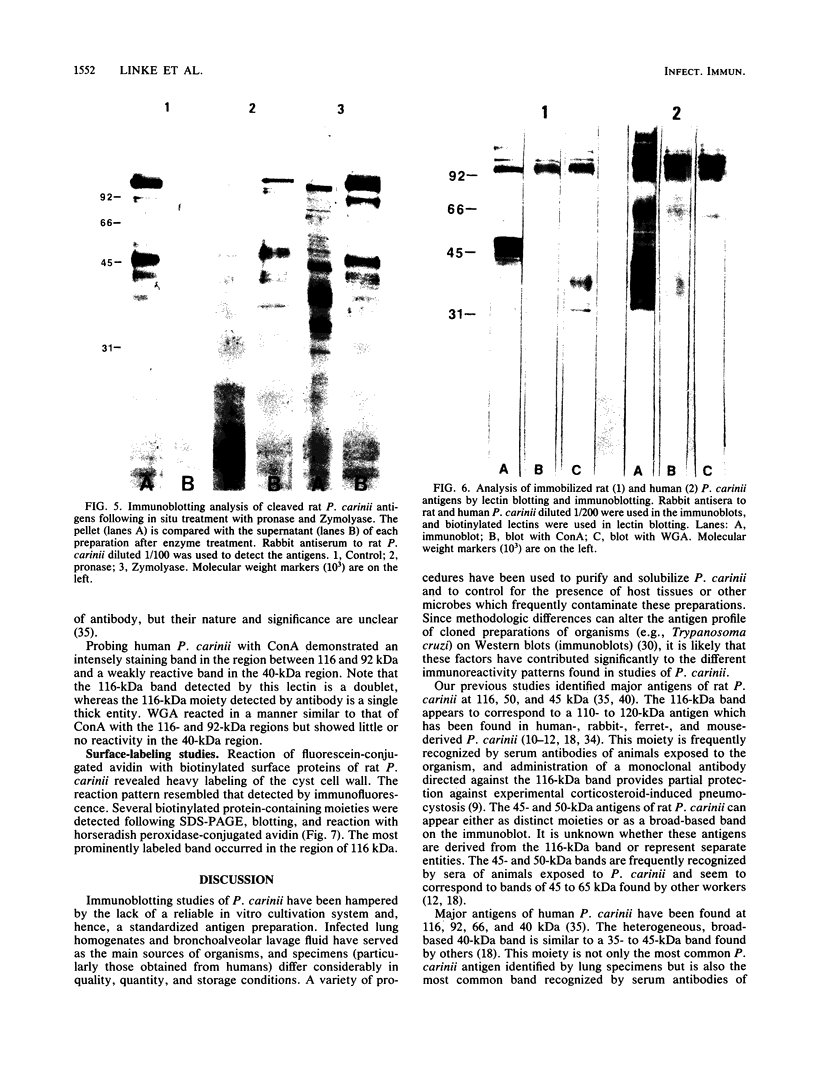
The major rat and human Pneumocystis carinii antigens were analyzed for their susceptibility to treatment with enzymes and other procedures by immunoblotting, immunofluorescence, and light microscopy. Carbohydrate residues were further analyzed by lectin-binding experiments. The 116-kilodalton (kDa) band of rat P. carinii was susceptible to proteolytic (e.g., trypsin) and glycolytic (e.g., Zymolyase) treatments but not to a variety of other procedures (e.g., lipase). This moiety reacted strongly with concanavalin A and wheat germ agglutinin, indicating the presence of mannosyl or glucosyl and N-acetylglucosamine residues. Immunofluorescence staining and surface labeling suggested that the 116-kDa antigen was located on the P. carinii cell wall. The 45- and 50-kDa bands were as sensitive as the 116-kDa band to degradative treatments when studied after immobilization onto nitrocellulose but were more resistant to proteolytic enzymes when studied in situ on whole organisms. These moieties exhibited poor binding to lectins and reactivity by surface-labeling procedures. The 116-kDa band of human P. carinii appeared to be a glycoprotein with characteristics similar to those of its counterpart in rats, whereas the human P. carinii 40-kDa band exhibited protein and carbohydrate properties more closely related to those of the 45- and 50-kDa rat-derived antigens. We conclude that P. carinii antigens are complex glycoproteins and that this information will be helpful in developing strategies for their isolation and purification and study of their function.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cabib E., Roberts R., Bowers B. Synthesis of the yeast cell wall and its regulation. Annu Rev Biochem. 1982;51:763–793. doi: 10.1146/annurev.bi.51.070182.003555. [DOI] [PubMed] [Google Scholar]
- Cushion M. T., DeStefano J. A., Walzer P. D. Pneumocystis carinii: surface reactive carbohydrates detected by lectin probes. Exp Parasitol. 1988 Dec;67(2):137–147. doi: 10.1016/0014-4894(88)90061-6. [DOI] [PubMed] [Google Scholar]
- Cushion M. T., Ruffolo J. J., Linke M. J., Walzer P. D. Pneumocystis carinii: growth variables and estimates in the A549 and WI-38 VA13 human cell lines. Exp Parasitol. 1985 Aug;60(1):43–54. doi: 10.1016/s0014-4894(85)80021-7. [DOI] [PubMed] [Google Scholar]
- Cushion M. T., Ruffolo J. J., Walzer P. D. Analysis of the developmental stages of Pneumocystis carinii, in vitro. Lab Invest. 1988 Mar;58(3):324–331. [PubMed] [Google Scholar]
- Cushion M. T., Stanforth D., Linke M. J., Walzer P. D. Method of testing the susceptibility of Pneumocystis carinii to antimicrobial agents in vitro. Antimicrob Agents Chemother. 1985 Dec;28(6):796–801. doi: 10.1128/aac.28.6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman J. C., Kovacs J. A., Masur H., Santi D. V., Elwood H. J., Sogin M. L. Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi. Nature. 1988 Aug 11;334(6182):519–522. doi: 10.1038/334519a0. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Ballou L. R., Hughes W. T., Mosley B. D. Purification and initial characterization of a ferret Pneumocystis carinii surface antigen. J Infect Dis. 1988 Oct;158(4):848–854. doi: 10.1093/infdis/158.4.848. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Hughes W. T. Passive immunoprophylaxis with specific monoclonal antibody confers partial protection against Pneumocystis carinii pneumonitis in animal models. J Clin Invest. 1988 Jun;81(6):1666–1668. doi: 10.1172/JCI113503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti F., Stokes D. C., Cheatham A. B., Davis D. S., Hughes W. T. Development of murine monoclonal antibodies to Pneumocystis carinii. J Infect Dis. 1986 Aug;154(2):315–322. doi: 10.1093/infdis/154.2.315. [DOI] [PubMed] [Google Scholar]
- Graves D. C., McNabb S. J., Ivey M. H., Worley M. A. Development and characterization of monoclonal antibodies to Pneumocystis carinii. Infect Immun. 1986 Jan;51(1):125–133. doi: 10.1128/iai.51.1.125-133.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves D. C., McNabb S. J., Worley M. A., Downs T. D., Ivey M. H. Analyses of rat Pneumocystis carinii antigens recognized by human and rat antibodies by using western immunoblotting. Infect Immun. 1986 Oct;54(1):96–103. doi: 10.1128/iai.54.1.96-103.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Hocking R. E. Stage-specific, strain-specific, and cross-reactive antigens of Leishmania species identified by monoclonal antibodies. Infect Immun. 1982 Jul;37(1):28–33. doi: 10.1128/iai.37.1.28-33.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm C., Meeks-Wagner D. W., Fangman W. L., Botstein D. A rapid, efficient method for isolating DNA from yeast. Gene. 1986;42(2):169–173. doi: 10.1016/0378-1119(86)90293-3. [DOI] [PubMed] [Google Scholar]
- Kim C. K., Foy J. M., Cushion M. T., Stanforth D., Linke M. J., Hendrix H. L., Walzer P. D. Comparison of histologic and quantitative techniques in evaluation of therapy for experimental Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1987 Feb;31(2):197–201. doi: 10.1128/aac.31.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. K., Hughes W. T., Feldman S. Studies of morphology and immunofluorescence of Pneumocystis carinii. Proc Soc Exp Biol Med. 1972 Oct;141(1):304–309. doi: 10.3181/00379727-141-36764. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Gill V., Swan J. C., Ognibene F., Shelhamer J., Parrillo J. E., Masur H. Prospective evaluation of a monoclonal antibody in diagnosis of Pneumocystis carinii pneumonia. Lancet. 1986 Jul 5;2(8497):1–3. doi: 10.1016/s0140-6736(86)92555-9. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Halpern J. L., Swan J. C., Moss J., Parrillo J. E., Masur H. Identification of antigens and antibodies specific for Pneumocystis carinii. J Immunol. 1988 Mar 15;140(6):2023–2031. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Bolinger C. D., Bartlett M. S., Kohler R. B., Wilde C. E., 3rd, Smith J. W. Production of monoclonal antibody against Pneumocystis carinii by using a hybrid of rat spleen and mouse myeloma cells. J Clin Microbiol. 1986 Mar;23(3):505–508. doi: 10.1128/jcm.23.3.505-508.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder E., Lundin L., Vorma H. Detection of Pneumocystis carinii in lung-derived samples using monoclonal antibodies to an 82 kDa parasite component. J Immunol Methods. 1987 Apr 2;98(1):57–62. doi: 10.1016/0022-1759(87)90435-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Amagai T., Yamada M., Imanishi J., Yoshida Y. Production of a monoclonal antibody with specificity for the pellicle of Pneumocystis carinii by hybridoma. Parasitol Res. 1987;73(3):228–233. doi: 10.1007/BF00578509. [DOI] [PubMed] [Google Scholar]
- Mead J. R., Arrowood M. J., Sterling C. R. Antigens of Cryptosporidium sporozoites recognized by immune sera of infected animals and humans. J Parasitol. 1988 Feb;74(1):135–143. [PubMed] [Google Scholar]
- Meuwissen J. H., Tauber I., Leeuwenberg A. D., Beckers P. J., Sieben M. Parasitologic and serologic observations of infection with Pneumocystis in humans. J Infect Dis. 1977 Jul;136(1):43–49. doi: 10.1093/infdis/136.1.43. [DOI] [PubMed] [Google Scholar]
- Milder J. E., Walzer P. D., Coonrod J. D., Rutledge M. E. Comparison of histological and immunological techniques for detection of Pneumocystis carinii in rat bronchial lavage fluid. J Clin Microbiol. 1980 Apr;11(4):409–417. doi: 10.1128/jcm.11.4.409-417.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. Pneumocystis carinii and Toxoplasma gondii infections in patients with AIDS. Rev Infect Dis. 1986 Nov-Dec;8(6):1001–1011. doi: 10.1093/clinids/8.6.1001. [DOI] [PubMed] [Google Scholar]
- Norman L., Kagan I. G. Some observations on the serology of Pneumocystis carinii infections in the United States. Infect Immun. 1973 Sep;8(3):317–321. doi: 10.1128/iai.8.3.317-321.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffolo J. J., Cushion M. T., Walzer P. D. Techniques for examining Pneumocystis carinii in fresh specimens. J Clin Microbiol. 1986 Jan;23(1):17–21. doi: 10.1128/jcm.23.1.17-21.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter M., Nogueira N. Variations induced by different methodologies in Trypanosoma cruzi surface antigen profiles. Mol Biochem Parasitol. 1988 May;29(1):37–45. doi: 10.1016/0166-6851(88)90117-x. [DOI] [PubMed] [Google Scholar]
- Stahr B. J., Walzer P. D., Yoneda K. Effect of proteolytic enzymes on Pneumocystis carinii in rat lung tissue. J Parasitol. 1981 Apr;67(2):196–202. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Kim C. K., Linke M. J., Pogue C. L., Huerkamp M. J., Chrisp C. E., Lerro A. V., Wixson S. K., Hall E., Shultz L. D. Outbreaks of Pneumocystis carinii pneumonia in colonies of immunodeficient mice. Infect Immun. 1989 Jan;57(1):62–70. doi: 10.1128/iai.57.1.62-70.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Linke M. J. A comparison of the antigenic characteristics of rat and human Pneumocystis carinii by immunoblotting. J Immunol. 1987 Apr 1;138(7):2257–2265. [PubMed] [Google Scholar]
- Walzer P. D., Powell R. D., Jr, Yoneda K., Rutledge M. E., Milder J. E. Growth characteristics and pathogenesis of experimental Pneumocystis carinii pneumonia. Infect Immun. 1980 Mar;27(3):928–937. doi: 10.1128/iai.27.3.928-937.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Rutledge M. E. Comparison of rat, mouse, and human Pneumocystis carinii by immunofluorescence. J Infect Dis. 1980 Sep;142(3):449–449. doi: 10.1093/infdis/142.3.449. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Rutledge M. E. Humoral immunity in experimental Pneumocystis carinii infection. I. Serum and bronchial lavage fluid antibody responses in rats. J Lab Clin Med. 1981 Jun;97(6):820–833. [PubMed] [Google Scholar]
- Walzer P. D., Rutledge M. E. Serum antibody responses to Pneumocystis carinii among different strains of normal and athymic mice. Infect Immun. 1982 Feb;35(2):620–626. doi: 10.1128/iai.35.2.620-626.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Stanforth D., Linke M. J., Cushion M. T. Pneumocystis carinii: immunoblotting and immunofluorescent analyses of serum antibodies during experimental rat infection and recovery. Exp Parasitol. 1987 Jun;63(3):319–328. doi: 10.1016/0014-4894(87)90179-2. [DOI] [PubMed] [Google Scholar]
- Wolska-Mitaszko B., Jakubowicz T., Kucharzewska T., Gasior E. An efficient technique for the isolation of yeast spores and the preparation of spheroplast lysates active in protein synthesis. Anal Biochem. 1981 Sep 15;116(2):241–247. doi: 10.1016/0003-2697(81)90351-1. [DOI] [PubMed] [Google Scholar]