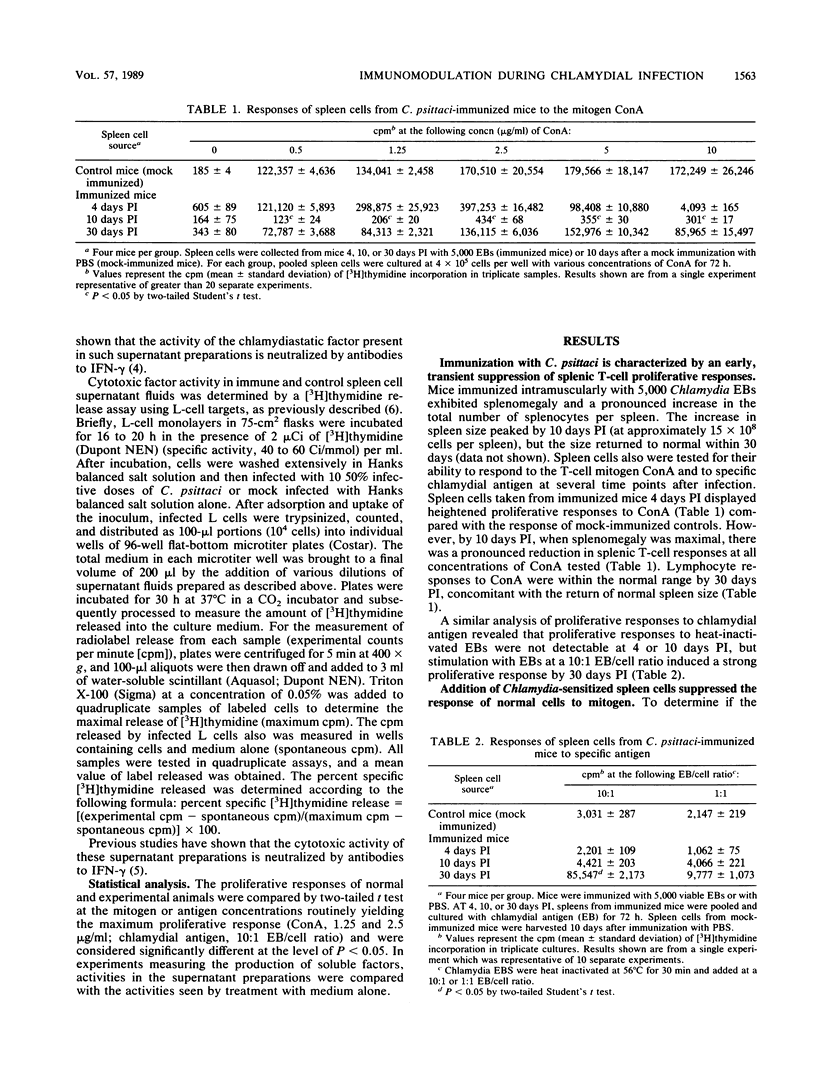
Abstract
A murine model was utilized to study immune responses occurring during the period of acquisition of immunity to chlamydial infection. C3H (H-2k) mice were immunized by intramuscular injection of 5 x 10(3) viable Chlamydia psittaci elementary bodies (EBs) by a protocol which permits animals to survive an otherwise lethal intraperitoneal challenge 10 days later with the homologous chlamydial strain. Spleen cells assayed during the 10-day period of development to immunity showed depressed proliferative responses in vitro to the T-cell mitogen, concanavalin A, and also exhibited suppressor cell activity. Spleen cell mitogen responses returned to normal levels by 30 days postimmunization, concomitant with the detectable development in vitro of responses to chlamydia-specific antigen. In marked contrast to the reduced proliferative responses, mitogen-stimulated production of the T-cell-derived lymphokines interleukin-2 and gamma interferon by spleen cells from immunized animals was within the normal range at 10 days postimmunization, and supernatant fluids containing these products had both microbicidal and microbistatic effects on chlamydial organisms in vitro. These results demonstrate that independent regulation of T-cell proliferation and lymphokine production occurs in vivo as part of the development of an antigen-specific protective immune response. These results also suggest that such differential modulation of T-cell responses may contribute to the development of protective immunity to chlamydiae in mice, perhaps through limited T-cell clonal expansion coupled with early or preferential maturation of cytokine-secreting helper T cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- Byrne G. I., Grubbs B., Marshall T. J., Schachter J., Williams D. M. Gamma interferon-mediated cytotoxicity related to murine Chlamydia trachomatis infection. Infect Immun. 1988 Aug;56(8):2023–2027. doi: 10.1128/iai.56.8.2023-2027.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Krueger D. A. In vitro expression of factor-mediated cytotoxic activity generated during the immune response to Chlamydia in the mouse. J Immunol. 1985 Jun;134(6):4189–4193. [PubMed] [Google Scholar]
- Byrne G. I., Krueger D. A. Lymphokine-mediated inhibition of Chlamydia replication in mouse fibroblasts is neutralized by anti-gamma interferon immunoglobulin. Infect Immun. 1983 Dec;42(3):1152–1158. doi: 10.1128/iai.42.3.1152-1158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Requirements for ingestion of Chlamydia psittaci by mouse fibroblasts (L cells). Infect Immun. 1976 Sep;14(3):645–651. doi: 10.1128/iai.14.3.645-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Harris D. T., Kozumbo W. J., Cerutti P., Cerottini J. C. Molecular mechanisms involved in T cell activation. I. Evidence for independent signal-transducing pathways in lymphokine production vs proliferation in cloned cytotoxic T lymphocytes. J Immunol. 1987 Jan 15;138(2):600–605. [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckford S. E., Gelmann E. P., Agnor C. L., Jacobson S., Zinn S., Matis L. A. Distinct signals are required for proliferation and lymphokine gene expression in murine T cell clones. J Immunol. 1986 Dec 1;137(11):3652–3663. [PubMed] [Google Scholar]
- Huebner R. E., Byrne G. I. In vivo-activated mononuclear phagocytes and protective immunity to chlamydiae in mice. Infect Immun. 1988 Jun;56(6):1492–1499. doi: 10.1128/iai.56.6.1492-1499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- Koster F. T., Williams J. C., Goodwin J. S. Cellular immunity in Q fever: modulation of responsiveness by a suppressor T cell-monocyte circuit. J Immunol. 1985 Aug;135(2):1067–1072. [PubMed] [Google Scholar]
- Kuo C. C., Grayston T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976 Apr;13(4):1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert J. K. Cytotoxic cells induced after Chlamydia psittaci infection in mice. Infect Immun. 1982 Mar;35(3):1011–1017. doi: 10.1128/iai.35.3.1011-1017.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammert J. K., Wyrick P. B. Modulation of the host immune response as a result of Chlamydia psittaci infection. Infect Immun. 1982 Feb;35(2):537–545. doi: 10.1128/iai.35.2.537-545.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D., Danen R., Bard J. Both species of chlamydia and two biovars of Chlamydia trachomatis stimulate mouse B lymphocytes. J Immunol. 1986 Jun 1;136(11):4249–4254. [PubMed] [Google Scholar]
- Robb R. J., Smith K. A. Heterogeneity of human T-cell growth factor(s) due to variable glycosylation. Mol Immunol. 1981 Dec;18(12):1087–1094. doi: 10.1016/0161-5890(81)90024-9. [DOI] [PubMed] [Google Scholar]
- Taylor C. E., Baker P. J. Production of soluble suppressor factor by spleen cells from mice immunized with type III pneumococcal polysaccharide. J Immunol. 1985 Oct;135(4):2551–2556. [PubMed] [Google Scholar]
- Watson R. R., MacDonald A. B., Murray E. S., Modabber F. Z. Immunity to chlamydial infections of the eye. 3. Presence and duration of delayed hypersensitivity to guinea pig inclusion conjuctivitis. J Immunol. 1973 Aug;111(2):618–623. [PubMed] [Google Scholar]


