Abstract
Cytotoxicity is an important virulence determinant in the pathogenesis of Vibrio vulnificus, and two cytotoxins, RTX (encoded by rtxA1) and cytolysin/hemolysin (encoded by vvhA), have been identified in this organism. We showed that the quorum-sensing regulator LuxO controlled the cytotoxicity of this organism: a ΔluxO mutant exhibited low cytotoxicity, whereas a constitutively activated luxO mutant, luxO(D47E), remained highly cytotoxic. The cytotoxicity of the ΔluxO mutant was restored when smcR, a Vibrio harveyi luxR homologue repressed by luxO, was further deleted. SmcR then was shown to repress the expression of both rtxA1 and vvhA. A DNA library of V. vulnificus was screened in Escherichia coli for clones that upregulated vvhA in the presence of SmcR, and hlyU, which has been shown to positively regulate rtxA1 and vvhA, was identified. We demonstrated that SmcR repressed the expression of hlyU and bound to a region upstream of hlyU in V. vulnificus. The deletion of hlyU resulted in the loss of cytotoxicity and reduced cytolysin/hemolysin production in the ΔsmcR mutant. The ΔsmcR ΔhlyU mutant regained cytotoxicity and cytolysin/hemolysin activity when hns, which has been shown to repress the transcription of rtxA1 and interfere with hlyU, was further removed. Collectively, our data suggest that SmcR mediates the regulation of cytotoxicity by quorum-sensing signaling in V. vulnificus by repressing hlyU, an activator of rtxA1 and vvhA.
INTRODUCTION
Vibrio vulnificus, a Gram-negative marine bacterium, is an opportunistic pathogen causing septicemia and wound infection in humans; it has a high mortality rate, particularly in those who suffer from chronic liver diseases or are immunocompromised. This organism produces two major cytotoxins, cytolysin/hemolysin (encoded by vvhA) and RTX (repeats in toxin; encoded by rtxA1), that are implicated in its virulence (19, 29). The cytolysin/hemolysin, which is an extracellular product, is lethal for mice at submicrogram levels. It lyses erythrocytes, increases vascular permeability (resulting in extensive extracellular edema in guinea pig skin), damages capillary endothelial cells, and causes mild inflammatory cell infiltration (7, 8). The RTX toxin, which forms pores on cell membranes only after the contact of the bacterium with the host cell (12, 13), is required for V. vulnificus virulence in mice by promoting bacterial colonization at the infection site and subsequent invasion into the bloodstream (12, 15).
Several regulators of vvhA and rtxA1 have been identified (1, 19). HlyU, which was identified by in vivo-induced antigen technology and is essential for V. vulnificus virulence in mice (11, 19), is required for the expression of vvhA and rtxA1 and binds directly to a region upstream of the operon where rtxA1 is located (19, 20).
Quorum-sensing (QS) signaling, which is widely used by bacteria to communicate with each other, regulates the virulence genes in a variety of microorganisms, including Vibrio species (23, 30). In Vibrio cholerae, the signals transduced from at least three QS sensory circuits integrate into LuxO, a repressor of hapR (a luxR homologue) that negatively regulate the virulence regulon (30). The repression of hapR by LuxO is mediated by a set of QS-regulatory small RNAs, qrr1-4. In detail, the activated LuxO, together with RpoN, turns on the expression of qrr1-4, and these small RNAs in turn destabilize hapR mRNA in the presence of the qrr-binding protein Hfq (18).
The homologues of most genes of QS signaling in V. cholerae, including luxS (coding for the synthesis of autoinducer AI-2), luxO, qrr1-4, and hapR, are found in the whole-genome sequences of V. vulnificus strains YJ016 (2) and CMCP6. Kim et al. have reported that, in the absence of AI-2 (caused by a deletion in luxS), the production of protease was delayed and cytolysin/hemolysin production was increased. Further, these changes were complemented by AI-2 contained in the log-phase spent medium of the wild-type V. vulnificus (10). These findings suggest that the expression of both the protease and cytolysin/hemolysin is regulated by quorum-sensing signaling. However, whether QS is involved in the regulation of cytotoxicity mediated by cytolysin/hemolysin and RTX toxin remains unclear. Therefore, we designed experiments to determine if the cytotoxicity of V. vulnificus is regulated by QS and further explore the mechanism of regulation.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers.
The bacterial strains and plasmids used are listed in Table 1, and the primers used are listed in Table S1 in the supplemental material. The nucleotide sequences of primers were derived from the whole-genome sequence of V. vulnificus strain YJ016 (NCBI accession no. NC 005139-40). The bacteria were grown at 37°C with shaking at 240 rpm in Luria-Bertani (LB) medium, to which ampicillin (100 μg/ml), polymyxin B (50 U/ml), or kanamycin (50 μg/ml) was added as appropriate. Throughout this study, a 4-h culture at 37°C, starting from a 100-fold dilution of an overnight culture, was used to infect the cultured cells.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| V. vulnificus | ||
| YJ016 | Clinical isolate | Laboratory collection |
| CP156 | YJ016 ΔsmcR | 27 |
| YJ016ΔsmcRΔvvp | CP156 deleted of vvp | This study |
| YJ016ΔrtxA1 | YJ016 deleted of rtxA1 | 21 |
| YJ016ΔluxO | YJ016 deleted of luxO | This study |
| YJ016luxO(D47E) | YJ016 with luxO replaced by luxO(D47E) | This study |
| YJ016ΔvvhA | YJ016 deleted of vvhA | This study |
| YJ016ΔluxOΔsmcR | YJ016ΔluxO deleted of smcR | This study |
| YJ016ΔluxOΔsmcR::psmcR | YJ016ΔluxOΔsmcR with psmcR integrated into the vicinity of deletion in smcR; Apr | This study |
| YJ016ΔluxOΔsmcRΔvvhA | YJ016ΔluxOΔsmcR deleted of vvhA | This study |
| YJ016ΔluxOΔsmcRΔvvhA::psmcR | YJ016ΔluxOΔsmcRΔvvhA with psmcR integrated into the vicinity of deletion in smcR; Apr | This study |
| YJ016ΔluxOΔsmcRΔrtxA1 | YJ016ΔluxOΔsmcR deleted of rtxA1 | This study |
| YJ016ΔluxOΔsmcRΔrtxA1::psmcR | YJ016ΔluxOΔsmcRΔrtxA1 with psmcR integrated into the vicinity of deletion in smcR; Apr | This study |
| YJ016ΔluxOΔsmcRΔvvhAΔrtxA1 | YJ016ΔluxOΔsmcRΔvvhA deleted of rtxA1 | This study |
| YJ016ΔluxOΔsmcRΔvvhAΔrtxA1::psmcR | YJ016ΔluxOΔsmcRΔvvhAΔrtxA1 with psmcR integrated into the vicinity of deletion in smcR; Ap | This study |
| YJ016luxO(D47E) ΔsmcR | YJ016luxO(D47E) deleted of smcR | This study |
| YJ016ΔvvhAΔrtxA1 | YJ016ΔrtxA1 deleted of vvhA | This study |
| YJ016ΔlacZ | YJ016 deleted of lacZ | This study |
| YJ016ΔsmcRΔlacZ | CP156 deleted of lacZ | This study |
| YJ016ΔhlyU | YJ016 deleted of hlyU | This study |
| YJ016 ΔsmcRΔhlyU | YJ016ΔhlyU deleted of smcR | This study |
| YJ016luxO(D47E) ΔhlyU | YJ016 luxO(D47E) deleted of hlyU | This study |
| YJ016ΔluxOΔsmcRΔhlyU | YJ016ΔluxOΔsmcR deleted of hlyU | This study |
| YJ016Δhns | YJ016 deleted of hns | This study |
| YJ016Δhns::phns | YJ016Δhns with phns integrated into the vicinity of deletion in hns; Apr | This study |
| YJ016ΔhlyUΔhns | YJ016ΔhlyU deleted of hns | This study |
| YJ016ΔhlyUΔhns::phns | YJ016ΔhlyUΔhns with phns integrated into the vicinity of deletion in hns; Apr | This study |
| YJ016ΔhlyUΔsmcRΔhns | YJ016ΔhlyUΔsmcR deleted of hns | This study |
| YJ016ΔhlyUΔsmcRΔhns::phns | YJ016ΔhlyUΔsmcRΔhns with phns integrated into the vicinity of deletion in hns; Apr | This study |
| E. coli | ||
| DH5α | supE44 lacU169(φ80lacZΔM15) hrdR17recA1endA1gyrA96thi-1relA | 9 |
| S17-1λpir | thi pro hsdR hsdM+recA::RP4-2-Tc::Mu λpir Kmr Nalr | 28 |
| KV372 | DH5α carrying pVH84 | This study |
| Plasmids | ||
| pJRD215 | Shuttle vector; Kmr | 3 |
| pUC19 | Cloning vector; Apr | Laboratory collection |
| pBR322 | Cloning vector; Apr Tcr | Laboratory collection |
| pGemT-easy | Cloning vector; Apr | Laboratory collection |
| pCVD442 | Suicide vector; Apr | 4 |
| pΔluxO | pCVD442 inserted with ΔluxO | This study |
| pΔrtxA1 | pCVD442 inserted with ΔrtxA1 | 21 |
| pΔvvhA | pCVD442 inserted with ΔvvhA | This study |
| pΔhlyU | pCVD442 inserted with ΔhlyU | This study |
| pΔhns | pCVD442 inserted with Δhns | This study |
| pGemT-hns | pGemT-easy inserted with hns+ | This study |
| pOU1 | pUC19 inserted with luxO+ | This study |
| pluxO(D47E) | pCVD442 inserted with luxO(D47E) | This study |
| psmcR | pCVD442 inserted with smcR+ | This study |
| phlyU | pJRD215 inserted with hlyU+ | This study |
| phns | pCVD442 inserted with hns+ | This study |
| pVR19 | pCVD442 inserted with ΔsmcR | 27 |
| pSI026 | pCVD442 inserted with Δvvp | 26 |
| pVP84 | pJRD215 containing a promoterless lacZ gene of YJ016 | Laboratory collection |
| pVH84 | pVP84 inserted with a 675-bp, vvhBA promoter-containing DNA fragment in front of lacZ | This study |
| pVU84 | pVP84 containing 128 bp of hlyU promoter region in front of lacZ | This study |
| pVR11 | pBR322 inserted with a 3.4-kb DNA fragment that contains smcR | 27 |
| pVR11.2 | pBR322 inserted with a 1-kb smcR-containing DNA fragment cloned from pVR11 | This study |
| pVR11.3 | pBR322 inserted with ΔsmcR | This study |
| pVR11.2-L24 | pVR11.2 inserted with a 3.6-kb DNA fragment that contains ORFs VV0681, VV0682, and hlyU | This study |
| pVR11.2-L241 | pVR11.2 inserted with a 3.6-kb fragment that contains ORF VV0681 | This study |
| pVR11.2-L242 | pVR11.2 inserted with a 2.4-kb fragment that contains ORF VV0682 | This study |
| pVR11.2-L243 | pVR11.2 inserted with a 2.38-kb fragment that contains ORFs VV0682 and hlyU | This study |
| pVR11.2-L244 | pVR11.2 inserted with a 0.77-kb fragment that contains ORF VV hlyU | This study |
| pVR11.3-L244 | pVR11.2-L244 ΔsmcR | This study |
| pET30b | Protein expression vector; Kmr | Laboratory collection |
| pVR282 | pET30b inserted with smcR+ for producing C-terminally His6-tagged SmcR | This study |
| pVR35 | pJRD215 inserted with His6-tagged smcR+ cloned from pVR282 | This study |
Abbreviations: Apr, Kmr, and Nalr are resistance to ampicillin, kanamycin, and nalidixic acid, respectively.
DNA preparation and manipulation.
The plasmid and genomic DNAs were purified by a Wizard plasmid/genomic DNA purification kit (Promega, Madison, WI). Standard techniques were used to construct the recombinant plasmids (25). DNA restriction endonucleases and T4 DNA ligase were from New England BioLabs. PCR was performed as described previously (26) with a thermocycler (Mastercycler gradient; Eppendorf AG, Hamburg, Germany).
Isolation of V. vulnificus mutants and their complemented strains.
V. vulnificus mutants with deletions in luxO, rtxA1, vvhA, hlyU, and hns, as well as the luxOD47E mutant, which contains an Asp-to-Glu mutation at the 47th residue that results in a constitutively active LuxO in V. harveyi (6), were isolated by in vivo allelic exchange (26). Briefly, for the isolation of deletion mutants, derivatives of suicide plasmid pCVD442, containing various DNA fragments with the desired deletions, first were constructed by the ligation of pCVD442 linearized by an appropriate restriction enzyme with the PCR-amplified upstream and downstream DNA fragments flanking the deletion. The corresponding primer pairs used to amplify the upstream (U) and downstream (D) DNA fragments were UVV1195F-UVV1195R and DVV1195F-DVV1196R for luxO, UVVA0965F1-UVVA0965R and DVVA0965F-DVVA0965R for vvhA, UVV0683F-UVV0683R and DVV0683F-DVV0683R for hlyU, and UVV1346F-UVV1346R and DVV1346F-DVV1346R for hns (see Table S1 in the supplemental material). The resultant plasmids were pΔluxO, pΔvvhA, pΔhlyU, and pΔhns (1,311, 1,032, 210, and 300 bp, respectively). In-frame deletions then were used to generate the specific gene knockout mutants. The ΔrtxA1 mutants were isolated as described previously (21).
The luxO(D47E) mutant was isolated similarly, except that the mutation was introduced into pOU1, a pUC19 derivative containing the luxO gene amplified with primers vlo7 and vlo10, by a QuikChange site-directed mutagenesis kit (Stratagene, CA) and primers D47EF and D47ER (see Table S1). The pCVD442 derivative carrying the luxO(D47E) mutation (pluxOD47E) was transferred to the ΔluxO mutant instead of the wild-type strain to facilitate the screening of mutants by PCR.
For the complementation of the ΔsmcR mutant, a 2.1-kb NheI-NheI DNA fragment that contained the entire smcR gene was excised from pVR11 (pBR322 with smcR inserted) and was inserted into the XbaI site of pCVD442 to generate psmcR. This plasmid then was transferred to the ΔsmcR mutants by conjugation, and the transconjugants with the whole plasmid integrated adjacent to the deletion in smcR by homologous recombination were selected. For the complementation of the ΔhlyU mutants, a 776-bp BglII-BamHI DNA fragment with the entire hlyU gene was cloned from pVR11.2-L24 (pBR322 derivative containing hlyU) into the BamHI site of pJRD215, a broad-host-range plasmid, and the recombinant plasmid then was transferred to the ΔhlyU mutants by conjugation. For the complementation of the Δhns mutants, a 2,041-bp, hns-containing DNA fragment amplified by PCR with primers UVV1346F and DVV1346R was cloned into the SacI site of pCVD442. The resultant plasmid subsequently was transferred to the Δhns mutants by conjugation, and the transconjugants with the whole plasmid integrated adjacent to the deletion in hns by homologous recombination were selected.
Cytotoxicity assay.
Monolayers of HEp-2 cells, about 1.5 × 104 cells per well in a 96-well microplate, were infected with phosphate-buffered saline (PBS)-washed bacteria harvested from a 4-h culture at a multiplicity of infection (MOI) of 10 for 4 h. The infected monolayer then was assayed for cell lysis with a CytoTox 96 nonradioactive cytotoxicity kit (Promega) that measures the activity of lactate dehydrogenase (LDH) released from lysed cells. The cytotoxicity is expressed as 100 × [(LDH activity of infected monolayer − LDH activity of monolayer treated with medium)/(the LDH activity of monolayer treated with lysis buffer − LDH activity of monolayer treated with medium)].
Cytolysin/hemolysin assay.
The amount of cytolysin/hemolysin produced in culture supernatant or conditioned medium of infected cells was assayed by the hemolysis of mouse red blood cells (RBCs). The sample was mixed with an equal volume of mouse RBC suspension (0.7 to 0.9% in PBS), and the mixture was incubated at 37°C for 30 min. The unlysed RBCs and cell debris were pelleted by centrifugation, and the optical density at 545 nm (OD545) of the supernatant, which represents the level of hemolysis, was measured. The cytolysin/hemolysin level is expressed as 100 × (OD545 of RBC suspension treated with sample/OD545 of RBC suspension treated with 1% Triton X-100).
RNA isolation and cDNA synthesis.
Total RNA was extracted from V. vulnificus cells by TRI reagent (Sigma-Aldrich), and the concentration of RNA was determined with a BioPhotometer (Eppendorf). DNA contaminants were removed by DNase (RQ1 RNase-free DNase; Promega) before RNA precipitation by ethanol. The cDNA of total RNA (2 μg per reaction mixture) was synthesized with a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA).
Real-time PCR.
Real-time PCR was performed with Mastercycler ep realplex (Eppendorf). The standard 1× real-time PCR mixture contained 12.5 μl 2× Power SYBR green PCR master mix (Applied Biosystems), 50 ng cDNA, and 200 nM (each) forward and reverse primers. The 23S rRNA gene was used as an internal control, and the experiment was performed in triplicate. The reaction consisted of an initial denaturing at 95°C for 10 min, followed by 40 cycles of denaturing at 95°C for 15 s and annealing at 60°C for 60 s. DNA polymerization was conducted in a range of temperatures from 60 to 95°C within 20 min to obtain the melting curve for determining the PCR amplification specificity. The threshold cycle (CT) value and relative change in expression level were determined by automated threshold analysis with Realplex software, v. 1.5 (Eppendorf).
Construction of the reporter strains and β-galactosidase assay.
The Escherichia coli DH5α reporter strains, which expressed lacZ under the control of various V. vulnificus promoters, were constructed for studying the regulation of these promoters. To construct the PvvhBA-lacZ fusion, a 675-bp DNA fragment that contained the promoter of the vvhBA operon and an NdeI restriction site with a start codon, ATG, was amplified by PCR with primers pvvhF and pvvhR. The PCR product was cloned into pVP84, which contained a promoterless lacZ gene from V. vulnificus, at the NdeI site to create pVH84. For the construction of the PhlyU-lacZ fusion, a 299-bp DNA fragment that contained part of VV0682, the entire intergenic region upstream of hlyU, and an NdeI site with a start codon, ATG, was amplified by PCR with primers phlyUF and phlyUR. The PCR product then was cloned into pVP84 at the NdeI site to create pVU84. For β-galactosidase assay of V. vulnificus, pVU84 was transferred from E. coli S17-1λpir to the various V. vulnificus strains by conjugation.
To assay the activity of the promoter fused with lacZ in a reporter strain, an overnight bacterial culture starting with a single colony picked from a 2-day plate culture was 100-fold diluted in fresh LB medium and incubated at 37°C with shaking. An aliquot of the culture then was collected at intervals, and the β-galactosidase activity in the culture supernatant was determined as described previously (22).
Library construction, screening, and subcloning.
The genomic DNA was extracted from V. vulnificus YJ016 and partially digested with Sau3AI, and the DNA fragments ranging from 3 to 6 kb were recovered and ligated to the BamHI-linearized pVR11.2, a pBR322 derivative containing the intact smcR gene. The resultant plasmids were introduced into E. coli strain KV372, which carried pVH84 that contained the PvvhBA-lacZ fusion, by transformation to generate a DNA library for screening the gene(s) that could mediate the regulation of vvhA. The blue colonies, which indicated enhanced vvhBA promoter activity by the gene cloned into pVR11.2 on 5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside (X-gal)-containing plates, were selected for further characterization.
Production and purification of SmcR with a His6 tag at C terminus.
The smcR gene with its promoter was amplified by PCR with primers VR22 and VR24, and the PCR product was cloned into pET30b between XbaI and XhoI, a site 5′ to the His6 tag, to generate pVR282. To express the SmcR-His6 protein in V. vulnificus, the smcR-His tag DNA fragment was amplified from pVR282 by PCR with primers VR22 and VRT7 and then cloned into pJRD215 to generate pVR35. To avoid the degradation of SmcR by the extracellular metalloprotease (Vvp) during purification, pVR35 was introduced into a Vvp-deficient V. vulnificus mutant. The ΔsmcR Δvvp V. vulnificus mutant was isolated by introducing a deletion in vvp of CP156 (ΔsmcR mutant) with pSI026, a suicide plasmid carrying Δvvp, as described previously (26). The resultant strain was grown in LB at 37°C to an OD600 of 5, and the bacterial cells were pelleted and then lysed by a French press to release SmcR-His6 recombinant protein. SmcR-His6 in the soluble fraction was purified by affinity chromatography with HisTrap HP columns (GE Healthcare). The concentration of purified SmcR-His6 was estimated by a Bio-Rad protein assay kit.
Gel mobility shift assay.
The biotin-labeled probes A, B, and C were amplified from the hlyU promoter region by PCR with biotin-labeled primer pairs phlyUF3-phlyURB, phlyUF3B-phlyUR1, and phlyUF4-phlyURB, respectively. Purified SmcR-His6 was incubated with the probe for 30 min in the presence of 1 μg poly(dI-dC) (used as a nonspecific competitor) as suggested by the manufacturer of the Panomics electrophoretic mobility shift assay (EMSA) kit (Redwood City, CA). The mixture then was fractionated by electrophoresis on a native polyacrylamide gel (6%) and transferred to a membrane, and the probe was visualized by chemiluminescence with the Panomics EMSA kit.
Statistical analysis.
The cytotoxicity and cytolysin/hemolysin activity in V. vulnificus strains and the levels of β-galactosidase from lacZ driven by vvhBA promoter in E. coli DH5α containing various pVR11.2 derivatives were compared using one-way analysis of variance (ANOVA) with Tukey's honestly significant difference (HSD) test. The mRNA levels and the effect of SmcR on the expression of hlyU in various V. vulnificus strains were compared using one-way ANOVA with Dunnett's post test and two-way ANOVA with Bonferroni's post test, respectively. All experiments were repeated three times, and the results from a representative experiment were expressed as means ± standard deviations (SD) for each group examined. All tests were performed with GraphPad Prism 4.0, and statistical significance was defined as P < 0.05.
RESULTS
Cytotoxicity diminished in ΔluxO mutant is restored when smcR is further deleted.
To investigate the involvement of LuxO in the expression of cytotoxicity in V. vulnificus, the cytotoxicity of a ΔluxO mutant and a constitutively active luxO mutant, luxO(D47E), was assessed. The ΔluxO mutant exhibited very low cytotoxicity to HEp-2 cells compared to that of the wild-type strain (P < 0.001), while the luxO(D47E) mutant was as cytotoxic as the wild-type strain after coincubation for 4 h at an MOI of 10 (Fig. 1A). This indicates that LuxO is associated with the expression of V. vulnificus cytotoxicity.
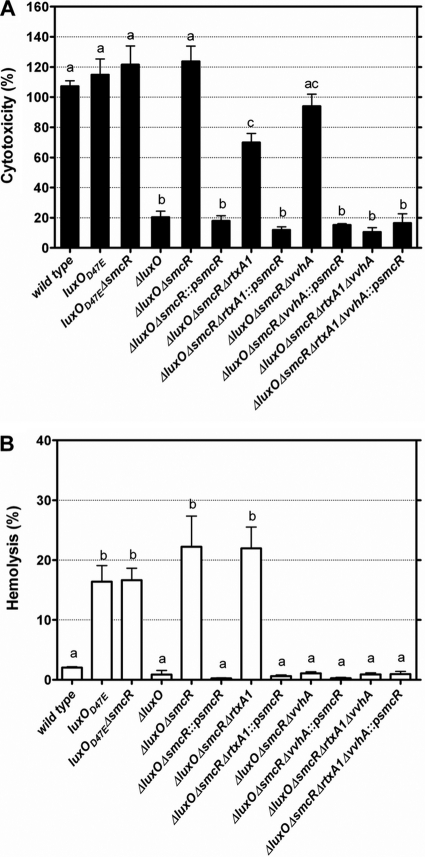
Fig. 1.
Cytotoxicity and cytolysin/hemolysin activity of V. vulnificus luxO and smcR mutants as well as their complemented strains cocultured with HEp-2 cells. The cytotoxicity (A) and cytolysin/hemolysin activity (B) in the conditioned medium were determined after coculture at an MOI of 10 for 4 h. ::psmcR, complementation with smcR. The bars represent means ± standard deviations; n = 3. Bars showing no significant difference are labeled with the same letters, and those showing significant difference (P < 0.05) are labeled with different letters based on one-way ANOVA with Tukey's HSD test.
While the expression of SmcR is negatively regulated by LuxO (24), to determine the role of SmcR in the regulation of cytotoxicity by LuxO, we further deleted smcR in the ΔluxO and luxO(D47E) mutants and determined the cytotoxicity of these double mutants. We found that the luxO(D47E) ΔsmcR mutant remained as cytotoxic as the luxO(D47E) mutant, but the cytotoxicity of the ΔluxO ΔsmcR mutant, compared to that of the ΔluxO mutant, was greatly enhanced (P < 0.001) (Fig. 1A). When the ΔluxO ΔsmcR mutant was complemented with smcR, the cytotoxicity was reduced to a level comparable to that of the ΔluxO mutant (Fig. 1A). These results suggest that the regulation of cytotoxicity by LuxO is mediated by SmcR, which could repress cytotoxicity.
Cytotoxicity downregulated by smcR is attributed to RTX and cytolysin/hemolysin.
To determine the contribution of RTX and cytolysin/hemolysin to the cytotoxicity of the ΔluxO ΔsmcR mutant, vvhA and rtxA1 of this mutant were further deleted, and the cytotoxicity of each resultant mutant was measured. The cytotoxicity of the ΔluxO ΔsmcR ΔrtxA1, ΔluxO ΔsmcR ΔvvhA, and ΔluxO ΔsmcR ΔvvhA ΔrtxA1 mutants to HEp-2 cells was reduced to about 54 (P < 0.001), 76 (P < 0.05), and 8.5% (P < 0.01), respectively, of that of the ΔluxO ΔsmcR mutant (Fig. 1A). The reintroduction of smcR into the ΔluxO ΔsmcR ΔrtxA1 or ΔluxO ΔsmcR ΔvvhA mutant resulted in reduced cytotoxicity (P < 0.01 and 0.001, respectively) to a level similar to that of the ΔluxO ΔsmcR ΔvvhA ΔrtxA1 mutant (Fig. 1A). However, the reintroduction of smcR into the ΔluxO ΔsmcR ΔvvhA ΔrtxA1 mutant did not reduce the cytotoxicity any further (Fig. 1A). These findings suggest that the cytotoxicity downregulated by SmcR is attributed to both cytolysin/hemolysin and RTX.
SmcR downregulates cytolysin/hemolysin production.
Mouse RBCs were lysed by treatment with the culture supernatant of a wild-type V. vulnificus; however, little hemolysis was detected in the culture supernatant of a cytolysin/hemolysin-deficient mutant (5). This indicates that cytolysin/hemolysin is an extracellular product but RTX is not. Since cytolysin/hemolysin contributed to the cytotoxicity detected in the coculture of bacteria and HEp-2 cells, we determined whether cytolysin/hemolysin was detectable in the conditioned medium by hemolysis assay with mouse RBCs. The wild-type strain exhibited a low level of hemolysis in the conditioned medium, but that of the luxO(D47E), luxO(D47E) ΔsmcR, or ΔluxO ΔsmcR mutant, in which SmcR was either deficient or downregulated, was higher (P < 0.001) (Fig. 1B). The deletion of vvhA, but not rtxA1, in the ΔluxO ΔsmcR mutant abolished the hemolytic activity in conditioned medium (Fig. 1B), showing that indeed cytolysin/hemolysin, and only it, was secreted in the conditioned medium. The hemolytic activity in conditioned medium became almost undetectable when the ΔluxO ΔsmcR and ΔluxO ΔsmcR ΔrtxA1 mutants were complemented with smcR (P < 0.001 compared to each parent strain) (Fig. 1B), indicating a negative correlation of smcR with the amount of cytolysin/hemolysin secreted.
LuxO and SmcR regulate expression of rtxA1 and vvhA at transcriptional level.
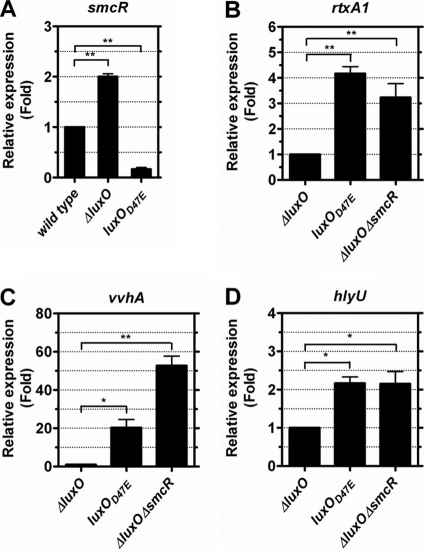
To determine if the regulation of cytotoxicity by LuxO and SmcR is through affecting the transcription of vvhA and rtxA1, the mRNA levels of these two genes in the various luxO and smcR mutants after coincubation with HEp-2 cells were estimated by real-time reverse transcription-PCR (RT-PCR). As was expected, compared to that of the wild-type strain, the mRNA level of smcR was 2-fold increased in the ΔluxO mutant (P < 0.01), but that in the luxO(D47E) mutant was reduced (P < 0.01) (Fig. 2A). Meanwhile, the mRNA levels of both rtxA1 and vvhA in the luxO(D47E) mutant, in which smcR was downregulated, were higher than those in the ΔluxO mutant (P < 0.01 and 0.05, respectively), in which smcR was upregulated (Fig. 2B and C). Nevertheless, when smcR in the ΔluxO mutant was further deleted, both rtxA1 and vvhA were upregulated (P < 0.01) (Fig. 2B and C). Taken together, these data indicate that SmcR negatively regulated the transcription of rtxA1 and, more evidently, vvhA, and in the absence of LuxO, SmcR was upregulated to result in the decreased expression of both rtxA1 and vvhA. In addition, the cytotoxicity to HEp-2 cells and hemolytic activity of the various luxO and smcR mutants (Fig. 1A and B) correlated well with the mRNA levels of rtxA1 and vvhA (Fig. 2B and C).
Fig. 2.
mRNA levels of smcR (A), rtxA1 (B), vvhA (C), and hlyU (D) in various V. vulnificus luxO and smcR mutants after coculture with HEp-2 cells. Washed bacteria prepared from a 4-h culture in LB were coincubated with the HEp-2 cell monolayer at an MOI of 10 for 4 h. The mRNA levels (represented by 2−ΔΔCT values) of various genes in the bacteria then were measured by real-time RT-PCR and expressed as the fold change from that measured in the wild-type strain (A) or the ΔluxO mutant (B, C, and D). The bar represents means ± standard deviations; n = 3. The significance of difference was analyzed by one-way ANOVA with Dunnett's post test. *, P < 0.05; **, P < 0.01.
HlyU mediates the regulation of vvhA by SmcR.
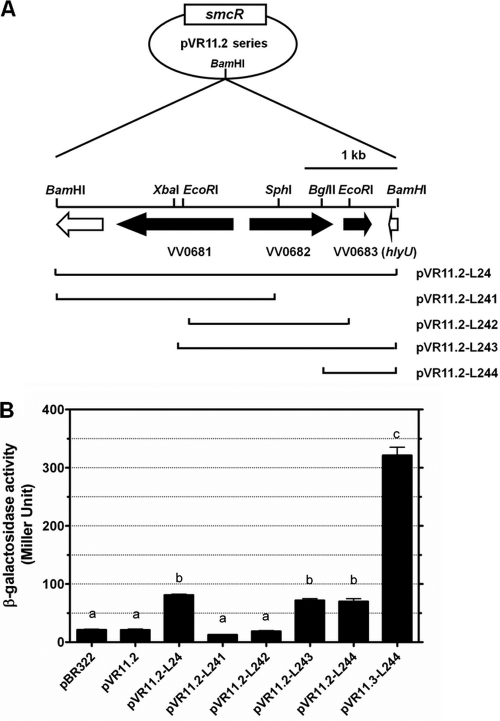
To identify the gene(s) that mediates the regulation of vvhA by SmcR, we screened a V. vulnificus DNA library in an E. coli strain, KV372, which contained a transcriptional fusion of the vvhBA promoter and lacZ (PvvhBA-lacZ) as a reporter, for clones showing upregulated PvvhBA-lacZ in the presence of SmcR. Three out of approximately 8,000 clones screened exhibited deep blue colonies on the X-gal plates after prolonged incubation. One of them, whose plasmid was designated pVR11.2-L24, contained three intact open reading frames (ORFs), VV0681, VV0682, and VV0683, while the others contained the lacZ gene. We further constructed four subclones from pVR11.2-L24 (Fig. 3A) and found that only those (pVR11.2-L243 and pVR11.2-L244) containing ORF VV0683, which encodes HlyU, could upregulate PvvhBA-lacZ (P < 0.001 compared to the vector control, pVR11.2) (Fig. 3B). When the intact smcR gene in pVR11.2-L244 was replaced by ΔsmcR (pVR11.3-L244), the expression of PvvhBA-lac was further increased (P < 0.001) (Fig. 3B).
Fig. 3.
Identification of regulator of vvhA. (A) The restriction map of pVR11.2 series. The three ORFs VV0681, VV0682, and VV0683 (hlyU), identified in the insert of pVR11.2-L24, are indicated with black arrows. Plasmids pVR11.2-L241, pVR11.2-L242, pVR11.2-L243, and pVR11.2-L244 are the derivatives of pVR11.2-L24 that contain various parts of the insert as indicated. (B) The levels of β-galactosidase encoded from lacZ driven by the promoter of the vvhBA operon in E. coli DH5α containing various pVR11.2 derivatives. The bacteria were grown in LB at 37°C for 2 h, and the β-galactosidase activity in the culture supernatant was determined. pBR322, vector; pVR11.3-L244, pVR11.2-L244 deleted of smcR. Bars represent means ± standard deviations; n = 3. Those showing no significant difference are labeled with the same letters, and those showing significant difference (P < 0.05) are labeled with different letters based on one-way ANOVA with Tukey's HSD test.
hlyU is involved in regulation of cytotoxicity by QS signaling.
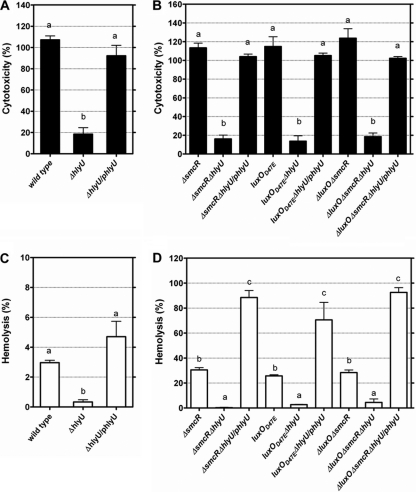
HlyU has been shown to activate the expression of both vvhA and rtxA1 (19). Consistently with this finding, the cytotoxicity and cytolysin/hemolysin levels of the ΔhlyU mutant were abolished (P < 0.001 and 0.05, respectively) compared to those of the wild-type strain, and the complementation of the ΔhlyU mutant with hlyU restored these properties (Fig. 4A and C).
Fig. 4.
Cytotoxicity and cytolysin/hemolysin activity of V. vulnificus hlyU mutants and complemented strains cocultured with HEp-2 cells. The cytotoxicity (A and B) and cytolysin/hemolysin activity (C and D) in the conditioned medium were determined after coculture at an MOI of 10 for 4 h. /phlyU, complementation with hlyU. The bars represent means ± standard deviations; n = 3. Those showing no significant difference are labeled with the same letters, and those showing significant difference (P < 0.05) are labeled with different letters based on one-way ANOVA with Tukey's HSD test.
To test whether hlyU is associated with the regulation of cytotoxicity and cytolysin/hemolysin production by QS signaling in V. vulnificus, this gene in the SmcR-deficient or downregulated mutants was deleted, and the resultant mutants were assayed for these properties. The cytotoxicity of the ΔsmcR, luxO(D47E), and ΔluxO ΔsmcR mutants was greatly reduced when their hlyU gene was further deleted (P < 0.001 for all of them) (Fig. 4B). Meanwhile, the hemolytic activity in the conditioned medium of cells infected by these ΔhlyU mutants was reduced in all of them (P < 0.01, 0.05, and 0.01 for the ΔsmcR ΔhlyU, luxO(D47E) ΔhlyU, and ΔluxO ΔsmcR ΔhlyU mutants, respectively) (Fig. 4D). The complementation of these mutants with hlyU restored these properties (Fig. 4B and D).
SmcR downregulates hlyU.
To test whether hlyU is regulated by SmcR, the hlyU expression levels in the ΔluxO, luxO(D47E), and ΔluxO ΔsmcR mutants after coincubation with HEp-2 cells were estimated by real-time RT-PCR. The transcriptional level of hlyU was increased in luxO(D47E), in which smcR was downregulated, and ΔluxO ΔsmcR mutants (P < 0.05) compared to that of the ΔluxO mutant in which smcR was upregulated (Fig. 2D).
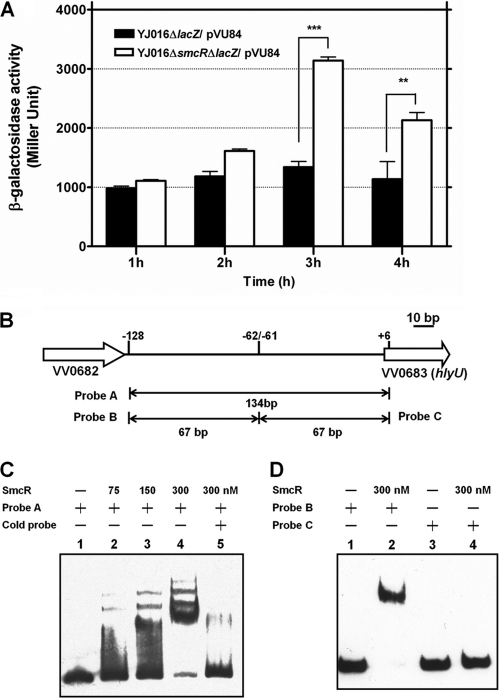
In another experiment, a DNA fragment containing the intergenic region between VV0682 and hlyU was cloned into pVP84 to generate PhlyU-lacZ as a reporter, and the resultant plasmid, pVU84, was introduced into the smcR+ and ΔsmcR isogenic strains. Compared to that of the smcR+ strain, the ΔsmcR mutant exhibited higher hlyU promoter activity at 3 and 4 h of growth in LB broth (P < 0.001 and 0.01, respectively) (Fig. 5A).
Fig. 5.
(A) Effect of SmcR on expression of hlyU. The β-galactosidase activity in various V. vulnificus strains carrying a PhlyU-lacZ fusion in pVU84 was determined after growth in LB for the indicated periods. The bars represent means ± standard deviations; n = 4. The significance of difference was analyzed by two-way ANOVA with Bonferroni's post test. **, P < 0.01; ***, P < 0.001. (B) Organization of VV0682, VV0683 (hlyU), and the intergenic region. The regions from which the various probes used in the gel mobility shift assay were derived are indicated. (C) Binding of SmcR to the intergenic region detected by gel mobility shift assay. Lane 1, probe A (2.5 nM) alone; lane 2, probe A plus 75 nM SmcR; lane 3, probe A plus 150 nM SmcR; lane 4, probe A plus 300 nM SmcR; lane 5, probe A plus 300 nM SmcR and 100-fold excess of unlabeled probe A. (D) Binding of SmcR to probes derived from the intergenic region. Lane 1, probe B (2.5 nM) alone; lane 2, probe B plus 300 nM SmcR; lane 3, probe C (2.5 nM) alone; lane 4, probe C plus 300 nM SmcR.
SmcR binds to hlyU promoter region.
A gel mobility shift assay then was performed with the purified His6-tagged SmcR to determine whether SmcR binds to the promoter of hlyU. A hlyU promoter-containing DNA fragment (probe A in Fig. 5B) was upshifted in the presence of increasing amounts (from 75 to 300 nM) of SmcR-His6 (Fig. 5C). The SmcR-binding site was further mapped to the region between −62 and −128 bp (probe B in Fig. 5B) upstream of hlyU, as the DNA fragment corresponding to this region caused a band shift but the other fragment (probe C in Fig. 5B), corresponding to a region between +6 and −61, did not (Fig. 5D).
H-NS represses cytotoxicity and cytolysin/hemolysin activity.
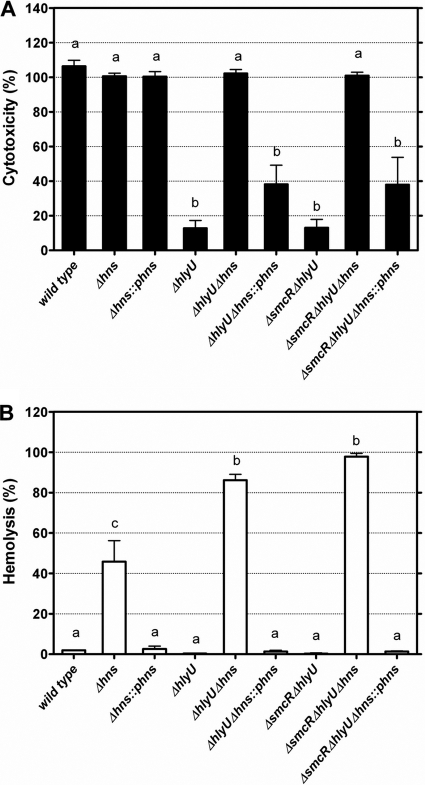
Recently, ORF VV12923 of V. vulnificus strain CMCP6 that encodes an H-NS universal regulator was shown to act as an rtxA1 repressor and compete with HlyU for binding to the rtxA1 promoter region (20). To test whether H-NS is a repressor of cytotoxicity and cytolysin/hemolysin production, the hns gene (ORF VV1346 in strain YJ016) of the wild-type, ΔhlyU, and ΔsmcR ΔhlyU strains was further deleted, and the resultant mutants were characterized. The deletion of hns resulted in increases of both the cytotoxicity (except for the wild-type strain, which already showed full cytotoxicity) and cytolysin/hemolysin production in all three strains (P < 0.001) (Fig. 6A and B). Moreover, the complementation of these Δhns mutants with hns led to a reduction of cytotoxicity (except for the Δhns mutant, which was HlyU proficient) and cytolysin/hemolysin production (P < 0.001).
Fig. 6.
Cytotoxicity and cytolysin/hemolysin activity of V. vulnificus Δhns mutants cocultured with HEp-2 cells. The cytotoxicity (A) and cytolysin/hemolysin activity (B) in the conditioned medium were determined after coculture at an MOI of 10 for 4 h. ::phns, complementation with hns. The bars represent means ± standard deviations; n = 3. Those showing no significant difference are labeled with the same letters, and those showing significant difference (P < 0.05) are labeled with different letters based on one-way ANOVA with Tukey's HSD test.
DISCUSSION
In this study, the requirement of the QS regulator, LuxO, in the expression of V. vulnificus cytotoxicity was confirmed, and the role of SmcR, a regulator repressed by LuxO (24), in the negative regulation of cytotoxicity was demonstrated (Fig. 1A). Both RTX and the cytolysin/hemolysin VvhA contributed to the cytotoxicity to HEp-2 cells regulated by QS signaling (Fig. 1A). Further, the transcriptional levels of both rtxA1 and vvhA in the luxO(D47E) mutant, which exhibited full cytotoxicity, were significantly higher than those of the ΔluxO mutant, which lost cytotoxicity (Fig. 2B and C). These results indicate that the induction of cytotoxicity by QS occurs by activating the transcription of these two cytotoxin genes.
The involvement of RTX in V. vulnificus cytotoxicity has been demonstrated, as RTX-deficient mutants exhibit reduced cytotoxicity (15, 19). Several possible mechanisms, including pore formation on the target cell membrane and inducing the apoptosis of the host cells, have been proposed for the cytotoxicity of RTX (12, 13, 15). The ΔluxO mutant was shown to be about 100-fold less virulent than the wild-type strain or luxO(D47E) mutant, and its spread into the bloodstream was much slower in mice infected subcutaneously (our unpublished data). These phenotypes of the ΔluxO mutant are similar to those of an RTX-deficient mutant (15, 19, 21), suggesting a close association of LuxO with RTX-mediated cytotoxicity and virulence in mice.
The role of cytolysin/hemolysin in cytotoxicity is equivocal. Although this protein can cause the cytotoxicity of cultured cells (17, 29), cytolysin/hemolysin-deficient mutants are as cytotoxic as the wild-type strain when they are coincubated with the cells (5, 12, 29). As has been noticed by Kim et al. previously (12), the RTX-deficient mutant still caused cell lysis, and cytolysin/hemolysin was detected in the conditioned medium when it was cocultured with the HEp-2 cells at an MOI of 100 for 4 h (data not shown). The cytotoxicity and cytolysin/hemolysin activity of the RTX-deficient mutants became more evident when cytolysin/hemolysin was overexpressed in mutants deficient in SmcR (Fig. 1A and B). As it has been shown that cytolysin/hemolysin is substantially produced in vivo (17), the role of this cytotoxin in pathogenesis needs to be reinvestigated.
We then found that hlyU, an activator of both vvhA and rtxA1 (11, 19), mediated the regulation of vvhA by SmcR in E. coli. In V. vulnificus, the deletion of hlyU in the ΔsmcR mutant resulted in the abolition of cytolysin/hemolysin activity (Fig. 4B), indicating that HlyU is required for the upregulation of vvhA in the ΔsmcR mutant. We further showed that the promoter activity of hlyU was increased in the absence of SmcR (Fig. 5A), and SmcR bound to a region (−128 to −62) upstream of hlyU in EMSA (Fig. 5D). In this region we also found a 22-bp sequence (−107 to −86) with 68% (15/22) identity to the consensus SmcR-binding sequence proposed by Lee et al. (14). These findings collectively suggest that SmcR negatively regulates the expression of vvhA by repressing hlyU. It has been shown that HlyU activates rtxA1 expression through competition with H-NS, a repressor of rtxA1, for binding to the promoter region of rtxA1 (20). We found in this study that the cytolysin/hemolysin activity in the conditioned medium of cells infected by the Δhns, ΔhlyU Δhns, or ΔsmcR ΔhlyU Δhns mutant was significantly higher than those infected by their hns+ parent strains (Fig. 6B), suggesting that H-NS also represses the vvhA promoter.
As it has been shown that HlyU increases the rtxA1 promoter activity only in the presence of H-NS (19), we supposed that HlyU regulates the vvhBA promoter in a similar manner. Surprisingly, the cytolysin/hemolysin activity of the ΔhlyU Δhns or ΔsmcR ΔhlyU Δhns mutant was much higher than, instead of comparable to, that of the Δhns mutant (Fig. 6B). This can be explained by the downregulation of the metalloprotease Vvp in the absence of SmcR (27) or HlyU (11). The cytolysin/hemolysin activity, which declines after late log phase in a wild-type strain, is sustained in the stationary phase in a Δvvp mutant (26), suggesting that cytolysin/hemolysin is inactivated in the presence of Vvp. Therefore, the higher cytolysin/hemolysin activity in the ΔhlyU Δhns or ΔsmcR ΔhlyU Δhns mutant compared to that of the Δhns mutant may be a consequence of reduced Vvp activity.
By epistasis analysis we showed that the reduced cytotoxicity of the ΔluxO mutant was restored when smcR was further deleted (Fig. 1A), and this double mutant lost cytotoxicity again when its hlyU gene was disrupted (Fig. 4A). Furthermore, the cytotoxicity of the ΔsmcR ΔhlyU mutant was regained by deleting the hns gene (Fig. 6A). Taken together, how LuxO regulates the cytotoxicity may be delineated: LuxO represses smcR to result in the derepression of hlyU, and the up-expressed HlyU then interferes with H-NS to cause the derepression of both vvhA and rtxA1.
Opposite findings on the roles of SmcR in cytotoxicity and virulence in mice have been reported (16). We previously characterized a ΔsmcR mutant of a clinical isolate, YJ016, and found that both the transcriptional level of vvhA and cytolysin/hemolysin activity in this mutant were remarkably increased (26). In addition, the virulence of this mutant in either normal or iron-overloaded C3H/HeN mice challenged by intraperitoneal injection was comparable to that of the wild-type strain. However, an smcR mutant derived from another clinical strain, ATCC 29307, exhibited decreased cytotoxicity to INT407 cells and was less virulent in ICR mice (16). These differences may result from the different bacterial strains, cell lines, or mouse strains used, and they also suggest that it is necessary to study the roles of SmcR at different sites in the host, such as the gastrointestinal tract and blood, during infection.
Supplementary Material
ACKNOWLEDGMENTS
This study was partly supported by grant NSC 95-2320-B-006-065 from the National Science Council of Taiwan.
We thank Ching-Liang Shen for helping C. P. Shao establish the laboratory of bacterial genetics and molecular biology.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 11 March 2011.
REFERENCES
- 1. Bang Y. B., Lee S. E., Rhee J. H., Choi S. H. 1999. Evidence that expression of the Vibrio vulnificus hemolysin gene is dependent on cyclic AMP and cyclic AMP receptor protein. J. Bacteriol. 181:7639–7642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen C. Y., et al. 2003. Comparative genome analysis of Vibrio vulnificus, a marine pathogen. Genome Res. 13:2577–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davison J., Heusterspreute M., Chevalier N., Ha-Thi V., Brunel F. 1987. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene 51:275–280 [DOI] [PubMed] [Google Scholar]
- 4. Donnenberg M. S., Kaper J. B. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fan J. J., Shao C. P., Ho Y. C., Yu C. K., Hor L. I. 2001. Isolation and characterization of a Vibrio vulnificus mutant deficient in both extracellular metalloprotease and cytolysin. Infect. Immun. 69:5943–5948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freeman J. A., Bassler B. L. 1999. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol. Microbiol. 31:665–677 [DOI] [PubMed] [Google Scholar]
- 7. Gray L. D., Kreger A. S. 1987. Mouse skin damage caused by cytolysin from Vibrio vulnificus and by V. vulnificus infection. J. Infect. Dis. 155:236–241 [DOI] [PubMed] [Google Scholar]
- 8. Gray L. D., Kreger A. S. 1985. Purification and characterization of an extracellular cytolysin produced by Vibrio vulnificus. Infect. Immun. 48:62–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557–580 [DOI] [PubMed] [Google Scholar]
- 10. Kim S. Y., et al. 2003. Regulation of Vibrio vulnificus virulence by the LuxS quorum-sensing system. Mol. Microbiol. 48:1647–1664 [DOI] [PubMed] [Google Scholar]
- 11. Kim Y. R., et al. 2003. Characterization and pathogenic significance of Vibrio vulnificus antigens preferentially expressed in septicemic patients. Infect. Immun. 71:5461–5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim Y. R., et al. 2008. Vibrio vulnificus RTX toxin kills host cells only after contact of the bacteria with host cells. Cell Microbiol. 10:848–862 [DOI] [PubMed] [Google Scholar]
- 13. Lee B. C., Choi S. H., Kim T. S. 2008. Vibrio vulnificus RTX toxin plays an important role in the apoptotic death of human intestinal epithelial cells exposed to Vibrio vulnificus. Microbes Infect. 10:1504–1513 [DOI] [PubMed] [Google Scholar]
- 14. Lee D. H., et al. 2008. A consensus sequence for binding of SmcR, a Vibrio vulnificus LuxR homologue, and genome-wide identification of the SmcR regulon. J. Biol. Chem. 283:23610–23618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee J. H., et al. 2007. Identification and characterization of the Vibrio vulnificus rtxA essential for cytotoxicity in vitro and virulence in mice. J. Microbiol. 45:146–152 [PubMed] [Google Scholar]
- 16. Lee J. H., et al. 2007. Identification and functional analysis of Vibrio vulnificus SmcR, a novel global regulator. J. Microbiol. Biotechnol. 17:325–334 [PubMed] [Google Scholar]
- 17. Lee S. E., et al. 2004. Production of Vibrio vulnificus hemolysin in vivo and its pathogenic significance. Biochem. Biophys. Res. Commun. 324:86–91 [DOI] [PubMed] [Google Scholar]
- 18. Lenz D. H., et al. 2004. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118:69–82 [DOI] [PubMed] [Google Scholar]
- 19. Liu M., Alice A. F., Naka H., Crosa J. H. 2007. The HlyU protein is a positive regulator of rtxA1, a gene responsible for cytotoxicity and virulence in the human pathogen Vibrio vulnificus. Infect. Immun. 75:3282–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu M., Naka H., Crosa J. H. 2009. HlyU acts as an H-NS antirepressor in the regulation of the RTX toxin gene essential for the virulence of the human pathogen Vibrio vulnificus CMCP6. Mol. Microbiol. 72:491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lo H. R., et al. RTX toxin enhances the survival of Vibrio vulnificus during infection by protecting the organism from phagocytosis. J. Infect. Dis., in press [DOI] [PubMed] [Google Scholar]
- 22. Miller J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 23. Ng W. L., Bassler B. L. 2009. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 43:197–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roh J. B., et al. 2006. Transcriptional regulatory cascade for elastase production in Vibrio vulnificus: LuxO activates luxT expression and LuxT represses smcR expression. J. Biol. Chem. 281:34775–34784 [DOI] [PubMed] [Google Scholar]
- 25. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 26. Shao C. P., Hor L. I. 2000. Metalloprotease is not essential for Vibrio vulnificus virulence in mice. Infect. Immun. 68:3569–3573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shao C. P., Hor L. I. 2001. Regulation of metalloprotease gene expression in Vibrio vulnificus by a Vibrio harveyi LuxR homologue. J. Bacteriol. 183:1369–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simon R., Priefer U., Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat. Biotechnol. 1:784–791 [Google Scholar]
- 29. Wright A. C., Morris J. G., Jr 1991. The extracellular cytolysin of Vibrio vulnificus: inactivation and relationship to virulence in mice. Infect. Immun. 59:192–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu J., et al. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 99:3129–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.