Abstract
We report here the physiological and genetic characterization of an orphan histidine kinase (HK) (OhkA, SCO1596) in Streptomyces coelicolor and its homolog (OhkAsav, SAV_6741) in Streptomyces avermitilis. The physiological analysis showed that the ohkA mutant of S. coelicolor exhibits impaired aerial mycelium formation and sporulation and overproduction of multiple antibiotics on mannitol-soy flour (MS) medium, especially actinorhodin (ACT) and calcium-dependent antibiotic (CDA), and disruption of ohkAsav in S. avermitilis also led to the similar phenotypes of impaired morphological differentiation and significantly increased oligomycin A production. DNA microarray analysis combined with real-time reverse transcription-PCR (RT-PCR) and RNA dot blot assay in the S. coelicolor ohkA deletion mutant confirmed the physiological results by showing the upregulation of genes involved in the biosynthesis of ACT, CDA, undecylprodigiosin (RED), a yellow type I polyketide (CPK, SCO6273-6289), and a sesquiterpene antibiotic, albaflavenone (SCO5222-5223). The results also suggested that the increased production of ACT and RED in the mutant could be partly ascribed to the enhanced precursor malonyl coenzyme A (malonyl-CoA) supply through increased transcription of genes encoding acetyl-CoA carboxylase (ACCase). Interestingly, DNA microarray analysis also showed that deletion of ohkA greatly downregulated the transcription of chpABCDEFGH genes essential for aerial mycelium formation by S. coelicolor on MS medium but significantly increased transcription of ramS/C/R, which is responsible for SapB formation and regulation and is normally absent on MS medium. Moreover, many other genes involved in development, such as bldM/N, whiG/H/I, ssgA/B/E/G/R, and whiE, were also significantly downregulated upon ohkA deletion. The results clearly demonstrated that OhkA is an important global regulator for both morphological differentiation and secondary metabolism in S. coelicolor and S. avermitilis.
INTRODUCTION
Streptomyces species are Gram-positive, soil-dwelling, and filamentous bacteria. They are well known for producing a wide variety of important natural antibiotics and bioactive compounds currently used in medicine and agriculture. In response to nutrient deprivation, the Streptomyces colonies begin producing many secondary metabolites (e.g., antibiotics) at the onset of aerial mycelium formation. Streptomyces coelicolor, as the model species, has been used in the studies of morphological differentiation and antibiotic regulation in the genus Streptomyces for many years (20). It can generate at least four antibiotics, including blue-pigmented polyketide actinorhodin (ACT), red-pigmented prodigiosins (RED), calcium-dependent antibiotic (CDA), and an SCP1 plasmid-encoded antibiotic, methylenomycin (Mmy) (30). The regulation of antibiotic biosynthesis and morphological differentiation in S. coelicolor has been found to involve many different regulatory proteins, such as bld- and whi-encoded regulatory proteins and eukaryotic serine/threonine protein kinase, etc. (5, 9).
The two-component system (TCS), which is the predominant signal transduction system widely distributed in bacteria (46), has also been involved in the regulation of antibiotic biosynthesis and morphological differentiation in S. coelicolor (22). Bioinformatic analysis of the S. coelicolor genome reveals the presence of at least 67 paired TCSs (4, 22), many of which have been identified as being involved in either the production of secondary metabolites (e.g., phoP-phoR, cutS-cutR, ecrA1-ecrA2, and rapA1-rapA2) (8, 22, 33, 34, 45), development (ragK-ragR) (43), or both antibiotic biosynthesis and morphological differentiation (e.g., afsQ1-afsQ2 and absA1-absA2) (3, 6, 23, 40, 44). In addition, there also exist 13 orphan response regulators (RRs) and 17 orphan histidine kinases (HKs) in S. coelicolor (22). Several of these orphan RRs have been characterized, and their roles in development and antibiotic production have been established (19, 49); however, no orphan HK has been studied so far.
In order to functionally identify the orphan HKs in S. coelicolor, we have constructed several deletion mutants of the orphan HK-encoding genes. After phenotype screening, we identified an orphan histidine kinase (designated OhkA, encoded by gene SCO1596) that plays global regulatory roles in both morphological differentiation and antibiotic biosynthesis in S. coelicolor. Deletion of ohkA in S. coelicolor led to drastically enhanced antibiotic biosynthesis, especially of ACT and CDA, and to impaired aerial hypha formation and sporulation on mannitol-soy flour (MS) medium. Disruption of an ohkA ortholog, ohkAsav, in Streptomyces avermitilis NRRL 8165 led to similar effects as those of ohkA deletion in S. coelicolor, suggesting that this orphan HK may represent a highly conserved signal transduction component involved in the regulation of both antibiotic biosynthesis and morphological differentiation among Streptomyces bacteria. In addition, DNA microarray analysis combined with real-time reverse transcription-PCR (RT-PCR) and RNA dot blot assay was employed to explore the possible regulatory mechanisms of ohkA in S. coelicolor.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. S. coelicolor M145 and S. avermitilis NRRL 8165 were cultivated at 30°C on mannitol-soy flour (MS) agar (29) for spore suspension preparation, conjugal transfer, and phenotype observation. For determination of oligomycin A and avermectin production, BioK seed medium and BioK fermentation medium were employed with some modifications (10). Escherichia coli was cultivated at 37°C in LB medium or on LB agar with 50 μg ml−1 apramycin, 50 μg ml−1 thiostrepton, 50 μg ml−1 kanamycin, or 100 μg ml−1 ampicillin when necessary. Genetic manipulation of S. coelicolor, S. avermitilis, and E. coli was carried out according to the methods described by Kieser et al. (29) and Sambrook et al. (42).
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) supE44 λ−thi-1 gyrA96 relA1 | Gibco-BRL |
| BW25113 | K-12 derivative; ΔaraBAD ΔrhaBAD | 13 |
| BW25113/pIJ790 | BW25113 containing temperature-sensitive plasmid pIJ790, which encodes the λ RED recombination system | 18 |
| ET12567 | dam-13::Tn9 dcm-6 hsdM | 35 |
| ET12567/pUZ8002 | ET12567 containing the nontransmissible RP4 derivative plasmid pUZ8002 | 18 |
| S. coelicolor strains | ||
| M145 | Wild type; SCP1− SCP2−pgl+ | 29 |
| M145ΔohkA | M145 ohkA::acc(3)IV | This work |
| M145/pSET152AT | M145 with the control vector pSET152AT | This work |
| M145ΔohkA/pSET152AT | M145ΔohkA with pSET152AT | This work |
| M145ΔohkA/pSETohkA | M145ΔohkA with the complementation vector pSETohkA | This work |
| M145ΔohkA/pSETohkAH159L | M145ΔohkA with the mutated complementation vector pSETohkAH159L | This work |
| S. avermitilis strains | ||
| NRRL 8165 | Wild type; the model avermectin-producing strain | NRRL |
| 8165ΔohkAsav | NRRL 8165 ohkAsav::acc(3)IV | This work |
| 8165/pIB141 | NRRL 8165 with the control vector pIB141 | This work |
| 8165ΔohkAsav/pIB141 | 8165ΔohkAsav with the control vector pIB141 | This work |
| 8165ΔohkAsav/pIBohkAsav | 8165ΔohkAsav with the complementation vector pIBohkAsav | This work |
| 8165/pSET152AT | NRRL 8165 with the control vector pSET152AT | This work |
| 8165ΔohkAsav/pSET152AT | 8165ΔohkAsav with the control vector pSET152AT | This work |
| 8165ΔohkAsav/pSETohkA | 8165ΔohkAsav with the complementation vector pSETohkA | This work |
| Plasmids | ||
| pIJ773 | Plasmid containing the apramycin resistance gene aac(3)IV and oriT of plasmid RP4, flanked by FRT sites | 18 |
| pSET152 | oriT RK2 plasmid carrying the apramycin resistance gene aac(3)IV | 29 |
| pSET152AT | pSET152 with insertion of the thiostrepton resistance gene (tsr) in the SphI site | This work |
| pIB139 | Derived from pSET152, with the constitutive promoter ermE*p | 52 |
| pIB141 | pIB139 modified by inserting the thiostrepton resistance gene (tsr) downstream of aac(3)IV | This work |
| pIBohkAsav | pIB141 with the ohkAsav ORF cloned between NdeI and XbaI sites, in which ohkAsav was under the control of the constitutive promoter ermE*p | This work |
| pSETohkA | pSET15AT with 2,120-bp DNA fragment containing the ohkA ORF, partial SCO1597 ORF, and the possible promoter region of SCO1597 | This work |
| pSETohkAH159L | ohkA ORF cloned in pSET152AT was mutated, resulting in pSETohkAH159L, in which histidine at position 159 in ohkA ORF encoding protein OhkA was changed to leucine | This work |
Construction of the ohkA and ohkAsav gene deletion mutants.
Gene deletion mutants of S. coelicolor M145 and S. avermitilis NRRL 8165 were constructed using a PCR targeting system previously established by Gust et al. (18). The mutant cosmids with ohkA or ohkAsav gene deletion were generated by electrotransforming E. coli BW25113 containing pIJ790 (with the genes encoding the λ Red system) and the cosmid harboring ohkA or ohkAsav gene with the PCR-amplified disruption cassette. The two disruption cassettes were obtained by PCR using template pIJ773 and primer pairs ohkAF/R and ohkAsavF/R, respectively (Table 1; see also Table S1 in the supplemental material). Cosmids with the deletion of ohkA or ohkAsav were verified by both PCR using primer pairs JohkAF/R and JohkAsavF/R (see Table S1 in the supplemental material), respectively, and SacI enzyme restriction analyses. The resulting cosmid [ΔohkA::aac(3)IV or ΔohkAsav::aac(3)IV] was electrotransformed into the nonmethylating E. coli ET12567/pUZ8002 and then transferred into S. coelicolor M145 or S. avermitilis NRRL 8165 by conjugal transfer. Finally, apramycin-resistant and kanamycin-sensitive exconjugants were selected, and the ohkA or ohkAsav knockout mutant (M145ΔohkA or 8165ΔohkAsav) was confirmed by colony PCR with primers JohkAF/R and JohkAsavF/R, respectively.
Genetic complementation.
The pSET152AT integrative vector, which was generated by inserting the thiostrepton resistance gene (tsr) in the SphI site of pSET152, was used for the construction of complementation vector with the wild-type (WT) ohkA gene. A 695-bp BglII-EcoRI fragment (containing the 5′ region of 198 bp encoding 66 amino acids [aa] of SCO1597, a 301-bp fragment including partial SCO1598, and the intergenic region between SCO1597 and SCO1598) was amplified with primers ohkAcomF1/R1 (see Table S1 in the supplemental material); a 1,425-bp EcoRI-BglII fragment (containing the 3′ region of 150 bp encoding 50 aa of SCO1597 and the whole ohkA gene) was amplified with primers ohkAcomF2/R2 (Table S1). No error in amplification was found by DNA sequencing. The two fragments were ligated together with pSET152AT, which was cut with BamHI and treated with alkaline phosphatase (calf intestinal alkaline phosphatase [CIAP]; Takara, Japan), respectively, resulting in the complementation vector pSETohkA.
For the construction of the complementation vector with the ohkAsav gene, pIB141 vector, modified by inserting the thiostrepton resistance gene (tsr) downstream of the apramycin resistance gene aac(3)IV of pIB139 (Table 1), was employed and expression of ohkAsav was under the control of the constitutive promoter ermE*p. The ohkAsav open reading frame (ORF) was amplified with primer pair ohkAsavcomF/R (see Table S1 in the supplemental material) and then cloned into NdeI and XbaI sites of pIB141, generating the complementation vector pIBohkAsav.
The resulting plasmids pSETohkA and pIBohkAsav were introduced into the ohkA and ohkAsav mutants, respectively, by E. coli-Streptomyces conjugation, and transformants were selected by thiostrepton resistance. pSET152AT and pIB141 were used as negative controls.
Site-directed mutagenesis of the putative phosphorylation site in OhkA.
The ohkA gene cloned in the complementation vector pSETohkA was mutated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), in which the putative phosphorylation site (histidine residue at position 159) of OhkA was replaced by leucine (L). Two complementary oligonucleotides, H159LF/R (see Table S1 in the supplemental material; mutation site is underlined; CACGAG was changed to CTCGAG, which is the XhoI enzyme site), were used. The clones with the correct site mutated were confirmed by DNA sequencing and then introduced into the ohkA mutant by conjugal transfer.
Microscopy.
Cultures for fluorescence microscopy and scanning electron microscopy (SEM) were obtained by inserting sterile coverslips at a 45° angle into MS agar which was inoculated with the spores of M145 or its derivatives. For fluorescence microscopic analysis, coverslips were removed after incubation at 30°C for 5 days and fixed with methanol followed by washing with phosphate-buffered saline (PBS; pH 7.4). The samples were then stained with 4′,6-diamidino-2-phenylindole (DAPI; 25 μg/ml) at room temperature for 30 min, washed with PBS buffer, and then observed using a laser scanning confocal microscope, Fluoview FV1000 (Olympus, Tokyo, Japan). For SEM analysis, the coverslips were taken after 4 days of incubation at 30°C and fixed with fresh 2% glutaraldehyde (pH 7.2) and 1% osmium tetroxide, followed by complete drying in HCP-2 (Hitachi, Japan); coated with gold with a Fine Coater JFC-1600 (JEOL, Tokyo, Japan); and then examined with scanning electron microscopy (JSM-6360LV; JEOL, Tokyo, Japan).
Determination of antibiotic production.
Actinorhodin (ACT) and undecylprodigiosin (RED) were determined as described before with some modifications (1, 34). Cultures of S. coelicolor wild-type M145 and its derivatives incubated on MS agar covered with plastic cellophane were collected at five time points (36, 48, 60, 72, and 94 h). For ACT measurement, KOH was added to the samples at a final concentration of 1 M; after 1 h at room temperature, the cultures were centrifuged at 8,000 × g for 10 min and absorbance of the supernatant was measured at the wavelength of 640 nm. For RED, the bacterial samples were first extracted with KOH to solubilize ACT as above, followed by centrifugation at 12,000 × g for 10 min, and the mycelial pellet was collected and washed with 0.9% NaCl twice. The resulting pellet was extracted with methanol (pH adjusted to 2.0 with HCl) overnight at room temperature, followed by centrifugation at 8,000 × g for 5 min, and absorbance of the supernatant was measured at the wavelength of 530 nm. The amount of ACT and RED was indicated as optical density at 640 nm (OD640)/g and OD530/g (wet weight), respectively.
Assay of CDA was carried out according to the method described by Kieser et al. (29). Briefly, M145 and its derivatives were grown on MS agar for 48 h and then overlaid with Staphylococcus aureus-seeded soft LB agar (0.5% agar) with or without Ca(NO3)2 (a final concentration of 12 mM). After incubation overnight, a zone of inhibition, which is absent when Ca(NO3)2 is omitted, is diagnostic for CDA production.
Production of oligomycin and avermectin in S. avermitilis was determined by high-pressure liquid chromatography (HPLC) as described previously (11). Briefly, spores of different S. avermitilis strains (with the same OD450) were inoculated into 30 ml BioK fermentation seed medium in 250-ml baffled flasks. After incubation for 40 h at 30°C on a rotary shaker at a speed of 200 rpm, cultures were transinoculated into three parallel 250-ml baffled flasks with 30 ml BioK fermentation medium by 5% inoculation and grown at 30°C, 200 rpm. Samples were taken at two time points, 5 and 10 days. For avermectin and oligomycin extraction, 7 ml methanol was added to 3 ml fermentation broth in a 20-ml tube and treated in an ultrasonic cleaner for 20 min. After centrifugation at 8,000 × g for 10 min, the supernatants were analyzed using an Agilent Eclipse XDB-C8 column (4.6 by 150 mm) maintained at 30°C, with a solvent system of methanol-water (85:15, vol/vol) and a flow rate of 0.9 ml/min. Commercial oligomycin A (98%, wt/wt) and avermectin B1a (95.9%, wt/wt) (Hisun Pharmaceutical Co. Ltd., Zhejiang, China) were used to make standard curves for quantitative determination.
RNA isolation and DNA microarray analysis.
RNA samples used for DNA microarray analysis were prepared as previously described (34). Briefly, cultures of S. coelicolor wild-type M145 and the ohkA mutant incubated on MS solid medium with plastic cellophane were collected at three time points (48, 64, and 88 h), frozen immediately in liquid nitrogen, and then ground into powder. RNA isolation was performed with Trizol (Invitrogen, Carlsbad, CA) according to the procedures recommended by the manufacturer. Subsequently, RNA samples were digested with DNase I (Takara) followed by purification with the RNeasy minikit (Qiagen, Valencia, CA) to remove the contamination of chromosomal DNA. The quality and integrity of the RNA samples were checked by 1% agarose gel electrophoresis and spectrophotometry.
For DNA microarray experiments, Agilent GeneChip S. coelicolor M145 DNA arrays (custom-specific design) were used. Each gene was represented by one specific 60-nucleotide (60-nt) oligonucleotide probe with two replicates. Microarray assays, including labeling, hybridization, and washing, and microarray data normalization were performed by Shanghai Biochip Co. Ltd. (Shanghai, China) according to standard protocols provided by Agilent Technologies. Briefly, RNA samples were reverse transcribed to cDNA using Moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen), followed by transcription with T7 RNA polymerase (New England BioLabs, Beverly, MA), resulting in aminoacyl-UTP (aaUTP; Ambion, Austin, TX)-labeled cRNA. After cRNA purification with an RNeasy minikit (Qiagen), cRNA was further labeled with Cy3 (for M145) and Cy5 (for the ohkA mutant), respectively. Hybridization was carried out at 65°C for 17 h with a constant rotation rate of 10 rpm. Arrays were scanned with 3-μm resolution using an Agilent DNA Microarray scanner (Agilent Technologies, Palo Alto, CA). Microarray data were normalized in the Agilent Feature Extraction software (Agilent Technologies) using total array signals and LOWESS algorithm options. The gene expression ratio (n-fold change; ohkA strain versus M145) was calculated from the normalized signal intensities.
qRT-PCR.
The quantitative real-time RT-PCR (qRT-PCR) was performed to validate selected microarray data. The SYBR Premix ExTaq (Toyobo, Japan) kit was used according to the instructions provided by the manufacturer. The primers used in qRT-PCR are listed in Table S1 in the supplemental material. The reactions were carried out in a Rotor-Gene RG-3000 thermal cycler (Corbett Research, Australia), using the following conditions: 95°C for 2 min, followed by 40 cycles of 95°C for 15 s, 65°C for 20 s, and 72°C for 20 s. Three PCRs were performed in parallel for each transcript. The hrdB gene was used as an internal control. The level of RNA transcript in different samples was measured according to the number of cycles (CT [threshold cycle]) needed for the reactions to reach a fixed threshold (T). The relative fold change of RNA transcript (mutant/WT) was determined using the 2−ΔΔCT method, in which ΔΔCT = (CTtested gene − CThrdB)mutant − (CTtested gene − CThrdB)WT. qRT-PCR was repeated three times with the same RNA samples.
RNA dot blot assay.
RNA dot blot assay was performed in accordance with the method described by Amon et al. (2). In RNA dot blot assay, antisense RNA probes were used. For the generation of these probes, internal DNA fragments with a size between 0.3 and 0.5 kb of the tested genes were amplified by PCR (primers are listed in Table S1 in the supplemental material). The reverse primers contained the promoter region for T7 RNA polymerase, which allowed in vitro transcription of probes using RNA digoxigenin (DIG) labeling mix (Roche, Mannheim, Germany) and T7 RNA polymerase (New England BioLabs). RNA samples (1 μg per time point) were spotted onto positively charged nylon membranes (Hy-Bond; Amersham, GE Healthcare, United Kingdom) using a Bio-Dot microfiltration apparatus (Bio-Rad Laboratories, Hercules, CA). Hybridization of DIG-labeled RNA probes was detected with X-ray films (Kodak, Rochester, NY) using alkaline phosphatase-conjugated anti-DIG Fab fragments and the chemiluminescent substrate disodium 3-(4-methoxyspiro {1,2-dioxetane-3,2′-(5′-chloro)tricyclo[3.3.1.13,7]decan}-4-yl)phenyl phosphate (CSPD) according to the manuals provided by the manufacturer (Roche). All experiments were carried out at least twice with RNA samples extracted from independent cultures. The hrdB gene, which encodes the principal sigma factor of S. coelicolor and is constantly transcribed throughout the time course, was used as a positive internal control.
Microarray data set accession number.
The raw DNA microarray data set analyzed in the present paper has been submitted to the NCBI Gene Expression Omnibus under the accession number GSE26426.
RESULTS AND DISCUSSION
Genomic organization of ohkA gene and its homologs in streptomycetes.
OhkA showed significant sequence identity to typical HKs (e.g., 35.4% identity in 257 amino acids overlapping with HydH in E. coli) (http://streptomyces.org.uk/). Bioinformatics analysis (http://smart.embl-heidelberg.de/) revealed that the domain architecture of OhkA was the same as the paradigmatic soluble, cytoplasmic-sensing HK NtrB, which was required for the assimilation of ammonia and the utilization of alternative N compounds in E. coli (36), indicating that OhkA might also be a soluble HK, in contrast with most of the membrane-bound HKs (36, 57). In addition to two typical domains, DHp (dimerization and histidine phosphotransfer domain) and CA (catalytic and ATP-binding domain), OhkA and NtrB both contain a PAS domain at the N terminus (see Fig. S1A in the supplemental material), which is an important signaling module, implicated in monitoring light, oxygen, and small ligands as well as protein-protein interactions for homodimer or heterodimer formation (36, 48). However, no gene encoding a putative RR is located close to ohkA, so it appears to be an orphan HK.
BLAST analysis showed that OhkA homologs with high amino acid identity (approximately 70 to 99%) are widely distributed in streptomycetes (see Fig. S1B in the supplemental material) and some other complex actinomycetes, such as Frankia and Thermobispora. The genetic organization of the ohkA gene is displayed in Fig. S1C. Upstream of ohkA is the SCO1597 gene, encoding a putative rRNA methylase. Although these two genes do not overlap, their shared intergenic region is very short (50 bp), suggestive of a possible operon (this was confirmed by RT-PCR analysis; data not shown). Immediately downstream of ohkA are pheS (SCO1595) and pheT (SCO1594), which encode alpha and beta chains of a putative phenylalanyl-tRNA synthetase, respectively. Interestingly, this genetic organization is highly conserved among closely related streptomycetes (Fig. S1C). The conserved domain structure, sequence similarity, and conserved genetic organization of ohkA and its homologs suggest that they may have similar physiological roles.
OhkA regulates both morphogenesis and antibiotic biosynthesis in S. coelicolor.
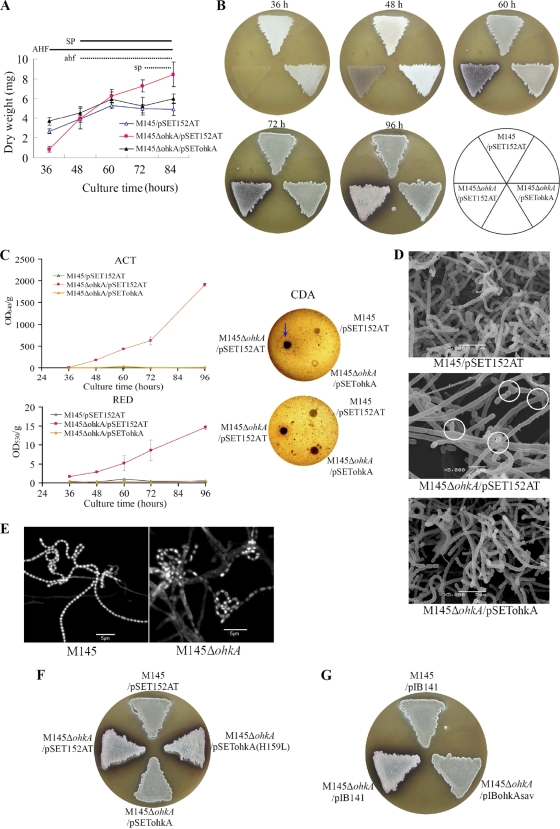
The mutant M145ΔohkA with the entire ohkA gene deleted in S. coelicolor was generated using a PCR targeting system (18). A growth experiment showed that the ohkA mutant (M145ΔohkA/pSET152AT) and M145 (M145/pSET152AT) had quite different growth rates. At the early stage (before 48 h), the mutant grew slower than M145; as time continued, from 60 h to 84 h it continued to grow and achieved a higher biomass, while M145 and the complemented strain (M145ΔohkA/pSETohkA) ceased growth (Fig. 1A). Meanwhile, aerial mycelium and spore formation were also quite different. M145 formed abundant white aerial mycelium at 36 h, followed by gray-color formation from 48 h. However, for the ohkA mutant, aerial mycelium appeared much later, as shown in Fig. 1B; at 48 h very sparse aerial hyphae were formed. Until 60 h, the ohkA mutant was covered with only a thin layer of aerial mycelium, and interestingly, from 72 h onward the colony surface began to show the pink color. In addition, compared with the wild-type strain M145, which normally produces very little or no pigmented antibiotic on MS agar, the ohkA deletion mutant produces much ACT and CDA and a low level of RED (Fig. 1C).
Fig. 1.
Phenotypes of the ohkA deletion mutant compared with the wild-type strain M145. (A) Pregerminated spore suspensions from S. coelicolor M145/pSET152AT (▵), the ohkA mutant (M145ΔohkA/pSET152AT; ▪), and the complemented strain (M145ΔohkA/pSETohkA; ▴) were cultured on MS agar with cellophane discs at 30°C. Cultures were taken and dried at the time points indicated. Experiments were done in triplicate. AHF and SP indicate phases of aerial hypha formation and sporulation, respectively, of M145/pSET152AT and the complemented strain, while ahf and sp indicate the corresponding phases of the ohkA mutant (M145ΔohkA/pSET152AT). Growth was calculated as mg (dry weight). (B) Phenotypes of M145/pSET152AT, M145ΔohkA/pSET152AT, and the complemented strain M145ΔohkA/pSETohkA on MS agar. The plates were incubated at 30°C for the indicated time points. The surface of the mutant strain M145ΔohkA/pSET152AT displayed pink color from 72 h onward. (C) Determination of antibiotic production in M145ΔohkA/pSET152AT, relative to M145/pSET152AT and the complemented strain M145ΔohkA/pSETohkA. For ACT and RED measurement, cultures were grown on MS agar at 30°C and taken at the indicated time course; ACT and RED production was calculated as OD640/g and OD530/g (wet weight), respectively. Experiments were performed in triplicate. For CDA, 1-μl spore suspensions with the same OD450 were grown on MS agar at 30°C for 48 h and then overlaid with Staphylococcus aureus-seeded soft LB agar with (upper panel) or without (lower panel) Ca(NO3)2. A zone of inhibition (indicative of CDA activity) was detected in the presence of Ca(NO3)2, which is indicated by the blue arrow. (D) Scanning electron micrographs (SEM) showing the developmental changes of the S. coelicolor ohkA mutant (with pSET152AT) compared with M145 (with pSET152AT) and the complemented strain M145ΔohkA/pSETohkA. Cultures were grown on MS medium for 4 days at 30°C. Branched aerial hyphae are shown by white circles. Scale bars are shown in the panels. (E) DNA content of S. coelicolor M145 and the ohkA mutant revealed by DAPI staining. The ohkA mutant is disturbed by DNA condensation and segregation. DNA staining was uneven in the ohkA mutant, with only a few spores very strongly stained (indicated by white arrows) and the majority of them weakly stained. Cultures were grown on MS agar for 4 days at 30°C. (F) Spore suspensions of M145/pIB141, M145ΔohkA/pIB141, the complemented strain M145ΔohkA/pSETohkA, and the mutant strain with plasmid-borne mutated ohkA (H159L) (M145ΔohkA/pSETohkAH159L) were plated on MS medium and incubated at 30°C for 4 days. The surface of the mutant strains M145ΔohkA/pSET152AT and M145ΔohkA/pSETohkAH159L showed pink color. (G) Spore suspensions of M145/pIB141, M145ΔohkA/pIB141, and the complemented strain M145ΔohkA/pIBohkAsav were plated on MS medium and incubated at 30°C for 4 days. The impaired morphological differentiation of the ohkA mutant could be complemented by the introduction of the ohkA-homologous gene ohkAsav from S. avermitilis.
When the ohkA-null mutant spores were inoculated on MS plates, a significant reduction in aerial mycelium formation and impaired sporulation were observed by scanning electron microscopy (SEM) after incubation for 4 days. As shown in Fig. 1D, in contrast to M145, which was covered with abundant straight or loosely coiled spore chains, the ohkA mutant might form two types of aerial hyphae: (i) nonsporulating, sparse, irregular long and straight ones and (ii) a minority (probably not so long) that were capable of developing into very short spore chains or single spores. In addition, a significant number of the mutant aerial hyphae are branched.
To further dissect the developmental differences between the ohkA mutant and the wild-type strain, they were stained with DAPI and visualized with confocal fluorescence microscopy. DNA staining clearly visualizes the completed segregation and condensed chromosomes in the wild-type strain. In contrast, the mutant strain formed very short spore chains and showed rather irregular chromosomal segregation and condensation. DNA staining was uneven in the mutant, with only a few spores very strongly stained and the majority of them weakly stained. Fluorescence microscopy also revealed, compared with M145, that chromosomal DNA in the spores of the mutant was distributed at irregular intervals (Fig. 1E).
To confirm that the mutant phenotype resulted from the absence of ohkA rather than random mutations elsewhere in the genome, the wild-type ohkA gene with its putative promoter present on the integrative vector pSET152AT was introduced into the mutant. The phenotype of the mutant could be restored to that of M145. After incubation on MS plates at 30°C for 4 days, in contrast to the mutant, both the wild type and the complemented strain were covered with abundant straight or loosely coiled spore chains which often contained several tens of spores (Fig. 1D) as observed by SEM, and antibiotic biosynthesis was restored to the wild-type levels (Fig. 1C). These results conclusively demonstrated that OhkA is involved in the regulation of both antibiotic biosynthesis and morphological differentiation in S. coelicolor.
The putative phosphorylation site (H159) is necessary for OhkA function.
Bioinformatics analysis showed that His at position 159 might be the phosphorylation site of OhkA (see Fig. S1B in the supplemental material). To verify this possibility, we studied the effect of inactivating the OhkA phosphorylation site on antibiotic production and morphological differentiation. The putative phosphorylation site (H159) of OhkA was mutated to leucine (L) by site-directed mutagenesis using the QuikChange site-directed mutagenesis kit, and the correct mutant cloned in pSET152AT [pSETohkA(H159L)] was transferred into M145ΔohkA by conjugation as described in Materials and Methods. The empty vector pSET152AT and pSETohkA were used as negative and positive controls, respectively. We found that, the mutant M145ΔohkA with introduction of pSETohkA(H159L) showed pink colony and antibiotic overproduction, the same phenotype as the mutant strain with empty vector (Fig. 1F), indicating that this histidine residue is functionally significant and required for the role of OhkA in vivo.
OhkAsav (SAV_6741) from S. avermitilis, a homolog of OhkA, has similar functions.
As described above, OhkA homologs from different streptomycetes had high sequence similarity and conserved genetic organization (see Fig. S1 in the supplemental material), prompting us to suggest that these homologs may have similar functions as OhkA does in S. coelicolor. To test this hypothesis, the ohkA-homologous gene ohkAsav from S. avermitilis was cloned into pIB141 vector under the control of ermE*p and transferred into the ohkA mutant. The results showed that introduction of the ohkAsav gene into the ohkA mutant could readily restore the phenotype changes of the S. coelicolor ohkA mutant (Fig. 1G), indicating that ohkAsav may function similarly to ohkA.
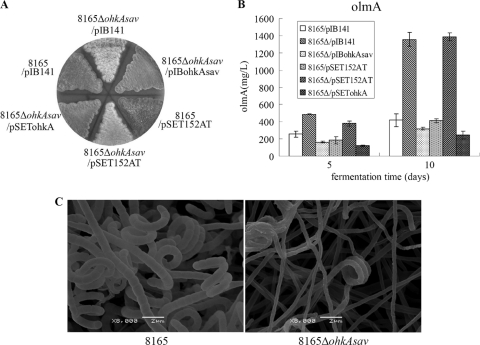
To further identify the function of ohkAsav, we constructed the ohkAsav deletion mutant in S. avermitilis and found that deletion of the ohkAsav gene also had global effects on both antibiotic biosynthesis (e.g., significantly increased oligomycin A production) and development (e.g., formation of thin aerial mycelium and impaired sporulation) (Fig. 2A to C) in S. avermitilis. The effects could be rescued by complementation with the wild-type ohkAsav or the ohkA gene from S. coelicolor (as shown in Fig. 2A and B). However, no notable difference in avermectin biosynthesis was found between the ohkAsav deletion mutant and the wild-type strain (data not shown).
Fig. 2.
Phenotypes of the ohkAsav mutant in contrast to the wild-type strain S. avermitilis NRRL 8165. (A) The defective sporulation of the ohkAsav mutant could be rescued by the complementation with the wild-type gene ohkAsav and ohkA from S. coelicolor when grown on MS medium. The white appearance of 8165ΔohkAsav with pSET152AT or pIB141 was due to the failure to produce the gray-pigmented spores. These strains were cultured on MS medium for 10 days. (B) Overproduction of oligomycin A (olmA) assayed by HPLC in the ohkAsav mutant (8165Δ/pSET152AT or 8165Δ/pIB141) was restored by the complementation with ohkAsav gene (strain 8165Δ/pIBohkAsav) or ohkA gene from S. coelicolor (strain 8165Δ/pSETohkA). After incubation in fermentation broth for 5 and 10 days, cultures were extracted with methanol and analyzed by HPLC. Error bars indicate the standard deviations from three parallel flasks. (C) Scanning electron micrographs of the aerial hyphae and spores of NRRL 8165 (left panel) and the ohkAsav deletion mutant (right panel). The ohkAsav mutant showed abnormal development, including formation of thin aerial hyphae and impaired sporulation. Both strains were grown on MS medium for 10 days. Scale bars are shown in both panels.
Comparison of transcription profiles of the ohkA mutant and its wild-type strain M145.
To explore the possible regulatory mechanisms of OhkA on both antibiotic production and morphological differentiation, we compared the global transcription profiles between the ohkA deletion mutant and its parental strain M145 using DNA microarrays. Strains were grown on MS agar with plastic cellophane, and cultures were harvested for RNA preparation at three time points, 48, 64, and 88 h. Pairwise comparison showed that, across three time points, there are a total of 1,078 genes upregulated and 1,277 downregulated at least 2-fold (ratio: mutant/wild type, ≥2.0 or ≤0.5) in the mutant compared with M145. However, it should be noted that only one sample was microarray analyzed for each time point (with two technical replicates); the significance of the gene expression changes needs to be further verified. To do so, we verified the DNA microarray data by qRT-PCR for 14 selected genes as shown in Table 2. In general, good consistency was found between DNA microarray and RT-PCR data in terms of the trends of the changes. Additionally, good consistency between the phenotype changes arising from ohkA deletion and the changes of the genes involved in these phenotypes also supported the overall quality of microarray analysis. Key functional genes whose transcription was down- or upregulated over 2-fold due to the deletion of the ohkA gene and further confirmed by qRT-PCR and RNA dot blot assay are described below (see Table S2 in the supplemental material).
Table 2.
qRT-PCR analysisa
| No. | Gene name | SCO no. | Function of gene product | Factor of transcription in real-time PCR (mean ±SD) |
DNA microarray (mean)b |
||
|---|---|---|---|---|---|---|---|
| 48 h | 64 h | 48 h | 64 h | ||||
| 1 | kasO | SCO6280 | Regulatory protein | 129.62 ± 25.51 | 6.85 ± 1.40 | 2.33 | 1.77 |
| 2 | actII-ORF4 | SCO5085 | Actinorhodin cluster activator protein | 2.23 ± 0.26 | 148.83 ± 70.12 | 5.65 | 13.65 |
| 3 | ramS | SCO6682 | Hypothetical protein | 31.55 ± 8.06 | 725.77 ± 61.37 | 4.31 | 7.42 |
| 4 | cdaR | SCO3217 | Transcriptional regulator | 12.70 ± 2.42 | 9.51 ± 1.00 | 2.31 | 2.22 |
| 5 | redD | SCO5877 | Transcriptional regulator | 31.36 ± 6.49 | 15.33 ± 2.34 | 2.93 | 1.62 |
| 6 | scbA | SCO6266 | ScbA protein | 13.07 ± 1.65 | 5.12 ± 1.58 | 2.21 | 3.74 |
| 7 | bldN | SCO3323 | RNA polymerase sigma factor | 0.09 ± 0.01 | 0.06 ± 0.01 | 0.18 | 0.09 |
| 8 | wblA | SCO3579 | Regulatory protein | 0.03 ± 0.01 | 0.07 ± 0.02 | 0.05 | 0.05 |
| 9 | chpB | SCO7257 | Secreted protein | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.05 | 0.07 |
| 10 | ssgA | SCO3926 | Regulator | 0.06 ± 0.01 | 0.17 ± 0.02 | 0.29 | 0.27 |
| 11 | bldD | SCO1489 | DNA-binding protein | 0.61 ± 0.05 | 0.46 ± 0.03 | 0.63 | 0.30 |
| 12 | whiG | SCO5621 | RNA polymerase sigma factor | 0.32 ± 0.07 | 0.22 ± 0.05 | 0.19 | 0.22 |
| 13 | cvnD9 | SCO1627 | ATP/GTP binding protein | 0.27 ± 0.03 | NDc | 0.28 | 0.32 |
| 14 | cvnD11 | SCO0585 | ATP/GTP binding protein | 0.08 ± 0.06 | ND | 0.14 | 0.19 |
RNA samples isolated from cultures of M145 and the ohkA mutant grown on MS agar covered with cellophane were used in qRT-PCR. The experiments were done in triplicate and repeated three times with RNA from independent cultures. The values reported are the means (± standard deviations) of three independent qRT-PCR analyses.
Averages of two microarray signal ratios (mutant/wild type) from the specific 60-nt probe replicates for each gene.
ND, not measured.
Effects of ohkA deletion on the transcription of secondary metabolism.
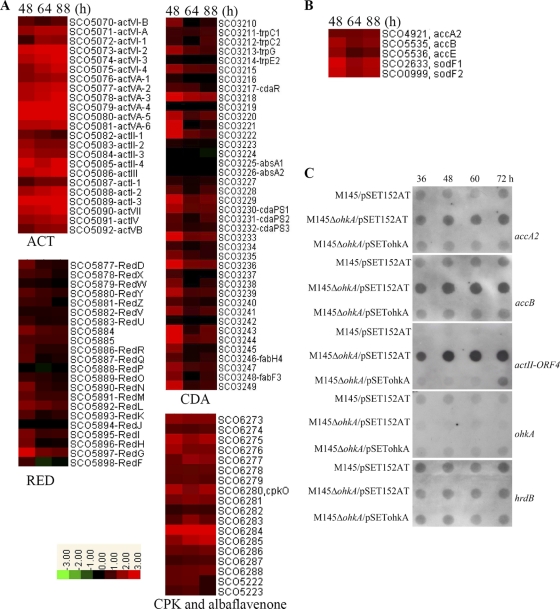
In agreement with the much higher production of ACT and CDA and slightly enhanced RED production in the ohkA mutant compared with M145, transcription profiles showed that, the majority of ACT and CDA biosynthetic gene clusters were upregulated more than 2-fold at the tested time points, while transcription of RED genes was mainly confined to the earliest time point (48 h), as shown in Fig. 3A. In addition, we found that the transcriptional levels of two other antibiotic biosynthetic gene clusters, a type I polyketide synthase gene cluster (SCO6273-6288) responsible for the biosynthesis of a novel yellow-pigmented secondary metabolite (CPK) with antibacterial activity (17, 39) and a gene cluster (SCO5222-5223) for biosynthesis of the sesquiterpene antibiotic albaflavenone (58), were enhanced as well (Fig. 3A).
Fig. 3.
Heat maps and RNA dot blot assays of selective gene clusters involved in antibiotic biosynthesis in S. coelicolor. (A and B) Transcription profiles of antibiotic biosynthetic gene clusters (ACT, RED, CDA, CPK, and albaflavenone) (A) and some primary metabolism genes in the ohkA mutant (B), compared with M145. RNA samples from M145 and the ohkA mutant were isolated at the indicated time points on MS medium. The relative changes of gene transcription are determined using log2(ratio of mutant/WT) and shown on a color scale, with red representing an increase in transcript abundance and green indicating a decrease in the ohkA mutant, relative to M145. Black represents unchanged expression levels. (C) RNA dot blot assay comparing the transcription of selected genes from panels A and B in M145/pSET152AT, M145ΔohkA/pSET152AT, and M145ΔohkA/pSETohkA. hrdB and ohkA were used as positive and negative controls, respectively. RNA samples were isolated at the indicated time points on MS medium, and 1 μg RNA per time point was spotted onto positive charged nylon membranes.
DNA microarray analysis also showed that the transcriptional levels of the pathway-specific regulators of four secondary metabolites (actII-ORF4 for ACT, redD for RED, cdaR for CDA, and cpkO for CPK) were all increased significantly in the ohkA-null mutant compared to M145, which was confirmed by qRT-PCR analysis (Table 2) and RNA dot blot assay (Fig. 3C). As shown from the RNA dot blot assay, transcription of actII-ORF4 was almost invisible in M145 throughout the time points tested; however, upon ohkA deletion, its transcription was drastically elevated and showed the characteristics of growth phase dependence. The level of hrdB transcript was constant in all of the three strains throughout the time points, thus providing an effective positive control in the RNA dot blot assay. As a negative control, the ohkA transcript was nearly undetected in the ohkA mutant as expected. We also found that, in M145, a low constant mRNA level of the ohkA gene was detected throughout the time course, indicating that ohkA is transcribed constitutively. Transcriptional alterations of actII-ORF4 and ohkA could be easily rescued by introduction of the ohkA gene (Fig. 3C). These data conclusively demonstrated that OhkA negatively regulated secondary metabolism by repressing directly or indirectly the pathway-specific activators in this microorganism.
Interestingly, there is no obvious transcriptional change of redZ encoding the orphan response regulator RedZ implicated in the positive control of redD transcription (19), suggesting that it might be one of the targets for OhkA. Since we have shown that there is no autophosphorylation activity of OhkA in vitro, other methods, such as yeast/bacterial two-hybrid assay, needed to be used to verify this possibility in the following study. We also showed that ohkA deletion had no effect on the transcription of TCS absA1/A2, which is clustered with CDA biosynthetic genes in the genome and can negatively regulate the biosynthesis of CDA, RED, and ACT (Fig. 3A).
DNA microarray analysis also revealed that the scbA gene involved in the synthesis of γ-butyrolactone signaling molecule SCB1 (47) was upregulated in the ohkA mutant, which was confirmed by qRT-PCR (Table 2). However, no obvious transcription change was found for the scbR gene, which encodes SCB1 binding protein. It was previously suggested that ScbR can repress the transcription of cpkO (pathway-specific regulator of CPK biosynthesis) through binding to its promoter region, resulting in lower CPK expression, and addition of SCB1 can eliminate the DNA binding and lead to high-level CPK production (47). It is thus speculative that upregulated transcription of cpk genes upon ohkA deletion might be the result of higher transcription of scbA that will elicit SCB1 accumulation and then relieve cpkO repression.
Effects of ohkA deletion on the transcription of primary metabolism genes.
Precursors for the secondary metabolite biosynthesis are generally formed from primary metabolism through the Embden-Meyerhof pathway (EMP) (55). From DNA microarray analysis combined with RNA dot blot assay (Fig. 3B and C), we showed that deletion of ohkA significantly induced the expression of a gene complex including accA2 (SCO4921), accB (SCO5535), and accE (SCO5536), which encodes the multienzyme complex acetyl coenzyme A (acetyl-CoA) carboxylase (ACCase) converting acetyl-CoA to malonyl-CoA in S. coelicolor (41). Malonyl-CoA and/or acetyl-CoA is the same precursor(s) for the biosynthesis of ACT and RED. Therefore, the increased expression of accA2/B/E should lead to higher malonyl-CoA formation and ACT and RED overproduction, which was in good agreement with a previous report on S. coelicolor (41), suggesting that OhkA plays a negative role in precursor supply for ACT and RED production in S. coelicolor.
We also found that two superoxide dismutase genes (sodF1/sodF2, SCO2633/SCO0999) which are involved in cellular resistance to toxic effects caused by oxidants were upregulated in the ohkA mutant (Fig. 3B). An earlier study demonstrated that transcription of sodF1/F2 in S. coelicolor was enhanced upon overexpression of pathway-specific regulator ActII-ORF4 (21). Recently, it was also reported that overexpression of two superoxide dismutase genes, sod1/sod2, from Streptomyces peucetius can increase the clavulanic acid (CA) level in Streptomyces clavuligerus and RED production in Streptomyces lividans (25).
Effects of ohkA deletion on transcription of developmental genes. (i) Genes associated with aerial mycelium formation.
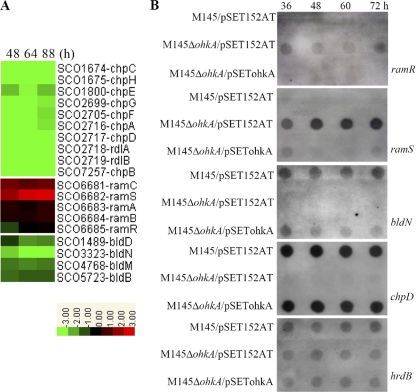
In S. coelicolor, there are two classes of surface-active molecules, SapB and the chaplins, which are important for aerial hypha formation by reducing the surface tension at the air-colony interface (7). Early studies showed that the biosurfactant activities of both SapB and the chaplins are needed for normal aerial hypha formation on rich medium, such as R2YE. However, on mannitol-containing medium (such as MS), S. coelicolor normally does not produce SapB molecules and needs only chaplins for aerial morphogenesis (7). Comparison of the global transcription profiles of M145 and the ohkA deletion mutant grown on MS medium revealed that transcription of all eight chaplin genes, chpA to chpH, was downregulated in the ohkA mutant, whereas the ram genes (ramC and ramS) responsible for the production of SapB (7, 54) were significantly elevated at all time points tested, 48, 64, and 88 h (Fig. 4A; Table 2). Although no obvious changes in expression of ramR were observed in the microarray data, ramR transcription in the mutant was up 2-fold compared with that in M145 as measured by qRT-PCR analysis (Table 2). These observations were in good agreement with previous evidence that ramC/S was under the positive regulation of ramR (26, 37). To further confirm these changes, an RNA dot blot assay for chpD and ramS/R was carried out. The results showed that, compared with high-level transcription of the chpD gene in M145, transcription of this chaplin gene was negligible in the ohkA mutant. In contrast, while ramS/R transcription was almost invisible in the wild-type strain, their expression was significantly enhanced upon ohkA deletion (Fig. 4B). These results clearly indicated that OhkA plays a differential role in the regulation of these two surface-active molecules, positive for the chaplin proteins and negative for SapB production.
Fig. 4.
Heat maps and RNA dot blot assays of selective gene clusters involved in morphological differentiation in S. coelicolor. (A) Transcription profiles of genes associated with aerial mycelium formation in the ohkA mutant, compared with M145. RNA samples from M145 and the ohkA mutant were isolated at the indicated time points on MS medium. The color representation is as described in the legend for Fig. 3. (B) RNA dot blot assay comparing the transcription of selected genes from panel A in M145/pSET152AT, M145ΔohkA/pSET152AT, and M145ΔohkA/pSETohkA. hrdB was used as a control for RNA loading levels.
It has been suggested that the formation of chaplins is under strict regulation by the bld cascade (7, 14). In this study, several bld genes in the ohkA mutant, including bldB, bldD, bldM, and bldN, were found to be downregulated, especially bldN, whose transcription was nearly undetectable, as verified by RNA dot blot assay (Fig. 4A and B). Previous reports showed that, on rich medium R2YE, the eight chaplin genes (chpA to chpH) were indirectly under the control of sigma factor σN and also its downstream target gene bldM, encoding the atypical response regulator BldM. Deletion of bldN or bldM would result in the drastically decreased expression of the chp genes (14). Here, we showed that in the ohkA-null mutant, transcription of both bldN and bldM was significantly reduced. Furthermore, transcription of all the genes which have been identified as being under the control of bldN (14), including the eight chp genes, SCO1088, SCO1089, SCO3714, SCO4002 (nepA), and SCO5819 (whiH), etc., was found to be significantly decreased in the ohkA mutant, implying that the regulation of ohkA on the chp genes was possibly mediated by the functions of bldN, and bldM may also be included. The data described above strongly suggested that when S. coelicolor was grown on MS agar, chaplin genes were positively regulated by bldN as well.
In addition, bldB is required for S. coelicolor aerial hypha formation on any medium and chaplins were not detectable in the bldB mutant (7), suggesting that downregulation of chp genes in the ohkA mutant might be partly associated with the lower transcription of bldB.
(ii) Genes associated with spore formation and maturation.
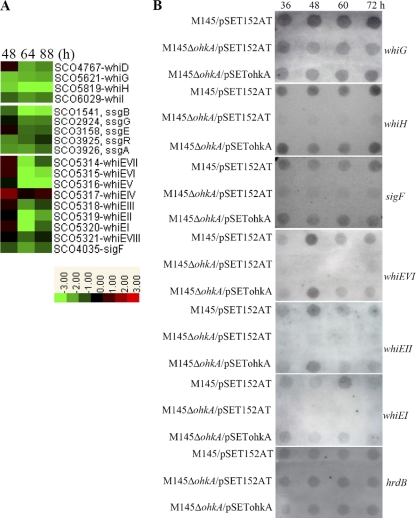
A number of regulatory genes have been identified as being necessary for initiating sporulation in S. coelicolor, such as whi and ssg genes. These genes could be divided into “early” and “late” sporulation genes depending on whether the relevant mutants undergo hyphal septum formation (9, 15). whiA/B/G/H/I/J and ssgA/B/R are considered early genes, which are required for the expression of late genes, including sigF and whiD. In the ohkA mutant, we found that mRNAs of one early whi gene, whiG, which encodes an early-sporulation-specific RNA polymerase sigma factor; two of its direct target genes, whiH and whiI; and one late sporulation gene, whiD, were significantly reduced at all three time points tested (Fig. 5A). In addition, transcription of ssgA and its regulatory gene ssgR, essential for sporulation-specific septum formation (50, 51, 53), was downregulated more than 2-fold. Moreover, several ssgA-like genes, ssgB, ssgE, and ssgG, involved in septum formation, autolytic spore separation, and exact septum localization (38, 53), respectively, were significantly decreased as well (Fig. 5A).
Fig. 5.
Heat maps and RNA dot blot assays of selective gene clusters involved in spore formation and maturation in S. coelicolor. (A) Transcription profiles of genes associated with spore formation and maturation in the ohkA mutant, compared with M145. RNA samples from M145 and the ohkA mutant were isolated at the indicated time points on MS medium. The color representation is as described in the legend for Fig. 3. (B) RNA dot blot assay comparing the transcription of selected genes from panel A in M145/pSET152AT, M145ΔohkA/pSET152AT, and M145ΔohkA/pSETohkA. hrdB was used as a control for RNA loading levels.
Transcription of the whiE gene cluster (ORFs I to VIII) responsible for the synthesis of spore pigment (a type II polyketide compound) in S. coelicolor, including a likely operon of seven genes (ORFs I to VII) and one divergently transcribed gene (ORF VIII) (27, 56), was significantly decreased in the ohkA mutant compared with M145, as shown in Fig. 5A and B. Moreover, a late-sporulation-specific RNA polymerase sigma factor, σF, encoded by sigF, which directs the expression of whiE-ORFVIII (27), was greatly downregulated as well in the mutant (Fig. 5A). Surprisingly, in the wild-type strain M145, transcription of sigF was detected earlier, at 36 h, which is inconsistent with previous work showing that sigF was expressed exclusively during sporulation when cultured on minimal medium (MM) (28), prompting us to suggest that transcription profiles of sigF might be quite different when S. coelicolor is grown on different media. An earlier study reported that disruption of whiE-ORFVIII resulted in spore color change (from gray to greenish). In addition, ectopic expression of different combinations of whiE and ORFs would lead to different spore pigment-related metabolites in or on the mycelium (56). So, it is speculative that the pink color of the ohkA mutant might be the result of decreased transcription of these whiE genes. Another possibility is the ectopic production of ACT and RED in aerial mycelium of the ohkA mutant, especially ACT, which has been described in the recent study carried out by Fowler-Goldsworthy (16).
DNA microarray data also revealed that deletion of ohkA not only nearly abolished expression of the chaplin genes but also led to drastically reduced transcription of the two rodlin genes encoding the other components identified as the S. coelicolor hydrophobic sheath, the rodlins RdlA and RdlB (Fig. 4A). It was previously demonstrated that expression of rdlA and rdlB is almost absent in the ΔchpABCDEFGH strain (12). We thus speculated that downregulation of rdlAB transcripts might be the result of significantly reduced expression of chaplin family genes. A previous report found that deletion of one or both rodlin genes did not affect the formation of aerial hyphae and spores; however, the spores of the rdl-null mutant had a disordered surface ultrastructure of fine chaplin filaments. However, due to the low resolving capability of the EM available, we have not seen any phenotype changes of the ohkA mutant spore surface. From these results presented here, we could conclude that OhkA had a pleiotropic impact on the control of aerial hypha formation and the sporulation process in S. coelicolor through the effect on bld, whi, and ssg genes.
Effects of ohkA deletion on other regulators involved in antibiotic biosynthesis.
In the S. coelicolor genome, there exist 13 conservons which typically consist of a cluster of four genes (cvnA to cvnD): cvnA, encoding a sensor histidine kinase homolog; cvnB and cvnC, encoding two proteins of unknown function; and cvnD, encoding an ATP/GTP-binding protein (22). In some cases, the conserved operons are clustered with cytochrome P450 genes (31). Previous study has revealed the involvement of cvn genes in antibiotic production; inactivation of cvnD9 in S. coelicolor resulted in overproduction of ACT and RED antibiotics (31). Interestingly, transcription of four conservons (cvn) (Fig. 6) was significantly reduced in the ohkA deletion mutant at three time points tested, which is confirmed by qRT-PCR (Table 2).
Fig. 6.

Heat maps of four conservons in S. coelicolor. Transcription profiles of genes from four conservons in the ohkA mutant, compared with M145. RNA samples from M145 and the ohkA mutant were isolated at the indicated time points on MS medium. The color representation is as described in the legend for Fig. 3.
DNA microarray analysis also revealed that two important pleiotropic regulatory genes, wblA (SCO3579) (16, 24) and nsdA (SCO5582) (32), which regulate both antibiotic production and morphological differentiation, were downregulated in the mutant. The decreased expression of wblA transcription was further confirmed using qRT-PCR analysis (Table 2). In a later study of the wblA gene in S. coelicolor (16), the wblA mutant showed a similar phenotype as that of the ohkA mutant in this study, such as ACT overproduction, red colony color, and a higher biomass at late growth stage, etc. In addition, there is a significant overlap in the transcriptional profiles upon the respective deletion of ohkA and wblA (16). So we speculated that the effects of ohkA exerted on antibiotic biosynthesis and development might be partly mediated by the wblA gene, a theory which needs to be verified in the future.
Conclusions.
In this study, we reported the characterization of a putative orphan histidine kinase, OhkA, and its roles in the regulation of both secondary metabolism and morphological differentiation in S. coelicolor and S. avermitilis. Genetic analysis showed that deletion of ohkA resulted in antibiotic overproduction and impaired aerial hypha and spore formation when strains were grown on MS agar. Disruption of the ohkA ortholog in S. avermitilis led to very similar effects as those of ohkA deletion in S. coelicolor, suggesting that this orphan HK may represent a highly conserved signal transduction component among Streptomyces bacteria. Further transcription profile analysis revealed that OhkA played a global role in antibiotic biosynthesis, by influencing precursor supply, pleiotropic and pathway-specific antibiotic regulators and also some structural genes involved in cellular resistance to toxic effects. The effects of OhkA on development might be mediated through the functions of bldN/M (regulation of chp genes), ramR/S (SapB formation and regulation), whiG/H/I and ssgA/R/G (required for aerial hypha, septum, and spore formation), and whiE (spore maturation). Further studies are required (i) to determine what signal the orphan histidine kinase OhkA responds to and (ii) to identify the downstream paired response regulator of OhkA and then to fully understand the molecular mechanism underlying OhkA's effects on antibiotic biosynthesis and morphological differentiation in S. coelicolor.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (30770023, 30970033, and 30830002), the National Basic Research Program of China (2007CB707803 and 2011CBA00806), and the Special Program for Biological Sciences Research of the Chinese Academy of Sciences (KSCX2-EW-J-12) and the Natural Science Foundation of Shanghai (11ZR1442700).
We are grateful to Keith F. Chater for providing the PCR targeting system and Zhongjun Qin for providing the S. coelicolor and S. avermitilis cosmids.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 22 April 2011.
REFERENCES
- 1. Adamidis T., Riggle P., Champness W. 1990. Mutations in a new Streptomyces coelicolor locus which globally block antibiotic biosynthesis but not sporulation. J. Bacteriol. 172:2962–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amon J., et al. 2008. Nitrogen control in Mycobacterium smegmatis: nitrogen-dependent expression of ammonium transport and assimilation proteins depends on the OmpR-type regulator GlnR. J. Bacteriol. 190:7108–7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson T. B., Brian P., Champness W. C. 2001. Genetic and transcriptional analysis of absA, an antibiotic gene cluster-linked two-component system that regulates multiple antibiotics in Streptomyces coelicolor. Mol. Microbiol. 39:553–566 [DOI] [PubMed] [Google Scholar]
- 4. Bentley S. D., et al. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147 [DOI] [PubMed] [Google Scholar]
- 5. Bibb M. J. 2005. Regulation of secondary metabolism in streptomycetes. Curr. Opin. Microbiol. 8:208–215 [DOI] [PubMed] [Google Scholar]
- 6. Brian P., Riggle P. J., Santos R. A., Champness W. C. 1996. Global negative regulation of Streptomyces coelicolor antibiotic synthesis mediated by an absA-encoded putative signal transduction system. J. Bacteriol. 178:3221–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Capstick D. S., Willey J. M., Buttner M. J., Elliot M. A. 2007. SapB and the chaplins: connections between morphogenetic proteins in Streptomyces coelicolor. Mol. Microbiol. 64:602–613 [DOI] [PubMed] [Google Scholar]
- 8. Chang H. M., Chen M. Y., Shieh Y. T., Bibb M. J., Chen C. W. 1996. The cutRS signal transduction system of Streptomyces lividans represses the biosynthesis of the polyketide antibiotic actinorhodin. Mol. Microbiol. 21:1075–1085 [PubMed] [Google Scholar]
- 9. Chater K. F. 2001. Regulation of sporulation in Streptomyces coelicolor A3(2): a checkpoint multiplex? Curr. Opin. Microbiol. 4:667–673 [DOI] [PubMed] [Google Scholar]
- 10. Chen L., et al. 2009. Transcriptomics analyses reveal global roles of the regulator AveI in Streptomyces avermitilis. FEMS Microbiol. Lett. 298:199–207 [DOI] [PubMed] [Google Scholar]
- 11. Chen L., et al. 2008. Characterization of a negative regulator AveI for avermectin biosynthesis in Streptomyces avermitilis NRRL8165. Appl. Microbiol. Biotechnol. 80:277–286 [DOI] [PubMed] [Google Scholar]
- 12. Claessen D., et al. 2004. The formation of the rodlet layer of streptomycetes is the result of the interplay between rodlins and chaplins. Mol. Microbiol. 53:433–443 [DOI] [PubMed] [Google Scholar]
- 13. Datsenko K. A., Wanner B. L. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elliot M. A., et al. 2003. The chaplins: a family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor. Genes Dev. 17:1727–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flardh K., Buttner M. J. 2009. Streptomyces morphogenetics: dissecting differentiation in a filamentous bacterium. Nat. Rev. Microbiol. 7:36–49 [DOI] [PubMed] [Google Scholar]
- 16. Fowler-Goldsworthy K., et al. 2011. The actinobacteria-specific gene wblA controls major developmental transitions in Streptomyces coelicolor A3(2). Microbiology doi:10.1099/mic.0.047555-0 [DOI] [PubMed] [Google Scholar]
- 17. Gottelt M., Kol S., Gomez-Escribano J. P., Bibb M., Takano E. 2010. Deletion of a regulatory gene within the cpk gene cluster reveals novel antibacterial activity in Streptomyces coelicolor A3(2). Microbiology 156:2343–2353 [DOI] [PubMed] [Google Scholar]
- 18. Gust B., Challis G. L., Fowler K., Kieser T., Chater K. F. 2003. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. U. S. A. 100:1541–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guthrie E. P., et al. 1998. A response-regulator-like activator of antibiotic synthesis from Streptomyces coelicolor A3(2) with an amino-terminal domain that lacks a phosphorylation pocket. Microbiology 144:727–738 [DOI] [PubMed] [Google Scholar]
- 20. Hopwood D. A. 1999. Forty years of genetics with Streptomyces: from in vivo through in vitro to in silico. Microbiology 145:2183–2202 [DOI] [PubMed] [Google Scholar]
- 21. Huang J., et al. 2005. Cross-regulation among disparate antibiotic biosynthetic pathways of Streptomyces coelicolor. Mol. Microbiol. 58:1276–1287 [DOI] [PubMed] [Google Scholar]
- 22. Hutchings M. I., Hoskisson P. A., Chandra G., Buttner M. J. 2004. Sensing and responding to diverse extracellular signals? Analysis of the sensor kinases and response regulators of Streptomyces coelicolor A3(2). Microbiology 150:2795–2806 [DOI] [PubMed] [Google Scholar]
- 23. Ishizuka H., Horinouchi S., Kieser H. M., Hopwood D. A., Beppu T. 1992. A putative two-component regulatory system involved in secondary metabolism in Streptomyces spp. J. Bacteriol. 174:7585–7594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang S. H., et al. 2007. Interspecies DNA microarray analysis identifies WblA as a pleiotropic down-regulator of antibiotic biosynthesis in Streptomyces. J. Bacteriol. 189:4315–4319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kanth B. K., Jnawali H. N., Niraula N. P., Sohng J. K. 2010. Superoxide dismutase (SOD) genes in Streptomyces peucetius: effects of SODs on secondary metabolites production. Microbiol. Res. doi:10.1016/j.micres.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 26. Keijser B. J., van Wezel G. P., Canters G. W., Vijgenboom E. 2002. Developmental regulation of the Streptomyces lividans ram genes: involvement of RamR in regulation of the ramCSAB operon. J. Bacteriol. 184:4420–4429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kelemen G. H., et al. 1998. Developmental regulation of transcription of whiE, a locus specifying the polyketide spore pigment in Streptomyces coelicolor A3(2). J. Bacteriol. 180:2515–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kelemen G. H., et al. 1996. The positions of the sigma-factor genes, whiG and sigF, in the hierarchy controlling the development of spore chains in the aerial hyphae of Streptomyces coelicolor A3(2). Mol. Microbiol. 21:593–603 [DOI] [PubMed] [Google Scholar]
- 29. Kieser T., Bibb M., Buttner M., Chater K. 2000. Practical Streptomyces genetics. John Innes Foundation, Norwich, England [Google Scholar]
- 30. Kirby R., Hopwood D. A. 1977. Genetic determination of methylenomycin synthesis by the SCP1 plasmid of Streptomyces coelicolor A3(2). J. Gen. Microbiol. 98:239–252 [DOI] [PubMed] [Google Scholar]
- 31. Komatsu M., et al. 2006. Proteins encoded by the conservon of Streptomyces coelicolor A3(2) comprise a membrane-associated hetero-complex that resembles eukaryotic G protein-coupled regulatory system. Mol. Microbiol. 62:1534–1546 [DOI] [PubMed] [Google Scholar]
- 32. Li W., et al. 2006. Identification of a gene negatively affecting antibiotic production and morphological differentiation in Streptomyces coelicolor A3(2). J. Bacteriol. 188:8368–8375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Y. Q., Chen P. L., Chen S. F., Wu D., Zheng J. 2004. A pair of two-component regulatory genes ecrA1/A2 in Streptomyces coelicolor. J. Zhejiang Univ. Sci. 5:173–179 [DOI] [PubMed] [Google Scholar]
- 34. Lu Y., et al. 2007. Characterization of a novel two-component regulatory system involved in the regulation of both actinorhodin and a type I polyketide in Streptomyces coelicolor. Appl. Microbiol. Biotechnol. 77:625–635 [DOI] [PubMed] [Google Scholar]
- 35. MacNeil D. J., et al. 1992. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene 111:61–68 [DOI] [PubMed] [Google Scholar]
- 36. Mascher T., Helmann J. D., Unden G. 2006. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 70:910–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nguyen K. T., et al. 2002. A central regulator of morphological differentiation in the multicellular bacterium Streptomyces coelicolor. Mol. Microbiol. 46:1223–1238 [DOI] [PubMed] [Google Scholar]
- 38. Noens E. E., et al. 2005. SsgA-like proteins determine the fate of peptidoglycan during sporulation of Streptomyces coelicolor. Mol. Microbiol. 58:929–944 [DOI] [PubMed] [Google Scholar]
- 39. Pawlik K., Kotowska M., Kolesinski P. 2010. Streptomyces coelicolor A3(2) produces a new yellow pigment associated with the polyketide synthase Cpk. J. Mol. Microbiol. Biotechnol. 19:147–151 [DOI] [PubMed] [Google Scholar]
- 40. Ryding N. J., Anderson T. B., Champness W. C. 2002. Regulation of the Streptomyces coelicolor calcium-dependent antibiotic by absA, encoding a cluster-linked two-component system. J. Bacteriol. 184:794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ryu Y. G., Butler M. J., Chater K. F., Lee K. J. 2006. Engineering of primary carbohydrate metabolism for increased production of actinorhodin in Streptomyces coelicolor. Appl. Environ. Microbiol. 72:7132–7139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 43. San Paolo S., Huang J., Cohen S. N., Thompson C. J. 2006. rag genes: novel components of the RamR regulon that trigger morphological differentiation in Streptomyces coelicolor. Mol. Microbiol. 61:1167–1186 [DOI] [PubMed] [Google Scholar]
- 44. Shu D., et al. 2009. afsQ1-Q2-sigQ is a pleiotropic but conditionally required signal transduction system for both secondary metabolism and morphological development in Streptomyces coelicolor. Appl. Microbiol. Biotechnol. 81:1149–1160 [DOI] [PubMed] [Google Scholar]
- 45. Sola-Landa A., Moura R. S., Martin J. F. 2003. The two-component PhoR-PhoP system controls both primary metabolism and secondary metabolite biosynthesis in Streptomyces lividans. Proc. Natl. Acad. Sci. U. S. A. 100:6133–6138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stock A. M., Robinson V. L., Goudreau P. N. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183–215 [DOI] [PubMed] [Google Scholar]
- 47. Takano E., et al. 2005. A bacterial hormone (the SCB1) directly controls the expression of a pathway-specific regulatory gene in the cryptic type I polyketide biosynthetic gene cluster of Streptomyces coelicolor. Mol. Microbiol. 56:465–479 [DOI] [PubMed] [Google Scholar]
- 48. Taylor B. L., Zhulin I. B. 1999. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63:479–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tian Y., Fowler K., Findlay K., Tan H., Chater K. F. 2007. An unusual response regulator influences sporulation at early and late stages in Streptomyces coelicolor. J. Bacteriol. 189:2873–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Traag B. A., Kelemen G. H., Van Wezel G. P. 2004. Transcription of the sporulation gene ssgA is activated by the IclR-type regulator SsgR in a whi-independent manner in Streptomyces coelicolor A3(2). Mol. Microbiol. 53:985–1000 [DOI] [PubMed] [Google Scholar]
- 51. van Wezel G. P., et al. 2000. ssgA is essential for sporulation of Streptomyces coelicolor A3(2) and affects hyphal development by stimulating septum formation. J. Bacteriol. 182:5653–5662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wilkinson C. J., et al. 2002. Increasing the efficiency of heterologous promoters in actinomycetes. J. Mol. Microbiol. Biotechnol. 4:417–426 [PubMed] [Google Scholar]
- 53. Willemse J., Borst J. W., de Waal E., Bisseling T., van Wezel G. P. 2011. Positive control of cell division: FtsZ is recruited by SsgB during sporulation of Streptomyces. Genes Dev. 25:89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Willey J., Santamaria R., Guijarro J., Geistlich M., Losick R. 1991. Extracellular complementation of a developmental mutation implicates a small sporulation protein in aerial mycelium formation by Streptomyces coelicolor. Cell 65:641–650 [DOI] [PubMed] [Google Scholar]
- 55. Wu G., Culley D. E., Zhang W. 2005. Predicted highly expressed genes in the genomes of Streptomyces coelicolor and Streptomyces avermitilis and the implications for their metabolism. Microbiology 151:2175–2187 [DOI] [PubMed] [Google Scholar]
- 56. Yu T. W., Hopwood D. A. 1995. Ectopic expression of the Streptomyces coelicolor whiE genes for polyketide spore pigment synthesis and their interaction with the act genes for actinorhodin biosynthesis. Microbiology 141:2779–2791 [DOI] [PubMed] [Google Scholar]
- 57. Zhang W., Shi L. 2005. Distribution and evolution of multiple-step phosphorelay in prokaryotes: lateral domain recruitment involved in the formation of hybrid-type histidine kinases. Microbiology 151:2159–2173 [DOI] [PubMed] [Google Scholar]
- 58. Zhao B., et al. 2008. Biosynthesis of the sesquiterpene antibiotic albaflavenone in Streptomyces coelicolor A3(2). J. Biol. Chem. 283:8183–8189 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.