Abstract
Ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) catalyzes the first step of CO2 fixation in the Calvin-Benson-Bassham (CBB) cycle. Besides its function in fixing CO2 to support photoautotrophic growth, the CBB cycle is also important under photoheterotrophic growth conditions in purple nonsulfur photosynthetic bacteria. It has been assumed that the poor photoheterotrophic growth of RubisCO-deficient strains was due to the accumulation of excess intracellular reductant, which implied that the CBB cycle is important for maintaining the redox balance under these conditions. However, we present analyses of cbbM mutants in Rhodospirillum rubrum that indicate that toxicity is the result of an elevated intracellular pool of ribulose-1,5-bisphosphate (RuBP). There is a redox effect on growth, but it is apparently an indirect effect on the accumulation of RuBP, perhaps by the regulation of the activities of enzymes involved in RuBP regeneration. Our studies also show that the CBB cycle is not essential for R. rubrum to grow under photoheterotrophic conditions and that its role in controlling the redox balance needs to be further elucidated. Finally, we also show that CbbR is a positive transcriptional regulator of the cbb operon (cbbEFPT) in R. rubrum, as seen with related organisms, and define the transcriptional organization of the cbb genes.
INTRODUCTION
The purple nonsulfur photosynthetic bacterium Rhodospirillum rubrum is metabolically diverse and can grow under photoautotrophic, photoheterotrophic, and chemoheterotrophic conditions. It can use different kinds of nitrogen and carbon sources, including N2 and CO2, through effective metabolic systems such as the nitrogenase and the Calvin-Benson-Bassham (CBB) cycles (2, 17, 21, 30). Both the nitrogenase system and the CBB cycle are very energy-demanding processes and are therefore usually tightly regulated and very sensitive to environmental signals (12, 20, 38, 40, 47).
The main role of the CBB cycle is to fix CO2 into organic carbon under photoautotrophic conditions where CO2 serves as the sole carbon source. Thus, most cbb genes have their highest expression levels under these conditions (2, 15, 28, 36, 47). However, under photoheterotrophic conditions in the presence of organic carbon such as malate or acetate, the CBB cycle also functions as a major electron sink in many photosynthetic bacteria, and it is assumed that this property maintains the redox balance in the cell (18, 24, 34, 57, 61, 62, 67). Recently, it was shown that the CBB cycle acts as an electron-accepting process to recycle the excess reduced cofactors under non-N2-fixing conditions in Rhodopseudomonas palustris (39). Thus, an R. rubrum cbbM mutant in which the CBB cycle is blocked by the elimination of its key enzyme, ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) (encoded by cbbM), not only fails to grow under photoautotrophic conditions but also grows poorly under photoheterotrophic conditions (66). Similar phenotypes have been seen for RubisCO-deficient strains of Rhodobacter sphaeroides and Rhodobacter capsulatus unless an alternate electron acceptor, such as dimethyl sulfoxide (DMSO), is supplied in the medium (18, 43, 62). It was hypothesized that the blockage of the CBB cycle causes an accumulation of excess reductant and that this redox imbalance perturbs cell growth (62). However, the actual mechanism behind the poor photoheterotrophic growth of cbbM mutants is unknown for any of these organisms.
The regulation of cbb operons has been extensively studied for R. sphaeroides, R. capsulatus, and R. palustris (6, 16, 22, 26, 46, 57, 64). These organisms all have two forms of RubisCO, form I and form II, encoded by cbbLS and cbbM, which are located in the cbbI and cbbII operons, respectively (32). The transcription of these cbb operons is activated by a LysR-type transcriptional regulator, CbbR (22, 43), which might need coinducers like ribulose-1,5-bisphosphate (RuBP) or its derivatives for its regulation (7, 13, 54, 63). In addition, cbb expression is also regulated by a two-component global regulatory system, RegB/RegA, which is also responsible for the regulation of nitrogen fixation, hydrogen metabolism, and energy generation in R. capsulatus and R. sphaeroides (10, 12, 14, 15, 27, 44, 51, 64). Models for the complex formation of RegA and CbbR at a cbb promoter were proposed for R. capsulatus and R. sphaeroides (8), and the phosphorylation of RegA is crucial for the transcription of cbb operons (11, 64). In R. palustris there is no RegB/RegA regulatory system. Instead, a three-protein CbbRRS system acts as a signal transduction system that regulates the transcription of cbb operons (46).
R. rubrum lacks both the RegB/RegA and the CbbRRS systems, and the mechanism of the regulation of its cbb operons is unknown. In addition, the CBB cycle of R. rubrum also has several features that distinguish it from those of other related photosynthetic bacteria: (i) R. rubrum has only one form of RubisCO (form II, encoded by cbbM), while many other organisms have two (32), and (ii) in R. rubrum, the cbbM gene is not located in the same operon with other major cbb genes as seen in other organisms (12, 42, 52). Instead, it is located at the 3′ end of cbbR, which encodes a putative regulatory protein. These genes are transcribed in the opposite direction from the main cbb gene cluster, which includes cbbE, cbbF, cbbP, and cbbT (Fig. 1A) (17). The linkage of cbbR with cbbM raises a question about the role of CbbR in the regulation of the expression of cbbM and other cbb operons.
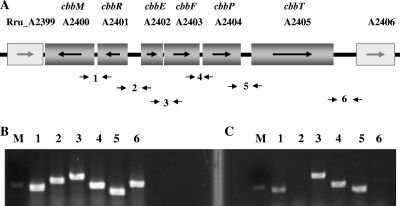
Fig. 1.
cbb gene cluster and flanking genes of R. rubrum and identification of transcripts of cbb operons by reverse transcription-PCR (RT-PCR). (A) Gene organization based on genome information from the National Center for Biotechnology Information (NCBI) database, where the genes shown at either end are not involved in the cbb regulon. The small arrows below the genetic map indicate the locations and directions of primers used for the regular PCR and RT-PCR, as described below. The bottom two panels show the results of the transcription analysis of the cbb operons in R. rubrum by RT-PCR. (B and C) Data for the intergenic spaces of cbbM-cbbR (lane 1), cbbR-cbbE (lane 2), cbbE-cbbF (lane 3), cbbF-cbbP (lane 4), cbbP-cbbT (lane 5), and cbbT-Rru_A2406 (lane 6) amplified by using genomic DNA of the wild type (UR2) as the PCR template (B) and cDNA of UR2 as the RT-PCR template (C). Lane M has a DNA marker indicating 550 bp.
Some insight into the basis of the poor photoheterotrophic growth of cbbM mutants was gained by seeking suppressors of that phenotype. A spontaneous mutant of an R. sphaeroides RubisCO-deficient strain (cbbM cbbLS) restored photoheterotrophic growth and also produced active nitrogenase in the presence of ammonia, and the expression of nitrogenase in this RubisCO-deficient strain presumably consumed excess reductant and allowed cell growth (27, 62, 63). A suppressor of an R. rubrum cbbM mutant that gained the ability to grow photoheterotrophically also had an altered regulation of nitrogenase activity, consistent with the redox balance hypothesis (66). These results strongly suggested a linkage between the CBB cycle and the nitrogenase system.
The nitrogenase system consists of nitrogenase (MoFe protein) and dinitrogenase reductase (Fe protein), whose expression and activity are regulated by NH4+ at both the transcriptional and posttranslational levels in R. rubrum (5, 38, 40, 74). The transcription of the nif genes requires active NifA (73). Under nitrogen-limiting conditions, NifA is activated by GlnB-UMP, one of the PII proteins in R. rubrum (72, 73, 76). The posttranslational regulation of nitrogenase is the result of the reversible ADP-ribosylation of dinitrogenase reductase in response to the addition of NH4+ or the depletion of energy (37, 70, 72). Two enzymes catalyze this reversible process. Dinitrogenase reductase ADP-ribosyl transferase (referred to as DRAT, the gene product of draT) carries out the transfer of the ADP-ribose from NAD to dinitrogenase reductase, inactivating that enzyme. The dinitrogenase reductase-activating glycohydrolase (referred to as DRAG, the gene product of draG) removes the ADP-ribose group attached to dinitrogenase reductase, thus restoring nitrogenase activity (38, 40). We have previously obtained mutants altered in nifA (named nifA*, encoding NifA-M173V) and draT in which the nitrogenase functions even in the presence of negative stimuli such as NH4+ (37, 77). When these nifA* and draT mutations were introduced into the R. rubrum cbbM mutant, the constitutive expression of active nitrogenase restored normal growth, which we interpreted to be due to the dissipation of the excess reductant caused by the cbbM mutation (66). Indeed, we also showed that the previously reported cbbM mutant of R. rubrum (strain I-19) also had a similar nifA mutation, which had not been recognized (66). However, the mechanisms of the effects of the reductant on the regulation of nitrogenase activity and cell growth are still unknown for R. rubrum.
In this paper, we further investigate the basis for the poor growth of R. rubrum cbbM mutants under photoheterotrophic conditions. We have performed Tn5 random mutagenesis and identified suppressor mutants that are able to restore a normal-growth phenotype to an R. rubrum cbbM mutant. To our surprise, the analysis of these suppressors suggests that the causative property for the poor growth is not a redox imbalance, as has been supposed, but instead the accumulation of the substrate of RubisCO, RuBP, or its derivatives. The results are potentially of broad significance to similar mutant phenotypes reported for other organisms. In the course of this analysis we have better defined the relevant gene products in the CBB pathway, the transcriptional organization of cbb operons, and the role of the apparent regulator, CbbR, in R. rubrum.
MATERIALS AND METHODS
Bacterial strains.
The R. rubrum strains used in this study are listed in Table 1. Antibiotics were used at the following concentrations: ampicillin (Ap) at 100 mg/liter, kanamycin (Km) at 25 mg/liter, gentamicin (Gm) at 5 mg/liter, chloramphenicol (Cm) at 25 mg/liter, and tetracycline (Tc) at 12.5 mg/liter for Escherichia coli and streptomycin (Sm) at 100 mg/liter, Km at 12.5 mg/liter, nalidixic acid (Nx) at 6 mg/liter, Tc at 1 mg/liter, Gm at 10 mg/liter, and Cm at 5 mg/liter for R. rubrum.
Table 1.
R. rubrum strains and their genotypes
| Strain | Relevant genotype and/or descriptiona | Reference |
|---|---|---|
| UR2 | Wild type | 19 |
| UR5251 | ΔcbbM1::aacC1; same orientation | 66 |
| UR5253 | UR5251 with a pRK404 derivative carrying cbbM and its own promoter region | This work |
| UR5311 | ΔcbbR2::aacC1; opposite orientation | This work |
| UR2553 | ΔcbbM1::aacC1 draT3 nifA12 (encoding NifA-M173V) | 66 |
| UR2555 | ΔcbbM1::aacC1 ΔcbbF1::kan | This work |
| UR2557 | ΔcbbM1::aacC1 ΔcbbP1::kan | This work |
| UR2562 | ΔcbbM1:: aacC1 nifA12 (encoding NifA-M173V) | 66 |
| UR2564 | ΔcbbF1::kan; opposite orientation | This work |
| UR2565 | ΔcbbP1::kan; opposite orientation | This work |
| UR2633 | ΔRru_A0595::aacC1 | This work |
| UR2651 | ΔcbbT1::kan; same orientation | This work |
| UR2653 | ΔcbbM1::aacC1 ΔcbbT1::kan | This work |
| UR2654 | PaacC1-cbbR integrated in the chromosome of the wild type (UR2) at the nifH draT region | This work |
| UR2657 | ΔcbbRM1::aacC1; same orientation | This work |
| UR2658 | ΔcbbRM2::aacC1; opposite orientation | This work |
“Same orientation” refers to the case where the direction of transcription of the inserted kan or aacC1 gene is the same as that of the mutated cbb gene, while “opposite orientation” refers to the transcription of the inserted gene being in the opposite direction.
Growth conditions.
E. coli cells were grown in LC medium (similar to Luria-Bertani medium but with 5 g/liter NaCl). R. rubrum was routinely grown in rich SMN (supplemented malate-ammonium) medium aerobically (19). To monitor cell growth under photoheterotrophic or photoautotrophic conditions, R. rubrum cultures were first grown in 5-ml aliquots of SMN medium aerobically without illumination at 30°C for 2 to 3 days. For photoheterotrophic growth, SMN cultures were diluted 60-fold into 25-ml anaerobic tubes that were almost completely filled with MG medium (a nitrogen-limiting, malate-glutamate minimal medium with glutamate as a nitrogen source) (41). These cultures had an initial optical density at 600 nm (OD600) of 0.04 to 0.05 and were incubated at 30°C under illumination. One milliliter of culture was taken out every 24 h to measure the optical density at 600 nm for 6 days. For photoautotrophic growth, SMN cultures were diluted 60-fold into 10 ml of Ormerod's minimal medium (41) supplemented with 0.1% NH4Cl and 0.05% yeast extract in a 100-ml serum bottle. After evacuation and replacement of the gas phase with CO2-H2 (2:98 [vol/vol] ratio), the cultures were incubated at 30°C under illumination on an orbital shaker with a speed of 120 rpm. Subsequently, extra CO2 gas was added into the gas phase of well-grown cultures every 24 h to keep the CO2 concentration at 1.5 to 3%, which was reported previously to result in the highest level of RubisCO activity (47).
Tn5 random mutagenesis and screen for suppressors of a cbbM mutation.
Plasmid pRL27 carries a hyperactive Tn5 transposase gene (tnp), a mini-Tn5 element containing both kanamycin resistance (Kmr) and the origin of replication from plasmid R6K (oriR6K) (33). This plasmid was transferred into an R. rubrum cbbM mutant (UR5251) through biparental conjugation as described previously (37). Transconjugants were enriched in SMN liquid medium with Nx, Gm, and Km aerobically and then inoculated into MG medium for anaerobic photoheterotrophic growth, where the parental cbbM mutant (UR5251) strain could not grow well (66). After incubation for 3 to 5 days, well-grown cultures in tubes with a pink or red color were plated onto SMN medium with Nx, Gm, and Km after a serial dilution. Nxr Gmr Kmr colonies were purified on SMN selection plates and then grown in MG medium again to verify their photoheterotrophic growth. The sites of the Tn5 insertions in 10 transconjugants were identified by DNA sequencing, as described previously (33, 71).
Construction of cbb mutants.
To construct ΔcbbP mutants, two 1.5-kb fragments immediately 5′ and 3′ of cbbP, respectively, were amplified by PCR with the chromosomal DNA of R. rubrum strain UR2 (wild type) as a template. These fragments have SalI/BamHI sites or BamHI/HindIII sites at both ends and were cloned into the SalI and HindIII sites of pSUP202 (53). kan (encoding Kmr) from pUC4K (65) was inserted in both orientations into a BamHI site between these two fragments, yielding pUX2918 and pUX2919. These plasmids were then transferred into R. rubrum UR2 by biparental conjugation as described previously (37). Smr Kmr R. rubrum colonies were selected and replica printed to identify Cm-sensitive (Cms) colonies resulting from a double-crossover recombination event (Cmr is encoded by pSUP202). Two ΔcbbP mutants were obtained with kan transcribed in either the same or the opposite direction as cbbP. Because these mutants behaved identically in subsequent studies, only the data for one of the mutants, UR2565 (ΔcbbP1::kan, with kan inserted in the opposite direction of the original cbbP), are reported in this study. To construct the cbbM cbbP double mutants, pUX2918 and pUX2919 were transferred into cbbM mutant strain UR5251. Smr Gmr Kmr Cms R. rubrum colonies resulting from a double-crossover recombination event were screened. Similarly, two double mutants were obtained, and only UR2557 (ΔcbbM1::aacC1 ΔcbbP1::kan) is reported in this study.
All other single and double mutants listed in Table 1 were constructed in the same way as described above, except for the single deletions/insertions lacking both cbbR and cbbM. These mutants were constructed by the deletion of the entire cbbRM region and its replacement by an aacC1 gene fragment from pUCGM (50), resulting in UR2657 (ΔcbbRM1::aacC1) and UR2658 (ΔcbbRM2::aacC1), which have different orientations of the aacC1 insertion. Unlike other constructed mutants, the insertion orientation affected the phenotypes of these two cbbRM mutants, so the data for both mutants are reported. All mutations were verified by PCR.
Complementation of a cbbM mutant with cbbM expressed from its own promoter between the cbbR and cbbM genes.
To study the expression of cbbM from its own promoter between the cbbR and cbbM genes, cbbM and 118 bp of the immediately adjacent 5′ region were cloned into the broad-host-range vector pRK404 (9) at BamHI and HindIII sites in the opposite orientation of the lac promoter on pRK404 to eliminate the possibility of cbbM expression from this promoter. This pRK404 vector carrying cbbM and its 5′ region was then introduced into a cbbM mutant strain (UR5251) by triparental conjugation as described previously (23), yielding UR5253.
Overexpression of cbbR in wild-type R. rubrum.
To overexpress cbbR in R. rubrum, cbbR was amplified by PCR with a pair of primers with NdeI and XhoI sites at the 5′ and 3′ ends, respectively, and was then cloned into plasmid pUX2519, which has the promoter of aacC1 cloned into pBSKS (Stratagene, La Jolla, CA), yielding pUX3197. The fragment including the aacC1 promoter and the cbbR gene was digested with BamHI and XhoI and then cloned into plasmid pYPZ261, which is a pUX19 derivative carrying a 2-kb nifH draT region of R. rubrum, yielding pUX3198. This plasmid was then integrated into the chromosome of wild-type R. rubrum (UR2) through biparental conjugation. Smr Kmr colonies were isolated and verified by PCR, indicating that a new copy of cbbR expressed from the aacC1 promoter was integrated into the chromosome of UR2 at the nifH draT region. The otherwise wild-type strain with the plasmid-carried cbbR was designated UR2654.
Quantitation of intracellular levels of RuBP.
Phenylhydrazine was used for the derivatization of RuBP and other sugars for better separation and detection by high-performance liquid chromatography (HPLC), as described previously (56). RuBP was then quantitated by HPLC using a method reported previously, with some modifications (45). R. rubrum strains were grown in MG medium under photoheterotrophic conditions for 2 to 3 days or until the OD600 reached 1. A 3-ml sample of the MG culture was collected by centrifugation at 20,000 × g for 1 min. The pellets were resuspended in 60 μl of 10% acetic acid to break the cells and extract RuBP, followed by another centrifugation at 20,000 × g for 5 min. The supernatant of each sample was then transferred into a new tube, and freshly made phenylhydrazine derivatization buffer (0.5% phenylhydrazine and 10% acetic acid) was added at a 10% (vol/vol) final concentration. The derivatization reaction was carried out at 80°C for 15 min.
A C18 Intersil HPLC column (10-μm particle size, ODS-4, 4.6 by 150 mm; GL Sciences Inc., Torrance, CA) was used with the Shimadzu CTO-20A HPLC system (Shimadzu Scientific Instruments, Columbia, MD). The mobile phase containing methanol, HPLC-grade water, and acetic acid at a 60:38.5:1.5 ratio and 5 mM tetrabutylammonium hydrogen sulfate was set at a flow rate of 1 ml per min. The HPLC oven temperature was set at 40°C, and detection was carried out at 325 nm. Twenty microliters of the derivatized samples and RuBP standards were injected into HPLC columns for detection and quantitation.
RNA isolation and reverse transcription.
Total RNA was prepared from harvested cells as previously described (60) and was then treated with RNase-free DNase I (Invitrogen, Carlsbad, CA) and purified by using an RNeasy minikit (Qiagen, Valencia, CA) as described by the manufacturer. The RNA concentration was quantitated with a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE).
The reverse transcription (RT) reaction was performed with the GoScript reverse transcription system (Promega, Madison, WI) according to the manufacturer's instructions. The random hexamer primers were synthesized by Integrated DNA Technologies (Coralville, IA). The cDNA was cleaned up by using a QIAquick PCR purification kit (Qiagen) after the reverse transcription reaction. The cDNA concentration was quantitated by using a NanoDrop ND-1000 spectrophotometer.
RT-PCR.
Six pairs of primers were designed to amplify the interspaces between the genes Rru_A2400 to Rru_A2406 in R. rubrum, which were tested by regular PCR using the genomic DNA of R. rubrum wild-type strain UR2 as a template. RT-PCR was performed by using cDNA as templates.
Quantitative real-time PCR.
Primers were designed with Primer3 (http://primer3.sourceforge.net/) to amplify 100- to 150-bp PCR products. GoTaq quantitative real-time PCR (qPCR) master mix (Promega) was used for qPCRs, which were performed with an ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA). The PCR was accomplished with a 2-min denaturation step at 95°C, followed by 40 cycles of 20 s at 95°C, 20 s at 50°C, and 40 s at 72°C. Primer dimers were evaluated by a melting-curve analysis reaction for 15 s at 95°C, 1 min at 60°C, and 15 s at 95°C.
rpoD, encoding the σ70 transcription factor, is considered to be stable in R. rubrum and many other bacteria (31, 49) and was used as a housekeeping gene control in qPCRs to normalize the results of relative comparisons of target gene expression levels. Because different PCR efficiencies (E) were obtained from each PCR, we calculated the relative expression ratio with the correction of efficiency (E) by the following formula, as described previously (48): relative expression ratio = (1 + Etarget)−ΔCT target (sample − control)/(1 + ErpoD)−ΔCT rpoD (sample − control).
cDNA from the wild type (UR2) was used as the control, and cDNA from the cbbR mutant (UR5311) was used as the sample for qPCR.
RESULTS AND DISCUSSION
Identification of genes and their encoded enzymes in the CBB pathway in R. rubrum.
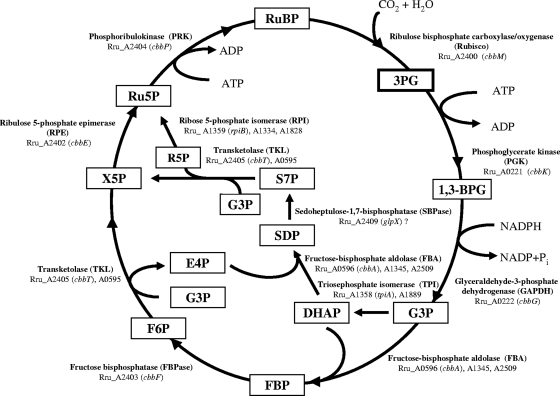
In collaboration with the U.S. Department of Energy Joint Genome Institute, the genome of R. rubrum ATCC 11170 was sequenced in 2004. Based on the genomic sequence information available at the NCBI website under accession number NC_007643 and primary pathway mapping by the Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.genome.jp/kegg-bin/show_pathway?rru00710), we were able to identify the putative CBB cycle in R. rubrum (Fig. 2). The cycle is similar to that found in many other organisms, except no gene for sedoheptulose-1,7-bisphosphatase was found in R. rubrum. However, in many organisms the fructose-1,6-bisphosphatases, especially of the class II type, also have sedoheptulose-1,7-bisphosphatase activity (1, 3, 55, 59, 69), and a cbbF homolog in R. rubrum, Rru_A2409 (glpX), is predicted to be of this type.
Fig. 2.
Calvin-Benson-Bassham cycle in R. rubrum. Abbreviations: 3PG, 3-phosphoglycerate; 1,3-BPG, 1,3-bisphosphoglycerate; G3P, glyceraldehyde-3-phosphate; DHAP, dihydroxy-acetone-phosphate; SDP, sedoheptulose-1,7-bisphosphate; S7P, sedoheptulose-7-phosphate; E4P, erythrose-4-phosphate; FBP, fructose-1,6-bisphosphate; F6P, fructose-6-phosphate; R5P, ribose-5-phosphate; X5P, xylulose-5-phosphate; Ru5P, ribulose-5-phosphate; RuBP, ribulose-1,5-bisphosphate. All enzymes and their putative encoding gene(s) in R. rubrum are also indicated.
As mentioned in the introduction, several cbb genes are located in a cluster in R. rubrum. cbbM is adjacent to cbbR, and these genes are transcribed in the opposite direction from the other cbb genes, cbbEFPT (Fig. 1A). Although cbbA, encoding fructose-bisphosphate aldolase, is typically found in the cbb gene cluster, this is not the case in R. rubrum. The gene 3′ of cbbT in R. rubrum, Rru_A2406, appears unrelated to cbb and encodes a diguanylate cyclase/phosphodiesterase with a PAS (Per-Arnt-Sim)/PAC (PAS-associated C-terminal motif) sensor(s), and it is separated from cbbT by a 325-bp gap. Instead, three cbbA homologs (Fig. 2, bottom right), including one (Rru_A0596) adjacent to another cbbT homolog (Rru_A0595), lie elsewhere in the R. rubrum genome. Multiple copies of rpiB (predicted to encode ribose 5-phosphate isomerase) and tpiA (predicted to encode triosephosphate isomerase) were also found in R. rubrum (Fig. 2), although the physiological roles of their products are unknown.
Mutations in several other cbb genes can suppress the poor growth of a cbbM mutant.
As reported previously, R. rubrum cbbM mutants grow poorly in MG medium (malate-glutamate minimal medium with glutamate as a poor nitrogen source) and MN medium (minimal malate-ammonium medium) under photosynthetic, anaerobic conditions (66). To gain insight into the cause of this growth defect, we sought mutations that suppress this phenotype. We randomly mutagenized a cbbM mutant (UR5251) with the Tn5 transposon and selected for fast-growing mutants in MG liquid medium under anaerobic conditions. The sites of the Tn5 insertion in 10 suppressors were identified by DNA sequencing as described previously (33, 71), and 8 out of 10 Tn5 insertions were found to be located in different sites in cbbR, cbbF, or cbbP (Table 2). This result strongly suggests that the disruption of other cbb genes in the CBB cycle can restore normal growth to a cbbM mutant under these anaerobic, photoheterotrophic growth conditions. We will focus on the cbbF and cbbP mutations first and discuss cbbR in a later section.
Table 2.
Locations of Tn5 suppressors of the R. rubrum cbbM mutation (UR5251)
| Strain | Locus of Tn5 insertion (positions, orientation)a | Encoded enzymeb |
|---|---|---|
| UR5281 | cbbR (61–62, +) | LysR-type transcription regulator (CbbR) |
| UR5282 | cbbR (360–361, +) | |
| UR5283 | cbbR (744–745, +) | |
| UR5284 | cbbF (215–216, −) | Fructose-1,6-bisphosphatase (FBPase or CbbF) |
| UR5285 | cbbF (418–419, +) | |
| UR5286 | cbbF (334–335, +) | |
| UR5287 | cbbP (33–34, +) | Phosphoribulokinase (PRK or CbbP) |
| UR5288 | cbbP (95–96, −) | |
| UR5289 | Rru_B0037 (2789–2790, −) | Hemolysin-type calcium-binding region |
| UR5290 | Rru_A3737 (928–929, +) | KAP-P loop |
The Tn5 insertion position is indicated as the number of nucleotides from the start codon of the structural gene to the Tn5 insertion site, including the 9-bp duplication. “+” indicates the same orientation of the gene and the inserted kan gene, while “−” indicates the opposite orientation.
The locations of Tn5 inserted into open reading frames (ORFs) were identified by BLAST searches at the NCBI website (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
To confirm that the loss of some other cbb functions could suppress a cbbM mutation, we constructed cbbM cbbF and cbbM cbbP double mutants. We used previously constructed cbbM::aacC1 mutants (UR5251 and UR5252, which have two different orientations of the aacC1 insertion) as host strains (66). We used the kan cassette from pUC4K (65) to replace deleted sections of cbbF or cbbP as described in Materials and Methods. With all double mutants, both orientations of the kan and aacC1 insertions were constructed and analyzed in all experiments described below. All mutants with different insertion orientations behaved identically, and only the data for one orientation are reported. As in previous experiments, a fairly heavy inoculum of the SMN-grown culture was used, which allowed cell growth in MG medium to reach an OD600 of approximately 0.2 to 0.3 (after 1 day) before the growth defects of cbbM mutants were apparent (66). The cbbM single mutant grew poorly in MG medium under heterotrophic anaerobic conditions, as seen previously (66), but the cbbM cbbP and cbbM cbbF double mutants grew much better than did the cbbM single mutant (Fig. 3B). These results confirm that the loss of either fructose-1,6-bisphosphatase (CbbF) or phosphoribulokinase (CbbP) activities can suppress a cbbM mutation. This demonstrates that a functional CBB pathway is not necessary for photoheterotrophic growth and suggests that the growth defect seen for cbbM mutants is likely caused by the accumulation of some pathway intermediate rather than a redox imbalance.
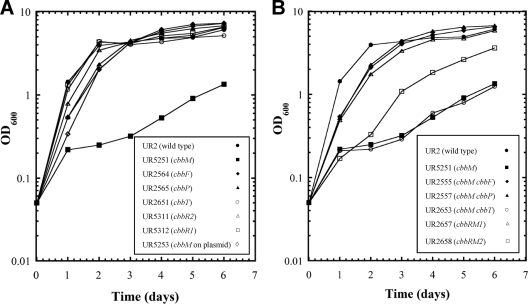
Fig. 3.
Photoheterotrophic growth of the R. rubrum wild type (UR2), the cbbM mutant (UR5251), and other cbb mutants. A culture grown in SMN medium (0.4 ml) was used to inoculate 25 ml of MG medium and then grown under phototrophic and anaerobic conditions. This fairly heavy inoculum of the culture grown in SMN medium allows growth in MG medium to an OD600 of approximately 0.2 to 0.3 (after 1 day) before growth defects of cbbM mutants are apparent. All single mutants are listed in A, and all double mutants are shown in B, while the wild type (UR2) and the cbbM mutant (UR5251) are shown in both panels for comparison.
Photoheterotrophic and photoautotrophic growths of various cbb mutants.
Because we detected suppressor mutations in only some cbb genes, we wanted to determine the phenotypes of loss-of-function mutations in other cbb genes. We were able to construct ΔcbbT (Rru_A2405), ΔcbbF (Rru_A2403), and ΔcbbP (Rru_A2404) single mutants but not a ΔcbbE (Rru_A2402) mutant.
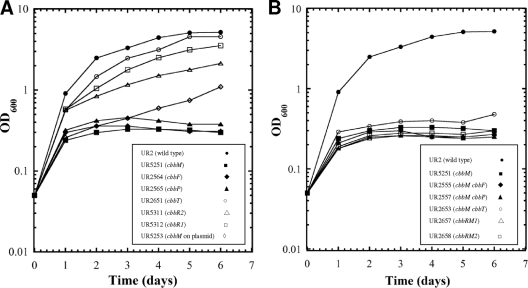
As shown in Fig. 3A, unlike the cbbM mutant, the cbbF (UR2564), cbbP (UR2565), and cbbT (UR2651) single mutants grew as well as the wild type in MG medium. Similar to the cbbM mutant, the cbbF (UR2564) and cbbP (UR2565) single mutants failed to grow under photoautotrophic conditions (Fig. 4A), indicating that the CBB cycle is blocked in these mutants. As expected, all double mutants containing a cbbM mutation failed to grow under photoautotrophic conditions (Fig. 4B). The inability of the cbbF single mutant to grow photoautotrophically indicates that the potential cbbF homolog (Rru_A2409 [glpX], encoding a putative class II type of fructose bisphosphatase) cannot fully replace the cbbF product in the CBB cycle. Presumably, GlpX functions as a sedoheptulose-1,7-bisphosphatase, as mentioned above. However, the cbbT mutant (UR2651) grew as well as the wild type under the photoautotrophic conditions, implying that the product of Rru_A0595, which has 53% amino acid sequence identity with CbbT (Rru_A2405), has sufficient transketolase activity to maintain a functional CBB cycle. Interestingly, we were unable to construct the cbbT Rru_A0595 double mutant under any of several growth conditions tested, indicating that some level of transketolase is essential in R. rubrum under these conditions.
Fig. 4.
Photoautotrophic growth of the R. rubrum wild type (UR2), the cbbM mutant (UR5251), and other cbb mutants. A culture grown in SMN medium (0.2 ml) was used to inoculate 10 ml of CO2-H2-NH4+ minimal medium supplemented with 0.05% yeast extract and then grown under phototrophic and anaerobic conditions. This fairly heavy inoculum of the culture grown in SMN medium and a small amount of yeast extract allows growth in CO2-H2-NH4+ minimal medium to an OD600 of approximately 0.2 to 0.3 (after 1 day) before growth defects of cbbM mutants are apparent. All single mutants are listed in A, and all double mutants are shown in B, while the wild type (UR2) and the cbbM mutant (UR5251) are shown in both panels for comparison.
We were unable to obtain a cbbE (Rru_A2402) mutant under any growth condition tested, including photoheterotrophic growth in MG or SMN rich medium or aerobic growth in SMN rich medium. We were also unable to delete the entire cbbEFPT region, even though cbbFPT were separately eliminated in the strains described above, suggesting that ribulose 5-phosphate epimerase (encoded by cbbE) is essential and might be involved in some other critical metabolic pathways in R. rubrum. Note that the lethality of a cbbE mutation is apparently not the result of an accumulation of its precursor, xylulose-5-phosphate (X5P) (Fig. 2), since the cbbEFPT deletion would not accumulate that molecule.
The normal growth of the cbbP and cbbF single mutants under photoheterotrophic conditions has the following implications. (i) It disproves the hypothesis that the poor growth of cbbM mutants is caused by the accumulation of excess reductant, since these single mutants are also compromised in the CBB cycle. (ii) It also clearly shows that the entire CBB cycle is not critical for cell growth under photoheterotrophic conditions. (iii) Unlike the cbbM mutant, the blockage of the CBB cycle at other steps does not cause significant growth defects under photoheterotrophic conditions, strongly suggesting that the growth defect of cbbM mutants is likely caused by the accumulation of some pathway intermediates.
The poor growth of the cbbM mutant is caused by the accumulation of RuBP in the cell.
The obvious intermediate that would accumulate in a cbbM mutant but be absent in the cbbM cbbP and cbbM cbbF double mutants is RuBP, the substrate of RubisCO (the cbbM product). It is well known that the accumulation of phosphorylated intermediates in the galactose pathway is also toxic for the cell (29, 68). Indeed, a recent report showed that this intermediate, UDP-galactose, might also serve as a stress signal under some conditions (35). It was also reported previously that an E. coli strain in which spinach phosphoribulokinase was overproduced had a high level of RuBP in the cells and grew very poorly (25). As shown in Fig. 2, RuBP is the substrate of RubisCO and the product of phosphoribulokinase (encoded by cbbP). Ribulose-5-phosphate 3-epimerase (encoded by cbbE), fructose-1,6-bisphosphatase (encoded by cbbF), and transketolase (encoded by cbbT) provide the substrate ribulose-5-phosphate (Ru5P) for the regeneration of RuBP. The mutational elimination of RubisCO in a cbbM mutant would lead to the accumulation of RuBP, while the disruption of cbbP or cbbF should block this and would explain why the cbbM cbbP and cbbM cbbF double mutants grow well in MG medium.
To test the hypothesis that the growth problem of cbbM mutants is caused by the accumulation of RuBP, we used an HPLC method to determine intracellular RuBP levels. As shown in Table 3, the cbbM mutant (UR5251) had more than a 30-fold-higher level of intracellular RuBP than did the wild type and all suppressor mutants displaying better growth, including the cbbM cbbF (UR2555) and cbbM cbbP (UR2557) double mutants as well as the cbbF (UR2564), cbbP (UR2565), and cbbT (UR2651) single mutants. These results strongly suggest that the poor growth of the cbbM mutants is caused by the accumulation of RuBP in the cell.
Table 3.
RuBP levels in R. rubrum cultures grown in MG medium
| Strain | Genotype | Growth ratea | Mean RuBP levelb (nmol per mg dry wt) ± SD |
|---|---|---|---|
| UR2 | Wild type | Fast | 0.5 ± 0.2 |
| UR5251 | ΔcbbM1::aacC1 | Very slow | 55.2 ± 1.2 |
| UR5311 | ΔcbbR2::aacC1 | Fast | 1.0 ± 0.4 |
| UR2564 | ΔcbbF1::kan | Fast | 1.5 ± 0.6 |
| UR2565 | ΔcbbP1::kan | Fast | 0.7 ± 0.3 |
| UR2651 | ΔcbbT1::aacC1 | Fast | 0.7 ± 0.2 |
| UR2657 | ΔcbbRM1::aacC1; same orientation | Fast | 1.2 ± 0.8 |
| UR2658 | ΔcbbRM2::aacC1; opposite orientation | Slow | 10.2 ± 0.1 |
| UR2555 | ΔcbbM1::aacC1 ΔcbbF1::kan | Fast | 1.3 ± 0.5 |
| UR2557 | ΔcbbM1::aacC1 ΔcbbP1::kan | Fast | 1.7 ± 0.2 |
| UR2653 | ΔcbbM1::aacC1 ΔcbbT1::aacC1 | Very slow | 64.2 ± 1.8 |
| UR2562 | cbbM nifA*(M173V) mutant | Fast | 1.8 ± 0.4 |
| UR2553 | cbbM draT nifA*(M173V) mutant | Fast | 0.9 ± 0.2 |
| UR2654 | PaacC1-cbbR | Slow | 25.5 ± 0.6 |
Growth rate refers to the growth rate on day 1 (when the initial nutrients have been exhausted) and was estimated based on data in Fig. 3, previously published data (66), or data not shown. Fast, a doubling time of 8 to 12 h; slow, a doubling time of 20 to 24 h; very slow, a doubling time of greater than 36 h.
The detection limit of RuBP by this HPLC method is 0.15 nmol, which is ∼0.5 nmol RuBP per mg dry weight.
Unlike the cbbM cbbF and cbbM cbbP double mutants, the cbbM cbbT double mutant (UR2653) grew poorly in MG medium (Fig. 3B) and also had a much higher level of RuBP than did the wild type (Table 3). This again implies that the other cbbT homolog (Rru_A0595) can provide the transketolase function to produce inhibitory levels of RuBP in the absence of cbbT (Rru_A2405).
Relationship of RuBP and reductant levels in the cbb mutants.
Although we found a correlation between poor growth and high levels of RuBP, previous studies suggested that the poor growth of cbbM mutants of R. rubrum and other related bacteria might be due to excess reductant (18, 27, 62, 67). Although this appears inconsistent with our results with the single cbb mutants noted above, we have reported a similar observation (66). Specifically, we showed that a constitutively active nitrogenase is able to suppress the photoheterotrophic growth defect of a cbbM mutant, and we interpreted this as a diversion of excess reductant to H2 production by nitrogenase (66). The constitutive nitrogenase activity in this strain was created by a mutation in nifA (termed nifA*), the positive transcriptional regulator of the nif genes, and another in draT, a posttranslational regulator of nitrogenase activity. Note that neither of these mutations has a direct effect on the cbb pathway. Under the hypothesis that the RuBP level is the key issue affecting growth in a cbbM mutant, we predicted that the RuBP level is also altered in the strains with constitutively active nitrogenase. The results in Table 3 show this to be the case. The cbbM nifA* (UR2562) and cbbM nifA* draT (UR2553) mutants both had low RuBP levels in the cell. Although this result does not disprove the original hypothesis that excess reductant is the direct cause of the growth defect, there is no longer any reason to support it. Instead, the simpler model of high RuBP levels in the cell being the direct cause of poor growth is more plausible.
Although the mechanism for the observed effect of nitrogenase, and therefore of the reductant, on RuBP levels is unknown, we can imagine two possibilities. One possibility is that one or more of the RuBP-regenerating enzymes are active only under strongly reducing conditions in R. rubrum and that expressing the nitrogenase system in cbbM nifA* mutants could make the conditions less reducing and thereby lower the enzyme activities in the CBB cycle for RuBP regeneration. Consistent with this idea, it was previously reported that the phosphoribulokinase (CbbP) and fructose bisphosphatase (CbbF) activities in plants are regulated by the redox state through a ferredoxin-thioredoxin system (4). Alternative models, such as differential RuBP stability under reducing conditions, cannot be ruled out, however.
There is another curiosity that should be explained. As noted above, we have shown previously that a cbbM mutant with the constitutive expression of active nitrogenase allows normal growth and that there is nitrogenase activity in a nif+ background in MG medium, so why is this activity not sufficient to allow good growth if there is a cbbM mutation? We assume that the explanation is one of timing. For unknown reasons, the level of nitrogenase activity of the wild type in MG medium is low until the culture density is above 1 OD unit, so we imagine that high levels of RuBP arise in a cbbM strain before nitrogenase activity can address the problem. In contrast, a nifA* draT strain has high levels of nitrogenase activity initially, which presumably prevents the appearance of RuBP even with a cbbM mutation.
Transcriptional organization of the cbb operon in R. rubrum.
As shown in Fig. 1, cbbR and cbbM in R. rubrum are transcribed in the opposite direction from that of the other cbb genes, including cbbE, cbbF, cbbP, and cbbT. This is quite different from the organization of cbb operons seen for other related photosynthetic bacteria, such as R. sphaeroides, R. capsulatus, and R. palustris (13, 32, 46), in which cbbM is adjacent to other cbb genes and cbbR is transcribed separately in the opposite direction. We therefore wished to define the transcriptional organization of these cbb genes in R. rubrum. First, we determined the transcriptional organization of cbbM, cbbR, cbbE, cbbF, cbbP, and cbbT by reverse transcription-PCR (RT-PCR), and the results are shown in Fig. 1B and C. Six pairs of primers were designed for amplifying the intergenic regions between cbbM and Rru_A2406: cbbM-cbbR, cbbR-cbbE, cbbE-cbbF, cbbF-cbbP, cbbP-cbbT, and cbbT-Rru_A2406 (Fig. 1A). When genomic DNA was used as a template, PCR products with the expected sizes were obtained with all corresponding pairs of primers (Fig. 1B), confirming that all primers annealed correctly. When cDNA synthesized from mRNA of the wild type (UR2) was used as the template, similarly sized PCR products were obtained for the gaps of cbbM-cbbR, cbbE-cbbF, cbbF-cbbP, and cbbP-cbbT (Fig. 1C). As expected, no PCR product was obtained for the gap of cbbR-cbbE, since these two genes are transcribed in opposite directions. This result also showed that there is no contamination of DNA in the cDNA sample. A negligible amount of PCR product was seen for the gap of cbbT-Rru_A2406, indicating that Rru_A2406 is not part of the cbb operon. These results indicate that cbbR and cbbM are cotranscribed, at least to some extent, and that cbbE cbbF, cbbP, and cbbT are cotranscribed in the same operon, in the opposite direction from the cbbRM operon.
The apparent cotranscription of cbbR and cbbM is surprising, since CbbR is predicted to be a regulatory protein (analyzed below) and would be expected to be synthesized at much lower levels than RubisCO, the product of cbbM. Indeed, previous studies using S1 mapping suggested that the initial site of transcription of R. rubrum cbbM is located in the space between cbbM and cbbR, and the size of the cbbM transcript determined by Northern blots was consistent with a monocistronic transcript (36). To examine the relative importance of the promoter 5′ of cbbR for cbbM expression, we created a clone that certainly lacks this promoter. As described in Materials and Methods, cbbM and 118 bp of the immediate 5′ region were cloned into the broad-host-range vector pRK404 and then introduced into a cbbM mutant (UR5251), yielding UR5253.
As noted previously and as described above, the cbbM mutant (UR5251) grew poorly under photoheterotrophic conditions (Fig. 3A) (66). Conversely, the strain carrying cbbM and its immediately proximal 5′ region (UR5253) grew well (Fig. 3A), suggesting the presence of a promoter between cbbR and cbbM. However, under photoautotrophic conditions, where high levels of RubisCO activity are required, cbbM harbored by the plasmid could not restore the normal growth of the cbbM mutant (Fig. 4A). Thus, while there does appear to be a promoter immediately 5′ of cbbM, it does not seem to be sufficient for the level of expression necessary for photoautotrophic growth.
As reported previously, Falcone and Tabita were unable to complement an R. rubrum cbbM mutant with various lengths of the cbbM region, including one fragment with cbbMR and cbbEFPT (17). Those authors interpreted this result with the model that the whole set of enzymes in the CBB pathway has to be “coordinately expressed” (17). In fact, that observation is better explained with the model that the reduced level of expression of cbbM relative to that of the cbbEFPT operon leads to an accumulation of RuBP and toxicity, even under heterotrophic conditions, as shown above.
CbbR is a positive transcriptional regulator for the cbb operon in R. rubrum.
In our original suppressor selection, another putative Tn5 suppressor in the cbb region was found in cbbR (Table 2). In R. sphaeroides, CbbR was shown previously to positively regulate the expression of the cbb operon, together with the two-component regulatory system RegB/RegA (10, 27, 44, 51). Although there are no RegB/RegA homologs in R. rubrum, and the mechanism for the regulation of the cbb operon is unknown, our Tn5 mutagenesis results are consistent with the idea that CbbR in R. rubrum positively regulates the transcription of cbbEFPT. A mutation in cbbR should decrease the expression levels of these genes and prevent the accumulation of RuBP. To test this hypothesis, two deletion mutants lacking both cbbR and cbbM were constructed with both orientations of the aacC1 insertion (UR2657 and UR2658), as described in Materials and Methods. These two mutants had quite different phenotypes: the cbbRM mutant (UR2658) with aacC1 inserted in the orientation opposite of that of cbbRM grew slowly in MG medium, while another cbbR cbbM mutant (UR2657) with aacC1 oriented in the same orientation as that of cbbRM transcription restored normal growth (Fig. 3B). Interestingly, all three of the cbbR Tn5 insertions originally isolated as suppressors had their kan genes transcribed in the same orientation as that of cbbRM (Table 2). Analysis of RuBP levels in these strains (UR2657 and UR2658) showed that poor growth again correlated with elevated RuBP levels (Table 3). We suppose that in the slow-growing cbbRM mutant (UR2658), transcription from the aacC1 insert drives sufficient cbbEFPT expression to allow some accumulation of RuBP. In contrast, because the orientation of the inserts in the Tn5 suppressors and in the rapidly growing cbbR cbbM mutant (UR2657) is directed away from cbbEFPT, there is no significant transcription into these genes and, therefore, no elevated RuBP level.
Because at least one of the Tn5 mutations in cbbR had the same suppression phenotype as that of Tn5 insertions in cbbF and cbbP, we predicted that the function of CbbR in R. rubrum is to positively regulate the transcription of the cbbF and cbbP genes. To test this hypothesis, we constructed two ΔcbbR single mutants with different orientations of the aacC1 insertion (UR5311 and UR5312), as described in Materials and Methods, and then employed quantitative real-time PCR (qPCR) to quantitate the mRNA levels of cbb genes in the wild type and the cbbR mutants. Both cbbR single mutants grew well in MG medium and were also able to grow under photoautotrophic conditions but less well than the wild type (Fig. 4A). This implies that some expression of the cbb genes is independent of CbbR and that this level is sufficient for moderate photoautotrophic growth. The following results of qPCR experiments are consistent with this.
The mRNA samples were extracted from photoautotrophically grown cells, since all genes in the CBB cycle should have the highest expression levels under these conditions (2, 28, 58). As shown in Table 4, there is a much higher level of mRNA accumulation, and, therefore, presumably gene expression, of cbbM, cbbE, and cbbP in the wild type (UR2) under photoautotrophic conditions than under photoheterotrophic conditions. This is consistent with a greater need for CBB proteins under the former conditions. As shown in Table 4, under photoautotrophic conditions, UR5311 (ΔcbbR2::aacC1) had a much lower level of accumulation of cbbE and cbbP mRNAs than did the wild type, consistent with CbbR acting as a positive transcriptional regulator of the cbbEFPT operon in R. rubrum albeit over a modest basal level of expression. A low level of accumulation of cbbM in cbbR mutants was also seen (Table 4), suggesting that the internal promoter located between cbbR and cbbM is not a very strong one or that it is also activated by CbbR. The modest photoautotrophic growth of the cbbR mutants also indicates that the low level of expression of cbbM and other cbb genes does not preclude even autotrophic growth, as long as the production and enzymatic activity of other CBB enzymes are balanced with those of CbbM.
Table 4.
Relative expression levels of cbb genes in the wild type (UR2) and the cbbR mutant (UR5311) based on qPCR data
| Gene | cDNA samplea | Mean CT ± SDc | Relative expressionb,c (%) |
|---|---|---|---|
| cbbM | UR2-auto | 16.2 ± 0.16 | 100 |
| UR5311-auto | 25.2 ± 0.02 | 1.4 | |
| UR2-hetero | 24.5 ± 0.06 | 0.8 | |
| cbbE | UR2-auto | 18.7 ± 0.04 | 100 |
| UR5311-auto | 22.0 ± 0.01 | 9.5 | |
| UR2-hetero | 22.8 ± 0.03 | 2.3 | |
| cbbP | UR2-auto | 21.4 ± 0.07 | 100 |
| UR5311-auto | 25.5 ± 0.03 | 6.7 | |
| UR2-hetero | 25.0 ± 0.12 | 3.7 |
cDNAs are from the R. rubrum wild type (UR2) and cbbR mutant (UR5311) grown under photoautotrophic growth conditions (auto) or photoheterotrophic conditions (hetero). The expressions of cbbM, cbbE, and cbbP in these two strains are compared.
Relative expression was calculated as described in Materials and Methods and then normalized by using the UR2-auto sample as a control for 100% expression.
The rank orders of samples for each of the genes in the “mean CT” (threshold cycle) and the “relative expression” analyses differ because the value for the reference gene, rpoD, varied slightly between heterotrophic and autotrophic growth conditions.
We then asked if the overproduction of CbbR in an otherwise wild-type background would provide some insight into the regulatory system. A second copy of cbbR was integrated into the chromosome of the wild type at a site away from the cbb gene cluster, as described in Materials and Methods. This second copy of cbbR was expressed from the aacC1 promoter (50), which is a constitutive, relatively strong promoter in R. rubrum (75). The resulting strain (UR2654) grew slowly under photoheterotrophic conditions (data not shown), with a high level of RuBP accumulation (Table 3). This strain failed to grow under photoautotrophic conditions (data not shown). The simplest hypothesis for this result is that elevated CbbR levels lead to disproportionately higher levels of the CbbEFPT proteins than of CbbM, since CbbR is a positive regulator for the expression of the cbbEFPT operon. The fact that poor growth again correlates with RuBP levels (Table 3), albeit by a completely different genetic mechanism, further supports the causative nature of elevated RuBP levels for that phenotype.
These results and those for the suppressor mutations show that the cbb genes are expressed under photoheterotrophic conditions, but it remains unclear why this should be the case. The normal growth of the cbbP and cbbF single mutants under photoheterotrophic conditions (Fig. 3A) clearly showed that the CBB cycle is not critical for growth under this condition. The qPCR data suggested that the CBB cycle in R. rubrum is not tightly regulated by CbbR. Our results indicate that although a functional CBB cycle is not essential for R. rubrum, some enzymes in this cycle, including ribulose 5-phosphate epimerase (encoded by Rru_A2405 [cbbE]) and transketolase (encoded by Rru_A2405 or Rru_A0595 [cbbT]), are critical so that cells should always have sufficient amounts of cbb expression to provide the necessary levels of these two enzymes. We also believe that the reducing conditions used for cells grown in MG medium likely keep many enzymes, such as RubisCO, CbbP, and CbbF, in active forms, since these enzymes in plants are induced to be active under reducing conditions and lost activities when the environment became oxidized (4, 25). Another possibility for the heterotrophic expression of the cbb operon is that a small amount of CO2 could be produced under photoheterotrophic conditions, which might induce the expression of cbb genes.
In summary, despite the plausibility of the model that growth defects in cbbM mutants are the result of a redox imbalance, we show that this does not appear to be the case for R. rubrum. Instead, we show that the high level of RuBP is the direct cause of the poor growth of these mutants, although we cannot discount the possibility that a derivative of RuBP is the toxic compound. Surprisingly, the accumulation of RuBP can apparently be affected by the reductant level in the cell, since a constitutively active nitrogenase can help reduce the RuBP level and suppress the poor growth of a cbbM mutant under photoheterotrophic conditions. We presume that the RuBP-regenerating enzymes may be active only under strongly reducing conditions in R. rubrum, but this needs further analysis. We cannot conclude that RuBP accumulation is also the cause of cell growth defects in other related organisms, since the organization and regulation of the cbb operons in these organisms are quite different, but this important possibility needs to be tested. We also confirmed that CbbR is a positive transcriptional regulator of the cbbEFPT operon and defined the transcriptional organization of the main cbb region in R. rubrum.
ACKNOWLEDGMENTS
This work was supported by NIGMS grant GM65891 to G.P.R., grant 30870059 from the Chinese National Natural Science Foundation and grant 2010CB126504 from the National Basic Research Program of China (973 Program) to J.L., and grant 2010SKLAB06-3 from the Key Laboratory for Agrobiotechnology of China Agricultural University to Y.Z.
We thank Eva Ziegelhoffer for her generous help with the qPCR experiments and Wayne Kontur for his help with gas chromatography. We also thank Timothy Paustian for assistance with the HPLC experiments.
Footnotes
Published ahead of print on 29 April 2011.
REFERENCES
- 1. Amachi T., Bowien B. 1979. Characterization of two fructose bisphosphatase isoenzymes from the hydrogen bacterium Nocardia opaca 1b. J. Gen. Microbiol. 113:347–356 [Google Scholar]
- 2. Anderson L., Fuller R. C. 1967. Photosynthesis in Rhodospirillum rubrum. III. Metabolic control of reductive pentose phosphate and tricarboxylic acid cycle enzymes. Plant Physiol. 42:497–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown G., et al. 2009. Structural and biochemical characterization of the type II fructose-1,6-bisphosphatase GlpX from Escherichia coli. J. Biol. Chem. 284:3784–3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buchanan B. B. 1991. Regulation of CO2 assimilation in oxygenic photosynthesis: the ferredoxin/thioredoxin system. Perspective on its discovery, present status, and future development. Arch. Biochem. Biophys. 288:1–9 [DOI] [PubMed] [Google Scholar]
- 5. Burris R. H. 1991. Nitrogenases. J. Biol. Chem. 266:9339–9342 [PubMed] [Google Scholar]
- 6. Chen J. H., Gibson J. L., McCue L. A., Tabita F. R. 1991. Identification, expression, and deduced primary structure of transketolase and other enzymes encoded within the form II CO2 fixation operon of Rhodobacter sphaeroides. J. Biol. Chem. 266:20447–20452 [PubMed] [Google Scholar]
- 7. Dangel A. W., Gibson J. L., Janssen A. P., Tabita F. R. 2005. Residues that influence in vivo and in vitro CbbR function in Rhodobacter sphaeroides and identification of a specific region critical for co-inducer recognition. Mol. Microbiol. 57:1397–1414 [DOI] [PubMed] [Google Scholar]
- 8. Dangel A. W., Tabita F. R. 2009. Protein-protein interactions between CbbR and RegA (PrrA), transcriptional regulators of the cbb operons of Rhodobacter sphaeroides. Mol. Microbiol. 71:717–729 [DOI] [PubMed] [Google Scholar]
- 9. Ditta G., et al. 1985. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid 13:149–153 [DOI] [PubMed] [Google Scholar]
- 10. Du S., Bird T. H., Bauer C. E. 1998. DNA binding characteristics of RegA*. A constitutively active anaerobic activator of photosynthesis gene expression in Rhodobacter capsulatus. J. Biol. Chem. 273:18509–18513 [DOI] [PubMed] [Google Scholar]
- 11. Dubbs J. M., Bird T. H., Bauer C. E., Tabita F. R. 2000. Interaction of CbbR and RegA* transcription regulators with the Rhodobacter sphaeroides cbbI promoter-operator region. J. Biol. Chem. 275:19224–19230 [DOI] [PubMed] [Google Scholar]
- 12. Dubbs J. M., Tabita F. R. 2004. Regulators of nonsulfur purple phototrophic bacteria and the interactive control of CO2 assimilation, nitrogen fixation, hydrogen metabolism and energy generation. FEMS Microbiol. Rev. 28:353–376 [DOI] [PubMed] [Google Scholar]
- 13. Dubbs P., Dubbs J. M., Tabita F. R. 2004. Effector-mediated interaction of CbbRI and CbbRII regulators with target sequences in Rhodobacter capsulatus. J. Bacteriol. 186:8026–8035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elsen S., Dischert W., Colbeau A., Bauer C. E. 2000. Expression of uptake hydrogenase and molybdenum nitrogenase in Rhodobacter capsulatus is coregulated by the RegB-RegA two-component regulatory system. J. Bacteriol. 182:2831–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elsen S., Swem L. R., Swem D. L., Bauer C. E. 2004. RegB/RegA, a highly conserved redox-responding global two-component regulatory system. Microbiol. Mol. Biol. Rev. 68:263–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Falcone D. L., Quivey R. G., Jr., Tabita F. R. 1988. Transposon mutagenesis and physiological analysis of strains containing inactivated form I and form II ribulose bisphosphate carboxylase/oxygenase genes in Rhodobacter sphaeroides. J. Bacteriol. 170:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Falcone D. L., Tabita F. R. 1993. Complementation analysis and regulation of CO2 fixation gene expression in a ribulose 1,5-bisphosphate carboxylase-oxygenase deletion strain of Rhodospirillum rubrum. J. Bacteriol. 175:5066–5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Falcone D. L., Tabita F. R. 1991. Expression of endogenous and foreign ribulose 1,5-bisphosphate carboxylase-oxygenase (RubisCO) genes in a RubisCO deletion mutant of Rhodobacter sphaeroides. J. Bacteriol. 173:2099–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fitzmaurice W. P., Saari L. L., Lowery R. G., Ludden P. W., Roberts G. P. 1989. Genes coding for the reversible ADP-ribosylation system of dinitrogenase reductase from Rhodospirillum rubrum. Mol. Gen. Genet. 218:340–347 [DOI] [PubMed] [Google Scholar]
- 20. Fridlyand L. E., Scheibe R. 1999. Regulation of the Calvin cycle for CO2 fixation as an example for general control mechanisms in metabolic cycles. Biosystems 51:79–93 [DOI] [PubMed] [Google Scholar]
- 21. Gest H., Kamen M. D., Beregoff H. M. 1950. Studies on the metabolism of photosynthetic bacteria. V. Photoproduction of hydrogen and nitrogen fixation by Rhodospirillum rubrum. J. Biol. Chem. 182:153–170 [Google Scholar]
- 22. Gibson J. L., Tabita F. R. 1993. Nucleotide sequence and functional analysis of CbbR, a positive regulator of the Calvin cycle operons of Rhodobacter sphaeroides. J. Bacteriol. 175:5778–5784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grunwald S. K., Lies D. P., Roberts G. P., Ludden P. W. 1995. Posttranslational regulation of nitrogenase in Rhodospirillum rubrum strains overexpressing the regulatory enzymes dinitrogenase reductase ADP-ribosyltransferase and dinitrogenase reductase activating glycohydrolase. J. Bacteriol. 177:628–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hallenbeck P. L., Lerchen R., Hessler P., Kaplan S. 1990. Phosphoribulokinase activity and regulation of CO2 fixation critical for photosynthetic growth of Rhodobacter sphaeroides. J. Bacteriol. 172:1749–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hudson G. S., Morell M. K., Arvidsson Y. B. C., Andrews T. J. 1992. Synthesis of spinach phosphoribulokinase and ribulose 1,5-bisphosphate in Escherichia coli. Aust. J. Plant Physiol. 19:213–221 [Google Scholar]
- 26. Joshi G. S., et al. 2009. Differential accumulation of form I RubisCO in Rhodopseudomonas palustris CGA010 under photoheterotrophic growth conditions with reduced carbon sources. J. Bacteriol. 191:4243–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joshi H. M., Tabita F. R. 1996. A global two component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation, and nitrogen fixation. Proc. Natl. Acad. Sci. U. S. A. 93:14515–14520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jouanneau Y., Tabita F. R. 1986. Independent regulation of synthesis of form I and form II ribulose bisphosphate carboxylase-oxygenase in Rhodopseudomonas sphaeroides. J. Bacteriol. 165:620–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalckar H. M. 1965. Galactose metabolism and cell “sociology.” Science 150:305–313 [DOI] [PubMed] [Google Scholar]
- 30. Kamen M. D., Gest H. 1949. Evidence for a nitrogenase system in the photosynthetic bacterium Rhodospirillum rubrum. Science 109:560. [DOI] [PubMed] [Google Scholar]
- 31. Kapanidis A. N., et al. 2005. Retention of transcription initiation factor σ70 in transcription elongation: single-molecule analysis. Mol. Cell 20:347–356 [DOI] [PubMed] [Google Scholar]
- 32. Kusian B., Bowien B. 1997. Organization and regulation of cbb CO2 assimilation genes in autotrophic bacteria. FEMS Microbiol. Rev. 21:135–155 [DOI] [PubMed] [Google Scholar]
- 33. Larsen R. A., Wilson M. M., Guss A. M., Metcalf W. W. 2002. Genetic analysis of pigment biosynthesis in Xanthobacter autotrophicus Py2 using a new, highly efficient transposon mutagenesis system that is functional in a wide variety of bacteria. Arch. Microbiol. 178:193–201 [DOI] [PubMed] [Google Scholar]
- 34. Lascelles J. 1960. The formation of ribulose 1:5-diphosphate carboxylase by growing cultures of Athiorhodaceae. J. Gen. Microbiol. 23:499–510 [DOI] [PubMed] [Google Scholar]
- 35. Lee S. J., et al. 2009. Cellular stress created by intermediary metabolite imbalances. Proc. Natl. Acad. Sci. U. S. A. 106:19515–19520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leustek T., Hartwig R., Weissbach H., Brot N. 1988. Regulation of ribulose bisphosphate carboxylase expression in Rhodospirillum rubrum: characteristics of mRNA synthesized in vivo and in vitro. J. Bacteriol. 170:4065–4071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liang J. H., et al. 1991. Mutations in the draT and draG genes of Rhodospirillum rubrum result in loss of regulation of nitrogenase by reversible ADP-ribosylation. J. Bacteriol. 173:6903–6909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ludden P. W., Roberts G. P. 1989. Regulation of nitrogenase activity by reversible ADP ribosylation. Curr. Top. Cell. Regul. 30:23–56 [DOI] [PubMed] [Google Scholar]
- 39. McKinlay J. B., Harwood C. S. 2010. Carbon dioxide fixation as a central redox cofactor recycling mechanism in bacteria. Proc. Natl. Acad. Sci. U. S. A. 107:11669–11675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nordlund S., Ludden P. W. 2004. Post-translational regulation of nitrogenase in photosynthetic bacteria, p. 175–196 In Klipp W., Masephol B., Gallon J. R., Newton W. E. (ed.), Genetics and regulation of nitrogen fixation in free-living bacteria. Kluwer Academic Publishers., Dordrecht, Netherlands [Google Scholar]
- 41. Ormerod J. G., Ormerod K. S., Gest H. 1961. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch. Biochem. Biophys. 94:449–463 [DOI] [PubMed] [Google Scholar]
- 42. Paoli G. C., Morgan N. S., Tabita F. R., Shively J. M. 1995. Expression of the cbbLcbbS and cbbM genes and distinct organization of the cbb Calvin cycle structural genes of Rhodobacter capsulatus. Arch. Microbiol. 164:396–405 [PubMed] [Google Scholar]
- 43. Paoli G. C., Vichivanives P., Tabita F. R. 1998. Physiological control and regulation of the Rhodobacter capsulatus cbb operons. J. Bacteriol. 180:4258–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qian Y., Tabita F. R. 1996. A global signal transduction system regulates aerobic and anaerobic CO2 fixation in Rhodobacter sphaeroides. J. Bacteriol. 178:12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Qureshi A. A., Burger W. C., Prentice N. 1979. Quantitation of potential flavoring compounds in worts and beers by HPLC. J. Am. Soc. Brew. Chem. 37:153–160 [Google Scholar]
- 46. Romagnoli S., Tabita F. R. 2006. A novel three-protein two-component system provides a regulatory twist on an established circuit to modulate expression of the cbbI region of Rhodopseudomonas palustris CGA010. J. Bacteriol. 188:2780–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sarles L. S., Tabita F. R. 1983. Derepression of the synthesis of D-ribulose 1,5-bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. J. Bacteriol. 153:458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schefe J. H., Lehmann K. E., Buschmann I. R., Unger T., Funke-Kaiser H. 2006. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression's CT difference” formula. J. Mol. Med. 84:901–910 [DOI] [PubMed] [Google Scholar]
- 49. Schnider U., et al. 1995. Amplification of the housekeeping sigma factor in Pseudomonas fluorescens CHA0 enhances antibiotic production and improves biocontrol abilities. J. Bacteriol. 177:5387–5392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schweizer H. P. 1993. Small broad-host-range gentamycin resistance gene cassettes for site-specific insertion and deletion mutagenesis. Biotechniques 15:831–834 [PubMed] [Google Scholar]
- 51. Sganga M. W., Bauer C. E. 1992. Regulatory factors controlling photosynthetic reaction center and light-harvesting gene expression in Rhodobacter capsulatus. Cell 68:945–954 [DOI] [PubMed] [Google Scholar]
- 52. Shively J. M., van Keulen G., Meijer W. G. 1998. Something from almost nothing: carbon dioxide fixation in chemoautotrophs. Annu. Rev. Microbiol. 52:191–230 [DOI] [PubMed] [Google Scholar]
- 53. Simon R., Priefer U. B., Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Biotechnology (NY) 1:784–791 [Google Scholar]
- 54. Smith S. A., Tabita F. R. 2002. Up-regulated expression of the cbbI and cbbII operons during photoheterotrophic growth of a ribulose 1,5-bisphosphate carboxylase-oxygenase deletion mutant of Rhodobacter sphaeroides. J. Bacteriol. 184:6721–6724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Springgate C. F., Stachow C. S. 1972. Fructose 1,6-diphosphatase from Rhodopseudomonas palustris. I. Purification and properties. Arch. Biochem. Biophys. 152:1–12 [DOI] [PubMed] [Google Scholar]
- 56. Streitwieser A., Heathcock C. H. 1976. Introduction to organic chemistry. Macmillan, New York, NY [Google Scholar]
- 57. Tabita F. R. 1988. Molecular and cellular regulation of autotrophic carbon dioxide fixation in microorganisms. Microbiol. Rev. 52:155–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tabita F. R., McFadden B. A. 1974. D-ribulose 1,5-diphosphate carboxylase from Rhodospirillum rubrum. I. Levels, purification, and effects of metallic ions. J. Biol. Chem. 249:3453–3458 [PubMed] [Google Scholar]
- 59. Tamoi M., Ishikawa T., Takeda T., Shigeoka S. 1996. Molecular characterization and resistance to hydrogen peroxide of two fructose-1,6-bisphosphatases from Synechococcus PCC 7942. Arch. Biochem. Biophys. 334:27–36 [DOI] [PubMed] [Google Scholar]
- 60. Tavano C. L., Podevels A. M., Donohue T. J. 2005. Identification of genes required for recycling reducing power during photosynthetic growth. J. Bacteriol. 187:5249–5258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tichi M. A., Tabita F. R. 2001. Interactive control of Rhodobacter capsulatus redox-balancing systems during phototrophic metabolism. J. Bacteriol. 183:6344–6354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tichi M. A., Tabita F. R. 2000. Maintenance and control of redox poise in Rhodobacter capsulatus strains deficient in the Calvin-Benson-Bassham pathway. Arch. Microbiol. 174:322–333 [DOI] [PubMed] [Google Scholar]
- 63. Tichi M. A., Tabita F. R. 2002. Metabolic signals that lead to control of CBB gene expression in Rhodobacter capsulatus. J. Bacteriol. 184:1905–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vichivanives P., Bird T. H., Bauer C. E., Tabita F. R. 2000. Multiple regulators and their interactions in vivo and in vitro with the cbb regulons of Rhodobacter capsulatus. J. Mol. Biol. 300:1079–1099 [DOI] [PubMed] [Google Scholar]
- 65. Vieira J., Messing J. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259–268 [DOI] [PubMed] [Google Scholar]
- 66. Wang D., Zhang Y., Welch E., Li J., Roberts G. P. 2010. Elimination of Rubisco alters the regulation of nitrogenase activity and increases hydrogen production in Rhodospirillum rubrum. Int. J. Hydrogen Energy 35:7377–7385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang X., Falcone D. L., Tabita F. R. 1993. Reductive pentose phosphate-independent CO2 fixation in Rhodobacter sphaeroides and evidence that ribulose bisphosphate carboxylase/oxygenase activity serves to maintain the redox balance of the cell. J. Bacteriol. 175:3372–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yarmolinsky M. B., Wiesmeyer H., Kalckar H. M., Jordan E. 1959. Hereditary defects in galactose metabolism in Escherichia coli mutants. II. Galactose-induced sensitivity. Proc. Natl. Acad. Sci. U. S. A. 45:1786–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yoo J. G., Bowien B. 1995. Analysis of the cbbF genes from Alcaligenes eutrophus that encode fructose-1,6-/sedoheptulose-1,7-bisphosphatase. Curr. Microbiol. 31:55–61 [DOI] [PubMed] [Google Scholar]
- 70. Zhang Y., Burris R. H., Ludden P. W., Roberts G. P. 1995. Comparison studies of dinitrogenase reductase ADP-ribosyl transferase/dinitrogenase reductase activating glycohydrolase regulatory systems in Rhodospirillum rubrum and Azospirillum brasilense. J. Bacteriol. 177:2354–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang Y., Pohlmann E. L., Conrad M. C., Roberts G. P. 2006. The poor growth of Rhodospirillum rubrum mutants lacking PII proteins is due to an excess of glutamine synthetase activity. Mol. Microbiol. 61:497–510 [DOI] [PubMed] [Google Scholar]
- 72. Zhang Y., Pohlmann E. L., Ludden P. W., Roberts G. P. 2001. Functional characterization of three GlnB homologs in the photosynthetic bacterium Rhodospirillum rubrum: roles in sensing ammonium and energy status. J. Bacteriol. 183:6159–6168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang Y., Pohlmann E. L., Ludden P. W., Roberts G. P. 2000. Mutagenesis and functional characterization of the glnB, glnA, and nifA genes from the photosynthetic bacterium Rhodospirillum rubrum. J. Bacteriol. 182:983–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang Y., Pohlmann E. L., Ludden P. W., Roberts G. P. 2003. Regulation of nitrogen fixation by multiple PII homologs in the photosynthetic bacterium Rhodospirillum rubrum. Symbiosis 35:85–100 [Google Scholar]
- 75. Zhang Y., Pohlmann E. L., Roberts G. P. 2009. Effect of perturbation of ATP level on the activity and regulation of nitrogenase in Rhodospirillum rubrum. J. Bacteriol. 191:5526–5537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang Y., Pohlmann E. L., Roberts G. P. 2004. Identification of critical residues in GlnB for its activation of NifA activity in the photosynthetic bacterium Rhodospirillum rubrum. Proc. Natl. Acad. Sci. U. S. A. 101:2782–2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zou X., et al. 2008. Identification and functional characterization of NifA variants that are independent of GlnB activation in the photosynthetic bacterium Rhodospirillum rubrum. Microbiology 154:2689–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]