Abstract
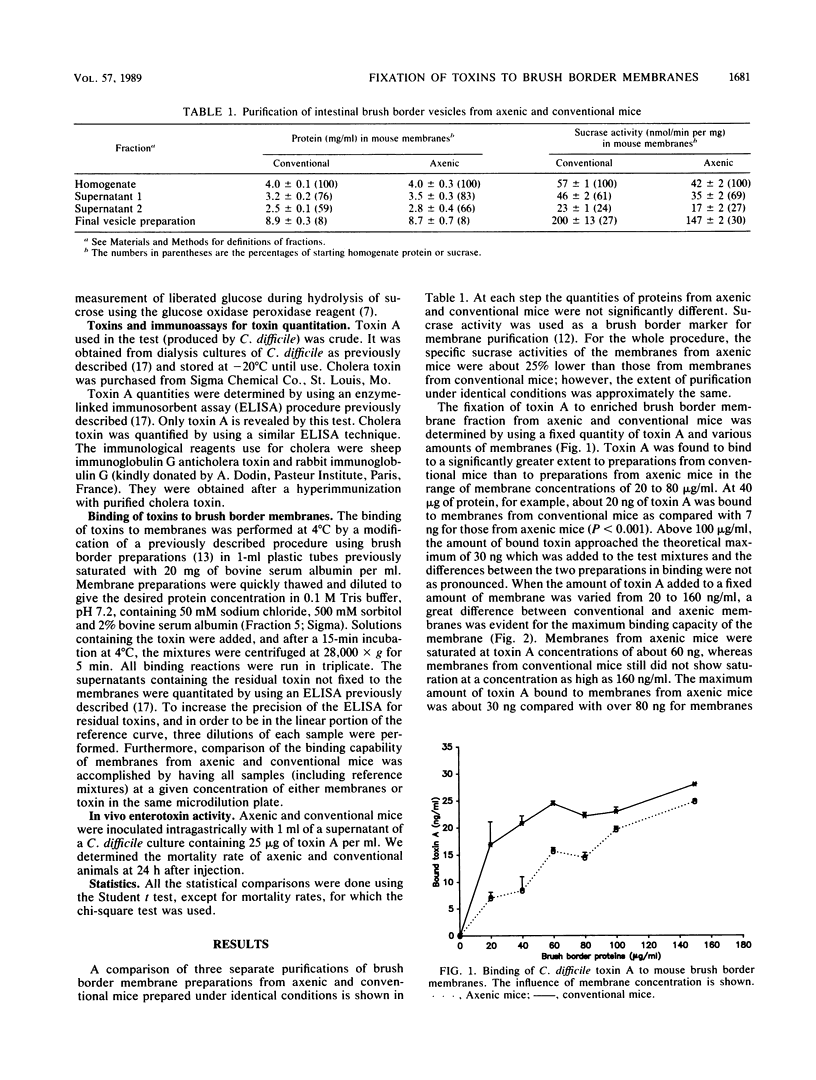
We have tested the in vitro binding of Clostridium difficile toxin A (enterotoxin) and cholera toxin to intestinal brush border membranes prepared from either conventional or axenic mice. Membranes from axenic mice were shown to be saturated at a lower toxin A concentration (at least 2.5 times lower). Because there were no significant differences between membranes from axenic and conventional mice in binding at low toxin A concentrations, the presence of the normal microflora seems to increase the number but not the affinity of brush border membrane receptors on the enterocyte surface. Corroborating the in vitro results, we observed that conventional mice were more sensitive to the pathological effects of toxin A given intragastrically than were axenic mice. In contrast, there was no difference in the binding characteristics of cholera toxin between membranes from conventional and axenic mice. We conclude that the presence of the mouse intestinal bacteria increases the number of C. difficile toxin A intestinal receptors but does not influence cholera toxin receptors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G. Antibiotic-associated pseudomembranous colitis. Rev Infect Dis. 1979 May-Jun;1(3):530–539. doi: 10.1093/clinids/1.3.530. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Onderdonk A. B., Cisneros R. L., Kasper D. L. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J Infect Dis. 1977 Nov;136(5):701–705. doi: 10.1093/infdis/136.5.701. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Bartlett J. G., Gorbach S. L., Onderdonk A. B. Clindamycin-induced enterocolitis in hamsters as a model of pseudomembranous colitis in patients. Infect Immun. 1978 May;20(2):526–529. doi: 10.1128/iai.20.2.526-529.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthier G., Dubos F., Raibaud P. Modulation of cytotoxin production by Clostridium difficile in the intestinal tracts of gnotobiotic mice inoculated with various human intestinal bacteria. Appl Environ Microbiol. 1985 Jan;49(1):250–252. doi: 10.1128/aem.49.1.250-252.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Dabard J., Dubos F., Martinet L., Ducluzeau R. Experimental reproduction of neonatal diarrhea in young gnotobiotic hares simultaneously associated with Clostridium difficile and other Clostridium strains. Infect Immun. 1979 Apr;24(1):7–11. doi: 10.1128/iai.24.1.7-11.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebright J. R., Fekety R., Silva J., Wilson K. H. Evaluation of eight cephalosporins in hamster colitis model. Antimicrob Agents Chemother. 1981 Jun;19(6):980–986. doi: 10.1128/aac.19.6.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P. H. Role of membrane gangliosides in the binding and action of bacterial toxins. J Membr Biol. 1982;69(2):85–97. doi: 10.1007/BF01872268. [DOI] [PubMed] [Google Scholar]
- George W. L., Rolfe R. D., Finegold S. M. Clostridium difficile and its cytotoxin in feces of patients with antimicrobial agent-associated diarrhea and miscellaneous conditions. J Clin Microbiol. 1982 Jun;15(6):1049–1053. doi: 10.1128/jcm.15.6.1049-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George W. L., Rolfe R. D., Harding G. K., Klein R., Putnam C. W., Finegold S. M. Clostridium difficile and cytotoxin in feces of patients with antimicrobial agent-associated pseudomembranous colitis. Infection. 1982;10(4):205–208. doi: 10.1007/BF01666910. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Clark G. F., Smith D. F., Wilkins T. D. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Gal alpha 1-3Gal beta 1-4GlcNAc. Infect Immun. 1986 Sep;53(3):573–581. doi: 10.1128/iai.53.3.573-581.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lyerly D. M., Saum K. E., MacDonald D. K., Wilkins T. D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985 Feb;47(2):349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Saum K. E., MacDonald D. K., Wilkins T. D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985 Feb;47(2):349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahe S., Corthier G., Dubos F. Effect of various diets on toxin production by two strains of Clostridium difficile in gnotobiotic mice. Infect Immun. 1987 Aug;55(8):1801–1805. doi: 10.1128/iai.55.8.1801-1805.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Thorne G. M., Bartlett J. G. Comparison of two toxins produced by Clostridium difficile. Infect Immun. 1981 Dec;34(3):1036–1043. doi: 10.1128/iai.34.3.1036-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toothaker R. D., Elmer G. W. Prevention of clindamycin-induced mortality in hamsters by Saccharomyces boulardii. Antimicrob Agents Chemother. 1984 Oct;26(4):552–556. doi: 10.1128/aac.26.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triadafilopoulos G., Pothoulakis C., O'Brien M. J., LaMont J. T. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology. 1987 Aug;93(2):273–279. doi: 10.1016/0016-5085(87)91014-6. [DOI] [PubMed] [Google Scholar]


