Abstract
Kinase suppressor of ras 1 (KSR1) is a molecular scaffold of the Raf/MEK/extracellular signal-regulated kinase (ERK) cascade that enhances oncogenic Ras signaling. Here we show KSR1-dependent, but ERK-independent, regulation of metabolic capacity is mediated through the expression of peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1α) and estrogen-related receptor α (ERRα). This KSR1-regulated pathway is essential for the transformation of cells by oncogenic Ras. In mouse embryo fibroblasts (MEFs) expressing H-RasV12, ectopic PGC1α was sufficient to rescue ERRα expression, metabolic capacity, and anchorage-independent growth in the absence of KSR1. The ability of PGC1α to promote anchorage-independent growth required interaction with ERRα, and treatment with an inhibitor of ERRα impeded anchorage-independent growth. In contrast to PGC1α, the expression of constitutively active ERRα (CA-ERRα) was sufficient to enhance metabolic capacity but not anchorage-independent growth in the absence of KSR1. These data reveal KSR1-dependent control of PGC1α- and ERRα-dependent pathways that are necessary and sufficient for signaling by oncogenic H-RasV12 to regulate metabolism and anchorage-independent growth, providing novel targets for therapeutic intervention.
INTRODUCTION
Metabolic reprogramming of cancer cells is recognized as an emerging hallmark of cancer biology (28). Oncogenes such as Ras, Myc, and PI3K have been shown to enhance the uptake and metabolism of nutrients in a manner that is independent of growth factors (65). Oncogenes such as K-Ras and B-Raf can provide a survival advantage under conditions of low nutrient availability by regulating glucose uptake via glucose transporter 1 (76). Although oncogenes can provide survival advantages through metabolic reprogramming, they can also increase the reliance of tumor cells on particular metabolic substrates (42). Oncogenic Akt can make glioblastoma cell lines dependent on glucose by reducing the ability of the cells to invoke oxidative phosphorylation (OXPHOS) in response to glucose depletion, which increases apoptosis (8). Oncogenic c-Myc can make cancer cells dependent on glutamine and sensitize cells to apoptosis following glutamine withdrawal (14, 71, 77). A more thorough understanding of metabolic regulation specific to cancer cells will improve understanding of mechanisms critical to tumor formation and maintenance and may provide novel targets for therapeutic intervention.
The Warburg effect is generally characterized by an enhanced rate of glucose uptake that is preferentially metabolized to lactate and secreted at the expense of oxidative phosphorylation. Numerous studies have demonstrated the ability of oncogenic Ras to induce aerobic glycolysis of tumor cells (i.e., the Warburg effect). Several groups have demonstrated that oncogenic Ras can signal through hypoxia-inducible factors (HIFs) to regulate numerous glycolytic enzymes and enhance glucose uptake, the rate of glycolysis, and lactate secretion (5, 12, 35, 56). Inhibition of Ras can decrease the expression of glycolytic enzymes and the rate of glycolysis through the downregulation of HIF1α (5). However, the role of oncogenic Ras in the regulation of OXPHOS has been dichotomous. Several studies have demonstrated the ability of oncogenic Ras to decrease OXPHOS. Transformation of NIH-3T3 cells with activated H-RasQ61L caused a functional defect in OXPHOS without altering mitochondrial content or mass (74). Transformation of mouse embryo fibroblasts (MEFs) with K-Ras caused a decrease in OXPHOS due to a decrease in mitochondrial complex 1 proteins (2). An additional study of H-Ras-induced transformation of fibroblasts reported that a rise in OXPHOS preceded an increase in glycolytic rate but that the cells became more tumorigenic as the glycolytic rate increased and the OXPHOS rate declined (15). However, recent evidence has highlighted the importance of OXPHOS to the biological consequences of Ras activation. In the yeast species Saccharomyces cerevisiae, activating Ras mutations increases mitochondrial content and respiratory capacity (16). The introduction of H-RasV12 into human bronchial epithelial cells increased flux through the tricarboxylic acid (TCA) cycle and oxygen consumption (62). Finally, in human cancer cells and mouse models of tumorigenesis, mitochondrial metabolism was necessary for anchorage-independent growth and tumorigenesis (67).
The metabolic characteristics of cells change throughout the process of transformation reflecting the dynamic nature of metabolic regulation. Metabolic profiling demonstrated that basal rates of glucose uptake, lactate production, and oxygen consumption increase progressively throughout the tumorigenic conversion of human fibroblasts following the serial addition of large T antigen, small T antigen, and H-Ras (27, 50). Furthermore, susceptibility to metabolic inhibitors changed throughout progression to the transformed state, providing an additional rationale for the study of oncogene-driven metabolic reprogramming as an essential step in tumor formation.
Oncogenic Ras requires signaling through numerous pathways to support transformation and tumorigenesis, such as Raf/MEK/extracellular signal-regulated kinase (ERK), PI3K/Akt, RalGDS, PLC-ε, etc. (54). Optimal signaling through Ras effector pathways requires support from additional proteins that help coordinate their actions (54). KSR1 is a molecular scaffold of the Ras/Raf/MEK/ERK signaling pathway that coordinates signaling through the Raf/MEK/ERK kinase cascade and supports Ras-induced transformation. KSR1 was identified in Drosophila and Caenorhabditis elegans as a loss-of-function allele that reversed the phenotype of a hyperactive Ras mutant, demonstrating that KSR1 was required for proper Ras signaling (34, 36, 61, 63, 64). Subsequent studies showed that KSR1 physically interacts with Raf/MEK/ERK to coordinate their activities in a variety of cellular processes (6, 9, 46, 51, 58). In vitro studies demonstrated that KSR1 is required by H-RasV12 to optimize ERK1/2 activation and transformation (38). Consistent with in vitro analyses, disruption of KSR1 in vivo decreased ERK1/2 activation and decreased tumor formation in mammary epithelial cells caused by polyomavirus MT or by treatment with 12-O-tetradecanoylphorbol-13-acetate (TPA) (41, 47).
KSR1 and KSR2 knockout mice possess metabolic defects. KSR2−/− mice become spontaneously obese and possess cellular defects in both glucose uptake and fatty acid oxidation (13). KSR1−/− mice have enlarged adipocytes and in vitro defects in adipogenesis and lipid accumulation (37). Because KSR proteins regulate both metabolism and tumorigenesis, we designed a study to determine how KSR1 impacts metabolism driven by oncogenic Ras. We used gene expression profiling to identify effectors that transduce Ras-induced and KSR1-dependent signals for metabolic regulation. We found that, in MEFs, KSR1 is essential for H-RasV12-induced expression of transcriptional regulators peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1α) and estrogen-related receptor α (ERRα). In the absence of KSR1, PGC1α was sufficient to increase glycolysis, OXPHOS, and anchorage-independent growth in a manner dependent upon interaction with ERRα. However, a constitutively active ERRα transgene increased only metabolic activity. These data reveal a novel KSR1-dependent but ERK1/2-indepedent pathway that requires PGC1α and ERRα to mediate the increased metabolism and anchorage-independent growth of oncogenic Ras.
MATERIALS AND METHODS
Cell culture and cell lines.
KSR1−/− MEFs were generated from 13.5-day embryos as described previously (47). Cell lines expressing various protein constructs were generated by infection with recombinant retroviruses containing the plasmid MSCV-IRES-GFP, which also carried a cDNA for each protein of interest. Infected cells were separated by flow cytometry according to increasing levels of fluorescence as described previously (38). Cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 0.1 mM minimum essential medium with nonessential amino acids, and 1% penicillin-streptomycin at 37°C in 5% CO2.
Western blot.
Cytoplasmic and nuclear extracts were made by lysing cells in cytoplasmic lysis buffer containing 0.5% NP-40, 25 mM HEPES, 5 mM KCl, 0.5 mM MgCl2, pH 7.4, with 10 μg/ml of aprotinin, 10 μg/ml of leupeptin, 2 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Lysates were lightly agitated at 4°C for 45 min followed by centrifugation at 15,000 rpm for 10 min. The supernatant was subjected a second time to centrifugation at 15,000 rpm for 10 min, and the cleared lysate was used as the cytoplasmic fraction. The pellet from the first centrifugation was washed once using cytoplasmic lysis buffer and then lysed in nuclear lysis buffer consisting of 40 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.25% sodium deoxycholate, pH 7.4, with 10 μg/ml of aprotinin, 10 μg/ml of leupeptin, 2 mM EDTA, and 1 mM PMSF. After 45 min, the nuclear fraction was cleared by centrifugation at 15,000 rpm for 10 min. Protein concentration was quantified by BCA protein assay (Promega). Proteins were resolved using SDS-PAGE and transferred to nitrocellulose membranes (LI-COR). Membranes were blocked in Odyssey blocking buffer (LI-COR) for 60 min. Transferred proteins were hybridized in Tris-buffered saline (TBS)-0.1% Tween 20 and detected using the Odyssey imaging system (LI-COR).
Reagents.
Primary antibodies were used at the indicated dilutions: KSR1 (H-70; Santa Cruz), 1:1,000; ERRα (V-19; Santa Cruz), 1:1,000; PGC1α (H-300; Santa Cruz), 1:1,000; β2 microglobin (M-10; Santa Cruz), 1:1,000; SDHA (C-18; Santa Cruz), 1:1,000; SDHB (FL-280; Santa Cruz), 1:1,000; AldoC (N-14; Santa Cruz), 1:500; α-tubulin (B-5-1-2; Santa Cruz), 1:2,000; β-actin (I-19; Santa Cruz), 1:2,000; H-Ras (OP23; EMD), 1:500; CytoC (556433; BD Transduction Labs), 1:2,000; HSP90 (610418; BD Transduction Labs), 1:4,000; HDAC2 (ab7029; Abcam), 1:5,000; SOD2 (06-984; Millipore), 1:2,000; VDAC (4866; Cell Signaling), 1:1,000. Anti-mouse, anti-rabbit, and anti-goat secondary antibodies conjugated to either AlexaFluor680 or IRDye800 (Rockland Immunochemicals) were used at 1:3,000. Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) and ERRα inverse agonist XCT790 were from Sigma-Aldrich.
Proliferation studies.
Cells (5 × 104) were seeded per 35-mm dish in triplicate in full serum medium. Cell number was determined using a Z2 particle counter and size analyzer (Beckman-Coulter) at the indicated times.
Anchorage-independent growth in soft agarose.
Cells were seeded at 5 × 103 cells per 35-mm dish in 1 ml of top agarose consisting of Iscove's modified Dulbecco's medium with 0.4% NuSieve GTG agarose suspended over a bottom layer consisting of 2 ml of Iscove's modified Dulbecco's medium with 0.8% NuSieve GTG agarose. Dimethyl sulfoxide (DMSO) or XCT790 was placed in both top and bottom layers at 10 μM. Colonies over 100 μm were counted and representative photomicrographs were taken after 14 days of incubation at 37°C in 5% CO2.
XF24 assays.
Cells were seeded at 5 × 104 cells/well in 24-well XF assay plates in full medium. Cells were switched to bicarbonate-free low-buffered DMEM with 25 mM glucose, 4 mM sodium pyruvate, and 4 mM l-glutamine for 1 h before being assayed. Triplicate measurements of the oxygen consumption rate (OCR) and the extracellular acidification rate (ECAR) were taken before and after three successive doses of 150 nM FCCP.
Radiometric glucose uptake assay.
Cells were cultured in six-well microplates and washed once before the addition of 0.1 mM 2-deoxy-d-[2,6-3H]glucose (1 μCi) in Krebs-Ringer's phosphate buffer for 5 min at 37°C. The assay was terminated by the rapid removal of assay buffer followed by four rapid washes with 1 ml of ice-cold buffer containing 500 μM phloretin. Cells were removed from each well with 0.4 ml of 0.1% SDS and counted for radioactivity after the addition of 4 ml of Optifluor (Packard Instrument Co).
Gene expression analysis.
Total RNA was isolated from cell lines using TRI reagent (Molecular Research Centers, Inc.), and its quality was verified using a Bioanalyzer (Agilent). A 2.5-μg portion of total RNA was reverse transcribed to generate cRNA using the Affymetrix 1-cycle target labeling kit. The resultant cRNA probes were hybridized to MG_U74Av2 arrays (Affymetrix) and scanned on a GeneChip Scanner 3000 7G (Affymetrix) in triplicate. The resultant .cel files were processed together using Robust Multichip Averaging using Expression FileCreator hosted by GenePattern to create a .res file that was analyzed using gene set enrichment analysis (Broad Institute) (4, 59, 60). Our cutoff for significant gene set enrichment was determined by a nominal (NOM) P value of <0.05, a family-wise error rate (FWER) P value of <0.05, and a false discovery rate (FDR) q value of <0.25.
Online deposition of microarray data.
Raw .chp and .cel files were deposited at NCBI Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/projects/geo/) and are freely accessible using the series identifier GSE28228.
RESULTS
H-RasV12-induced expression of PGC1α and ERRα is KSR1 dependent.
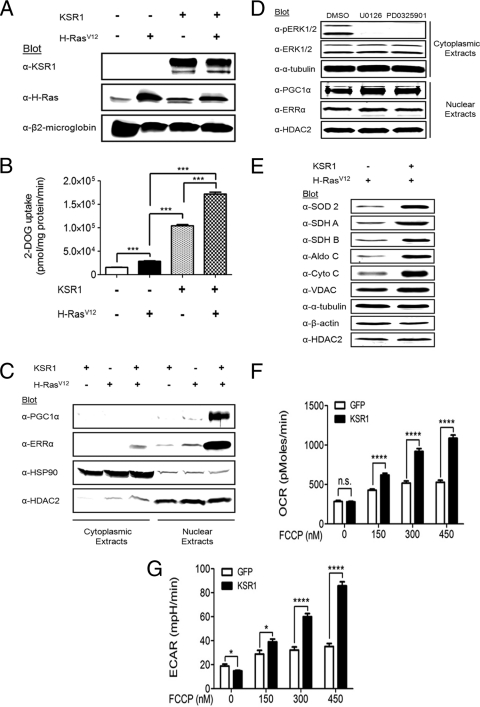
Disruption of KSR1 inhibits anchorage-independent growth by activated H-RasV12 (38). Given the effects of KSR proteins on normal cellular metabolism (13, 37), we assessed the ability of KSR1 to modulate metabolic pathways regulated by H-RasV12 in transformed cells. We engineered KSR1−/− MEFs to express green fluorescent protein (GFP) alone, KSR1 or H-RasV12 and GFP, or KSR1, H-RasV12, and GFP (Fig. 1A). In KSR1−/− MEFs the addition of H-RasV12 caused a small (1.8-fold) increase in glucose uptake while the addition of KSR1 stimulated uptake 6.7-fold compared to the result in control KSR1−/− MEFs (Fig. 1B). The combination of KSR1 and H-RasV12 increased glucose uptake 11-fold above that of KSR1−/− MEFs (Fig. 1B). As KSR1 regulates glucose uptake best in the presence of H-RasV12, a cardinal feature of the oncogene-induced Warburg effect, we tested whether gene expression profiling might identify RasV12-induced and KSR1-dependent effectors of cancer cell metabolism. Gene set enrichment analysis (GSEA) of microarray data from KSR1−/− MEFs expressing H-RasV12 ± KSR1 revealed highly significant KSR1-dependent pathways regulating glycolysis, the TCA cycle, and OXPHOS (Table 1). An additional gene set contained genes responsive to the transcriptional coactivator PGC1α. In contrast, GSEA of microarray data from KSR1−/− MEFs ± KSR1 alone revealed no enrichment of metabolic pathways (data not shown). GSEA of transcriptional cis-element gene sets revealed enrichment of the estrogen-related receptor responsive element (ERRE) (31, 44) TGACCTTG only in the presence of KSR1 and H-RasV12 (Table 2). These data suggest that H-RasV12-induced and KSR1-dependent transcription of genes is responsive to the ERR family of transcription factors. In the absence of H-RasV12, KSR1 enhanced expression of genes with cis elements responsive to the E2F transcription factor family (Table 3) (49). This likely reflects the ability of ectopic KSR1 to enhance proliferation of MEFs (38). The lack of significant overlap in gene set enrichment with KSR1 ± H-RasV12 highlights the unique properties of KSR1 in the presence of oncogenic Ras that supports anchorage-independent growth.
Fig. 1.
KSR1 enhances the expression of metabolic regulators PGC1α and ERRα only in the presence of H-RasV12. KSR1−/− MEFs were infected with recombinant retroviruses encapsulating bicistronic vectors carrying genes for KSR1 and GFP, GFP alone (control), H-RasV12 and puromycin resistance, or puromycin resistance alone (control). (A) Whole-cell extracts were immunoblotted with the indicated antibodies to confirm the expression of KSR1 and H-RasV12 in cells prior to testing. (B) KSR1−/− MEFs ± H-RasV12 ± KSR1 were assessed for glucose uptake by the incorporation of [3H]-2-deoxyglucose. (C) KSR1−/− MEFs ± H-RasV12 ± KSR1 were lysed and separated into cytoplasmic and nuclear extracts and immunoblotted with the indicated antibodies. (D) KSR1−/− MEFs expressing both H-RasV12 and KSR1 were treated with 20 μM U0126 or PD0325901 for 24 h and lysed and separated into cytoplasmic and nuclear extracts and immunoblotted with the indicated antibodies. (E) Whole-cell extracts from KSR1−/− MEFs expressing H-RasV12 and either KSR1 and GFP or GFP alone (control) were probed on Western blots with the indicated antibodies. (F and G) The rates of oxygen consumption (F) and extracellular acidification (G) were assessed in KSR1−/− MEFs expressing H-RasV12 ± KSR1. Data shown are the result of triplicate measurements for ECAR and OCR ± standard deviation taken at baseline and after increasing doses of FCCP. n.s., not significant (P > 0.05); *, P < 0.05; ****, P < 0.0001 by two-tailed Student's t test.
Table 1.
Enrichment of curated pathway gene sets in the presence of KSR1 and H-RasV12
| Pathway name | NOM P | FWER P | FDR q |
|---|---|---|---|
| OXIDATIVE_PHOSPHORYLATION | <0.001 | <0.001 | <0.001 |
| PGC RESPONSIVE GENES | <0.001 | <0.001 | <0.001 |
| ELECTRON_TRANSPORT_CHAIN | <0.001 | <0.001 | <0.001 |
| HUMAN_MITODB_6_2002 | <0.001 | <0.001 | <0.001 |
| IDX_TSA_UP_CLUSTER5 | <0.001 | <0.001 | <0.001 |
| GLYCOLYSIS_AND_ GLUCONEOGENESIS | <0.001 | <0.001 | <0.001 |
| MOOTHA_VOXPHOS | <0.001 | <0.001 | <0.001 |
| MITOCHONDRIA | <0.001 | <0.001 | <0.001 |
| KREBS_TCA_CYCLE | <0.001 | <0.001 | <0.001 |
| OXIDATIVE_PHOSPHORYLATION | <0.001 | <0.001 | <0.001 |
| IDX TSA UP CLUSTER6 | <0.001 | <0.001 | <0.001 |
| MENSE HYPOXIA UP | <0.001 | <0.001 | <0.001 |
| HIF1 TARGETS | <0.001 | <0.001 | <0.001 |
| GLUCONEOGENESIS | <0.001 | <0.001 | <0.001 |
| GLYCOLYSIS | <0.001 | <0.001 | <0.001 |
| CITRATE CYCLE TCA CYCLE | <0.001 | <0.001 | <0.001 |
| HSA00010 GLYCOLYSIS AND GLUCONEOGENESIS | <0.001 | <0.001 | <0.001 |
| HYPOXIA FIBRO UP | <0.001 | <0.001 | <0.01 |
| HYPOXIA REG UP | <0.001 | <0.001 | <0.01 |
| HYPOXIA REVIEW | <0.001 | <0.05 | <0.01 |
| PYRUVATE METABOLISM | <0.001 | <0.05 | <0.01 |
| FATTY ACID DEGRADATION | <0.001 | <0.05 | <0.01 |
| HSA00051 FRUCTOSE AND MANNOSE METABOLISM | <0.001 | <0.05 | <0.01 |
Table 2.
Enrichment of transcription factor binding cis elements in the presence of KSR1 and H-RasV12
| Binding motif | Transcription factor | NOM P | FWER P | FDR q |
|---|---|---|---|---|
| TGACCTTGa | ERRα | <0.001 | <0.01 | <0.01 |
Table 3.
Enrichment of transcription factor binding cis elements in the presence of KSR1 and the absence of H-RasV12
| Binding motif | Transcription factor | NOM P | FWER P | FDR q |
|---|---|---|---|---|
| TTTSGCGCGMNR | E2F | <0.001 | <0.05 | <0.05 |
| NCSCGCSAAAN | E2F | <0.001 | <0.05 | <0.05 |
GSEA suggests that PGC1α and ERREs have enhanced activity in KSR1−/− MEFs expressing both H-RasV12 and KSR1. PGC1α and ERRα form a functionally active complex that activates transcription of genes involved in mitochondrial metabolism (26, 30, 53). In light of the strong relationship between PGC1α, ERRα, and mitochondrial metabolism, we investigated the status of these proteins in relation to KSR1 and H-RasV12. Consistent with the data generated from gene expression profiling, Western blot analysis revealed that PGC1α and ERRα were upregulated by KSR1 only in the presence of H-RasV12 (Fig. 1C). The KSR1-dependent expression of PGC1α and ERRα was unaffected by treatment with MEK1/2 inhibitors U0126 and PD0325901 (Fig. 1D). Together these data identify both PGC1α and ERRα as downstream proteins that are induced by KSR1 in an H-RasV12-dependent but ERK1/2-independent manner.
Multiple metabolic enzymes identified by gene expression profiling to be regulated by PGC1α and ERRα were upregulated by H-RasV12 only in the presence of KSR1 (Fig. 1E). To determine the functional impact of KSR1 on H-RasV12-driven metabolism, the basal and maximal rates of aerobic glycolysis and OXPHOS were determined. In addition to basal rates, the maximal rate of OXPHOS in response to a bioenergetic challenge can be an important metabolic parameter in determining biological outcomes (11, 23, 48). FCCP is a commonly used mitochondrial uncoupler that creates a bioenergetic challenge to the cell that maximizes rates of aerobic glycolysis and OXPHOS in multiple cell lines (1, 11, 21, 73). The reexpression of KSR1 had no effect on the basal oxygen consumption rate (OCR), an index of OXPHOS, but led to a significant increase in the OCR after addition of the FCCP (Fig. 1F). Additionally, KSR1 caused a small but significant decrease in basal extracellular acidification rate (ECAR), an index of aerobic glycolysis, but led to a significant increase in ECAR after FCCP stimulation (Fig. 1G). Together these data indicate that KSR1 is required for H-RasV12 to maximize cellular capacity for OXPHOS and glycolysis.
PGC1α enhances anchorage-independent growth and metabolic capacity.
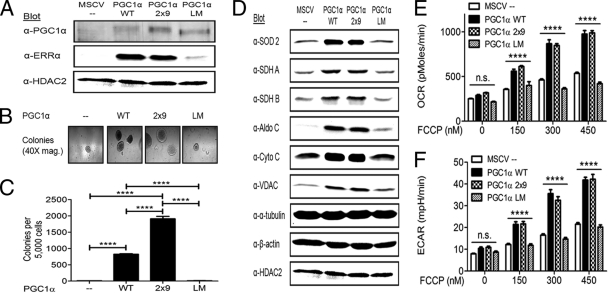
The role of PGC1α in anchorage-independent growth has not been elucidated (22, 29). We tested three PGC1α constructs (Fig. 2A) to determine the role of PGC1α in anchorage-independent growth. Wild-type PGC1α (PGC1α WT) was sufficient to rescue anchorage-independent growth of KSR1−/− MEFs expressing H-RasV12 (Fig. 2B and C). PGC1α WT also stimulated ERRα expression, consistent with published data from other model systems (53). To further define the role of PGC1α with respect to ERRα, two additional PGC1α constructs were used. PGC1α contains leucine-rich motifs that mediate interactions with nuclear receptors. Mutations of leucine-rich domains 2 and 3 alter the ability of PGC1α to bind ERRα (29, 53). In PGC1α 2×9, leucine-rich domains 2 and 3 were replaced with small peptide sequences that preferentially bind ERRα and eliminate other nuclear receptor interactions (25, 57). In PGC1α LM, the leucine-rich domains 2 and 3 were mutated to prevent ERRα interaction while retaining other critical interactions (24). PGC1α 2×9 induced ERRα expression and anchorage-independent growth comparable to those induced by PGC1α WT (Fig. 2A to C). However, PGC1α LM was unable to induce either ERRα expression or anchorage-independent growth (Fig. 2A to C). These data indicate that PGC1α expression and its interaction with ERRα are required for anchorage-independent growth.
Fig. 2.
PGC1α stimulates anchorage-independent growth and metabolism in KSR1−/− MEFs expressing H-RasV12. KSR1−/− MEFs with stable expression of H-RasV12 were infected with recombinant retroviruses encapsulating bicistronic retroviral vectors carrying genes for PGC1α constructs and GFP or GFP alone (control). (A) Nuclear extracts were immunoblotted with the indicated antibodies to confirm the expression of PGC1α constructs and to determine ERRα expression. (B and C) Cells were assessed for anchorage-independent growth by plating 5,000 cells/dish in 0.4% agar and counting colonies over 100 μm after 14 days. Representative photomicrographs of colonies (B) and colony count (C) are shown. (D) Whole-cell extracts from KSR1−/− MEFs expressing H-RasV12 and either PGC1α constructs and GFP or GFP alone (control) were probed on Western blots with the indicated antibodies. (E and F) The rates of oxygen consumption (E) and extracellular acidification (F) were assessed in KSR1−/− MEFs expressing H-RasV12 and either PGC1α constructs and GFP or GFP alone (control). Data shown are the result of triplicate measurements for ECAR and OCR ± standard deviation taken at baseline and after increasing doses of FCCP. n.s., not significant (P > 0.05); ****, P < 0.0001 by two-tailed Student's t test.
Cell lines expressing PGC1α constructs were analyzed for their ability to regulate metabolism in a manner similar to that of KSR1. Metabolic enzymes regulated by KSR1 (Fig. 1E) were also regulated by PGC1α in an ERRα-dependent manner (Fig. 2D). To determine the functional impact of PGC1α on H-RasV12-driven metabolism, the basal and FCCP-stimulated rates of aerobic glycolysis and OXPHOS were determined. Basal OCR was unaffected by each PGC1α construct. However, PGC1α WT and PGC1α 2×9 promoted significant FCCP-stimulated increases in OCR, while expression of PGC1α LM or the empty vector control had no effect (Fig. 2E). Similarly, PGC1α WT and PGC1α 2×9 promoted FCCP-stimulated ECAR, while PGC1α LM or the empty vector control could not (Fig. 2F). These data indicate that PGC1α expands the metabolic capacity of H-RasV12-transformed cells in an ERRα-dependent manner and that KSR1-dependent effects on RasV12-driven metabolism are mediated by PGC1α and ERRα.
ERRα enhances metabolic capacity but not anchorage-independent growth.
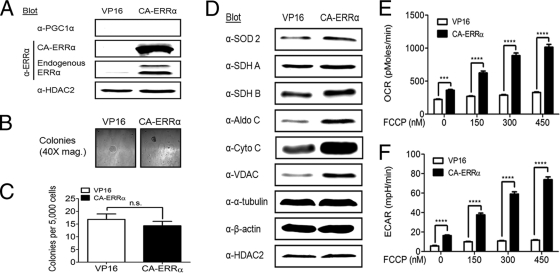
To determine whether ERRα expression was sufficient for anchorage-independent growth, a constitutively active ERRα (CA-ERRα) construct was expressed in KSR1−/− MEFs expressing H-RasV12 in the absence of KSR1. ERRα activity is thought to be modulated by coregulators, such as PGC1α, in the absence of a natural ligand (26, 32, 53). To make a transgene whose activity was independent of PGC1α, CA-ERRα was constructed by fusing the herpes simplex virus type 1 coactivator VP16 to ERRα (55). ERRα has an ERRE in its promoter and enhances its own transcription (39, 40, 44). CA-ERRα stimulates expression of endogenous ERRα but not PGC1α (Fig. 3A), demonstrating that CA-ERRα is transcriptionally active in the absence of PGC1α (53). However, compared to VP16 alone, CA-ERRα was unable to restore anchorage-independent growth to KSR1−/− MEFs expressing H-RasV12, producing an average of only 14 colonies from 5,000 cells assayed (Fig. 3B and C).
Fig. 3.
Constitutively active ERRα (CA-ERRα) stimulates metabolism but not anchorage-independent growth in KSR1−/− MEFs expressing H-RasV12. KSR1−/− MEFs with stable expression of H-RasV12 were infected with recombinant retroviruses encapsulating bicistronic retroviral vectors carrying genes for CA-ERRα and GFP or VP16 and GFP (control). (A) Nuclear extracts were immunoblotted with the indicated antibodies to confirm the expression of CA-ERRα and to determine endogenous PGC1α and ERRα expression. (B and C) Cells were assessed for anchorage-independent growth by plating 5,000 cells/dish in 0.4% agar and counting colonies over 100 μm after 14 days. Representative photomicrographs of colonies (B) and colony count (C). (D) Whole-cell extracts from KSR1−/− MEFs expressing H-RasV12 and either CA-ERRα and GFP or VP16 and GFP (control) were probed on Western blots with the indicated antibodies. (E and F) The rates of oxygen consumption (E) and extracellular acidification (F) were assessed in KSR1−/− MEFs expressing H-RasV12 and either CA-ERRα and GFP or VP16 and GFP (control). Data shown are the result of triplicate measurements for ECAR and OCR ± standard deviation taken at baseline and after increasing doses of FCCP. n.s., not significant (P > 0.05 by two-tailed Student's t test); ****, P < 0.0001 by 2-way analysis of variance (ANOVA).
Cell lines expressing VP16 or CA-ERRα were also analyzed for their ability to facilitate H-RasV12-driven metabolism in a manner similar to those of KSR1 and PGC1α. CA-ERRα was able to weakly enhance expression of some (e.g., SOD 2, SDH B, aldolase C) but not all of the metabolic proteins regulated by KSR1 and PGC1α (Fig. 3D). To determine the functional impact of CA-ERRα on H-RasV12-driven metabolism, basal and FCCP-stimulated rates of aerobic glycolysis and OXPHOS were determined in cells expressing the activated construct. Despite its limited effect on metabolic gene expression, CA-ERRα caused a significant increase in basal OCR and ECAR and a strong, dose-dependent increase in FCCP-stimulated OCR and ECAR (Fig. 3E and F). These data demonstrate that, while CA-ERRα maximized OXPHOS and glycolytic potential in cells expressing Ras, this restoration in metabolic capacity is not sufficient for H-RasV12-induced anchorage-independent growth.
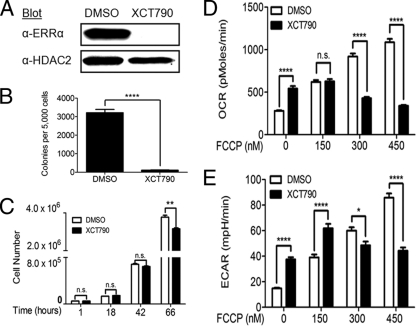
Inhibition of ERRα prevents H-RasV12-induced and KSR1-dependent effects on anchorage-independent growth and metabolism.
To determine if ERRα activity is necessary for KSR1-dependent and H-RasV12-induced anchorage-independent growth, we inhibited ERRα activity with a highly selective ERRα inverse agonist, XCT790 (7, 69). XCT790 decreased ERRα expression and anchorage-independent growth (Fig. 4A and B) but had a minimal effect on the growth rate of MEFs expressing KSR1 and H-RasV12 (Fig. 4C). XCT790 was tested for its ability to reverse KSR1-dependent metabolism in H-RasV12-containing MEFs. Twenty-four-hour treatment with 10 μM XCT790 caused a significant increase in basal OCR and ECAR but caused a dose-dependent decrease in FCCP-stimulated OCR and ECAR (Fig. 4D and E). In combination with analysis of CA-ERRα, the ability of XCT790 to inhibit ERRα activity, anchorage-independent growth, and maximal metabolic rates in MEFs expressing KSR1 and H-RasV12 suggests that ERRα is necessary but not sufficient for transformation by H-RasV12.
Fig. 4.
ERRα inverse agonist XCT790 inhibits anchorage-independent growth in KSR1−/− MEFs expressing H-RasV12 and KSR1. KSR1−/− MEFs with stable expression of H-RasV12 and KSR1 were treated with 10 μM XCT790 or DMSO (control) for the indicated times and then assayed. (A) Nuclear extracts were immunoblotted with the indicated antibodies to determine the expression of ERRα 24 h after treatment. (B) Cells were assessed for anchorage-independent growth by plating 5,000 cells/dish in 0.4% agar and counting colonies over 100 μm after 14 days. Cells were pretreated with drug for 24 h before plating and drug was included in both top and bottom layers of agar. (C) 50,000 cells were plated in the presence of XCT790 or DMSO, and cell numbers were determined at the indicated times. (D and E) The rates of oxygen consumption (D) and extracellular acidification (E) were assessed in KSR1−/− MEFs expressing H-RasV12 and KSR1 treated with either XCT790 or DMSO for 24 h. Data shown are the result of triplicate measurements for ECAR and OCR ± standard deviation taken at baseline and after increasing doses of FCCP. n.s., not significant (P > 0.05); *, P < 0.05; **, P < 0.01; ****, P < 0.0001 by two-tailed Student's t test.
DISCUSSION
The data presented here demonstrate that KSR1 promotes cell transformation by oncogenic Ras through the expression of PGC1α and ERRα. PGC1α and ERRα are also essential to the KSR1-dependent elevation in glycolytic and OXPHOS potential of Ras-transformed cells. This pathway is inhibited by the ERRα inverse agonist XCT790, which may reveal new approaches for targeting Ras-driven tumorigenesis.
KSR1 increases cell proliferation and anchorage-independent growth in the presence of H-RasV12 but enhances only cell proliferation in the absence of the oncogene (38). This observation suggested that KSR1 may mediate oncogene-specific signaling pathways that are essential to properties, like anchorage-independent growth, that are characteristic of transformed cells but distinct from those regulating proliferation. Consistent with this hypothesis, transcriptional targets of the E2F family comprised the only gene set significantly regulated by KSR1 in the absence of H-RasV12 (Table 3). E2F transcription factors have a well-described role in regulating the G1/S transition to increase cell proliferation and could be a mechanism used by KSR1 to enhance cell proliferation in untransformed cells (10). Enhanced expression of E2F transcription factors are associated with certain types of cancers but were not identified as effectors in support of KSR1-dependent transformation by H-RasV12. These data suggest an altered role for KSR1 in Ras-transformed cells.
In the presence of H-RasV12, KSR1 potently upregulated gene sets for glycolysis, OXPHOS, and the TCA cycle, including the transcriptional regulators PGC1α and ERRα and their targets (Table 2; Fig. 1C). The observation that PGC1α and ERRα are able to increase glycolytic and OXPHOS capacity in KSR1−/− MEFs expressing H-RasV12 but only PGC1α rescued anchorage-independent growth suggests that additional PGC1α-dependent pathways distinct from those regulating metabolism contribute to the transformed phenotype. However, mutation of PGC1α indicated that nonmetabolic pathways contributing to transformation result from transcription that requires interaction between PGC1α and ERRα. Those pathways have not been identified, but a comparison of gene expression profiles from cells expressing CA-ERRα and PGC1α 2×9 constructs may reveal candidates.
KSR1 has been most thoroughly studied as a scaffold for the Raf/MEK/ERK kinase cascade (38, 43, 45, 47, 58). The role of this signaling pathway in KSR1-dependent regulation of RasV12-induced expression of PGC1α and ERRα is not clear. Our data (Fig. 1D) demonstrate that the MEK inhibitors U0126 and PD0325901, while blocking ERK activation, have no effect on PGC1α and ERRα expression. This observation suggests that KSR1 may have additional functions independent of its role as a scaffold for ERK signaling. This ability of KSR1 to affect diverse signaling pathways is evident in reports that KSR1 assembles inducible nitric oxide synthase (iNOS) with Hsp90 to release NO during infection with Pseudomonas (78). Furthermore, the KSR1 paralog KSR2 interacts with and directs the activation of the energy sensor AMP-activated protein kinase (AMPK) in addition to its ability to regulate ERK signaling (13, 19). Thus, KSR1 may regulate multiple pathways to affect cell fate.
KSR1-enhanced anchorage-independent growth parallels an increase in the cellular capacity for both glycolysis and OXPHOS, consistent with the stepwise metabolic profiling of human fibroblasts (50). However, we were able to identify PGC1α and ERRα as key effectors necessary for metabolic regulation in transformation. Recent studies reveal the critical contribution of aerobic glycolysis in helping tumor cells achieve the high rates of nucleotide and fatty acid synthesis necessary for the production of daughter cells. In contrast, OXPHOS is often observed to be decreased in tumor cells showing increased aerobic glycolysis. To our knowledge, however, the OXPHOS capacity of tumor cells, irrespective of basal oxygen consumption, has not been examined. Increased capacity for oxidative metabolism may contribute to the generation of NADPH and substrates for nucleotide biosynthesis. The KSR1-dependent increase in OXPHOS capacity may also reflect an enhanced potential to respond to oxidative stress. In ovarian epithelial cells, RasV12 increases tolerance to reactive oxidative species (ROS) by upregulating antioxidant production in mitochondria to protect the transformed cells from high levels of ROS associated with the uncontrolled growth potential of tumor cells (75). In matrix-detached cells, increased ROS production inhibits mitochondrial β-oxidation of fatty acids leading to decreased ATP levels, and treatment with antioxidants was sufficient to promote anchorage-independent growth (52). Consistent with these data, we observed an increase in SOD2 following expression of KSR1 and PGC1α, but not CA-ERRα, which parallels their ability to enhance anchorage-independent growth.
Colon cancers bearing activating mutations in Ras or Raf oncogenes are completely resistant to the beneficial effects of cetuximab or panitumamab treatment due to the hyperactivation of downstream signaling components of epidermal growth factor receptor (EGFR) signaling (17, 18, 33). These findings highlight the importance of discovering new therapeutics for use in tumors bearing activating Ras mutations. In our model system, we focused on the molecular scaffold KSR1 to understand the necessary components for Ras-induced transformation. Our studies with the highly selective inverse agonist, XCT790, indicate that ERRα is necessary for KSR1 to promote anchorage-independent growth. ERRα inverse agonists can inhibit the growth of cells from multiple tumor types, including breast, lung, liver, and prostate, but the compound has never been specifically implicated in tumor cells with activating Ras mutations (3, 20, 66, 72). Thus, targeting ERRα may be a useful therapy against tumors bearing Ras oncogenes.
ACKNOWLEDGMENTS
We thank Ching-yi Chang and Donald P. McDonnell at Duke University for providing PGC1α and ERRα plasmids. We thank Ming-Hoi Wu for help with the gene expression profiling experiments. Deanna Volle is thanked for help preparing the manuscript. We thank Charles Kuszynski, Megan Michalak, and Victoria Smith in the UNMC Cell Analysis Facility for their technical expertise in the generation of GFP-expressing cell lines. We thank the Genomics Core Research Facility at the University of Nebraska in Lincoln for their technical expertise in gene expression profiling.
This work was supported by grants CA90400 from the NCI to R.E.L. and a Physician-Scientist Training Grant from the American Diabetes Association to K.W.F.
Footnotes
Published ahead of print on 25 April 2011.
REFERENCES
- 1. Abe Y., et al. 2010. Bioenergetic characterization of mouse podocytes. Am. J. Physiol. Cell Physiol. 299: C464–C476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baracca A., et al. 2010. Mitochondrial complex I decrease is responsible for bioenergetic dysfunction in K-ras transformed cells. Biochim. Biophys. Acta 1797: 314–323 [DOI] [PubMed] [Google Scholar]
- 3. Bianco S., et al. 2009. Modulating estrogen receptor-related receptor-α activity inhibits cell proliferation. J. Biol. Chem. 284: 23286–23292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bild A., Febbo P. G. 2005. Application of a priori established gene sets to discover biologically important differential expression in microarray data. Proc. Natl. Acad. Sci. U. S. A. 102: 15278–15279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blum R., Jacob-Hirsch J., Amariglio N., Rechavi G., Kloog Y. 2005. Ras inhibition in glioblastoma down-regulates hypoxia-inducible factor-1α, causing glycolysis shutdown and cell death. Cancer Res. 65: 999–1006 [PubMed] [Google Scholar]
- 6. Brennan J. A., Volle D. J., Chaika O. V., Lewis R. E. 2002. Phosphorylation regulates the nucleocytoplasmic distribution of kinase suppressor of Ras. J. Biol. Chem. 277: 5369–5377 [DOI] [PubMed] [Google Scholar]
- 7. Busch B. B., et al. 2004. Identification of a selective inverse agonist for the orphan nuclear receptor estrogen-related receptor alpha. J. Med. Chem. 47: 5593–5596 [DOI] [PubMed] [Google Scholar]
- 8. Buzzai M., et al. 2005. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid β-oxidation. Oncogene 24: 4165–4173 [DOI] [PubMed] [Google Scholar]
- 9. Cacace A. M., et al. 1999. Identification of constitutive and ras-inducible phosphorylation sites of KSR: implications for 14-3-3 binding, mitogen-activated protein kinase binding, and KSR overexpression. Mol. Cell. Biol. 19: 229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen H.-Z., Tsai S.-Y., Leone G. 2009. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat. Rev. Cancer 9: 785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi S. W., Gerencser A. A., Nicholls D. G. 2009. Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J. Neurochem. 109: 1179–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chun S., et al. 2010. Oncogenic KRAS modulates mitochondrial metabolism in human colon cancer cells by inducing HIF-1α and HIF-2α target genes. Mol. Cancer 9: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Costanzo-Garvey D. L., et al. 2009. KSR2 is an essential regulator of AMP kinase, energy expenditure, and insulin sensitivity. Cell Metab. 10: 366–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dang C. V. 2010. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res. 70: 859–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Groof A., et al. 2009. Increased OXPHOS activity precedes rise in glycolytic rate in H-RasV12/E1A transformed fibroblasts that develop a Warburg phenotype. Mol. Cancer 8: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dejean L., Beauvoit B., Bunoust O., Guérin B., Rigoulet M. 2002. Activation of Ras cascade increases the mitochondrial enzyme content of respiratory competent yeast. Biochem. Biophys. Res. Commun. 293: 1383–1388 [DOI] [PubMed] [Google Scholar]
- 17. De Roock W., et al. 2008. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann. Oncol. 19: 508–515 [DOI] [PubMed] [Google Scholar]
- 18. Di Nicolantonio F., et al. 2008. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J. Clin. Oncol. 26: 5705–5712 [DOI] [PubMed] [Google Scholar]
- 19. Dougherty M. K., et al. 2009. KSR2 is a calcineurin substrate that promotes ERK cascade activation in response to calcium signals. Mol. Cell 34: 652–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duellman S. J., et al. 2010. A novel steroidal inhibitor of estrogen-related receptor α (ERRα). Biochem. Pharmacol. 80: 819–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferrick D. A., Neilson A., Beeson C. 2008. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov. Today 13: 268–274 [DOI] [PubMed] [Google Scholar]
- 22. Finck B. N., Kelly D. P. 2006. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J. Clin. Invest. 116: 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Flynn J. M., et al. 2011. Impaired spare respiratory capacity in cortical synaptosomes from Sod2 null mice. Free Radic. Biol. Med. 50: 866–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaillard S., Dwyer M. A., McDonnell D. P. 2007. Definition of the molecular basis for estrogen receptor-related receptor-α-cofactor interactions. Mol. Endocrinol. 21: 62–76 [DOI] [PubMed] [Google Scholar]
- 25. Gaillard S., et al. 2006. Receptor-selective coactivators as tools to define the biology of specific receptor-coactivator pairs. Mol. Cell 24: 797–803 [DOI] [PubMed] [Google Scholar]
- 26. Giguere V. 2008. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr. Rev. 29: 677–696 [DOI] [PubMed] [Google Scholar]
- 27. Hahn W. C., et al. 1999. Creation of human tumour cells with defined genetic elements. Nature 400: 464–468 [DOI] [PubMed] [Google Scholar]
- 28. Hanahan D., Weinberg R. A. 2011. Hallmarks of cancer: the next generation. Cell 144: 646–674 [DOI] [PubMed] [Google Scholar]
- 29. Hock M. B., Kralli A. 2009. Transcriptional control of mitochondrial biogenesis and function. Annu. Rev. Physiol. 71: 177–203 [DOI] [PubMed] [Google Scholar]
- 30. Huss J. M., Kopp R. P., Kelly D. P. 2002. Peroxisome proliferator-activated receptor coactivator-1α (PGC-1α) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-α and -γ. J. Biol. Chem. 277: 40265–40274 [DOI] [PubMed] [Google Scholar]
- 31. Johnston S. D., et al. 1997. Estrogen-related receptor alpha 1 functionally binds as a monomer to extended half-site sequences including ones contained within estrogen-response elements. Mol. Endocrinol. 11: 342–352 [DOI] [PubMed] [Google Scholar]
- 32. Kallen J., et al. 2004. Evidence for ligand-independent transcriptional activation of the human estrogen-related receptor α (ERRα). J. Biol. Chem. 279: 49330–49337 [DOI] [PubMed] [Google Scholar]
- 33. Karapetis C. S., et al. 2008. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 359: 1757–1765 [DOI] [PubMed] [Google Scholar]
- 34. Karim F. D., et al. 1996. A screen for genes that function downstream of Ras1 during Drosophila eye development. Genetics 143: 315–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kikuchi H., Pino M. S., Zeng M., Shirasawa S., Chung D. C. 2009. Oncogenic KRAS and BRAF differentially regulate hypoxia-inducible factor-1α and -2α in colon cancer. Cancer Res. 69: 8499–8506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kornfeld K., Hom D. B., Horvitz H. R. 1995. The ksr-1 gene encodes a novel protein kinase involved in Ras-mediated signaling in C. elegans. Cell 83: 903–913 [DOI] [PubMed] [Google Scholar]
- 37. Kortum R. L., et al. 2005. The molecular scaffold kinase suppressor of Ras 1 (KSR1) regulates adipogenesis. Mol. Cell. Biol. 25: 7592–7604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kortum R. L., Lewis R. E. 2004. The molecular scaffold KSR1 regulates the proliferative and oncogenic potential of cells. Mol. Cell. Biol. 24: 4407–4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laganière J., et al. 2004. A polymorphic autoregulatory hormone response element in the human estrogen-related receptor α (ERRα) promoter dictates peroxisome proliferator-activated receptor γ coactivator-1α control of ERRα expression. J. Biol. Chem. 279: 18504–18510 [DOI] [PubMed] [Google Scholar]
- 40. Liu D., Zhang Z., Teng C. T. 2005. Estrogen-related receptor-γ and peroxisome proliferator-activated receptor-γ coactivator-1α regulate estrogen-related receptor-α gene expression via a conserved multi-hormone response element. J. Mol. Endocrinol. 34: 473–487 [DOI] [PubMed] [Google Scholar]
- 41. Lozano J., et al. 2003. Deficiency of kinase suppressor of Ras1 prevents oncogenic ras signaling in mice. Cancer Res. 63: 4232–4238 [PubMed] [Google Scholar]
- 42. Marin-Valencia I., DeBerardinis R. J. 2011. Targeting the metabolic flexibility of cancer cells: straighten up and die right. Cell Cycle 10: 191. [PubMed] [Google Scholar]
- 43. Michaud N. R., et al. 1997. KSR stimulates Raf-1 activity in a kinase-independent manner. Proc. Natl. Acad. Sci. U. S. A. 94: 12792–12796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mootha V. K., et al. 2004. Errα and Gabpa/b specify PGC-1α-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc. Natl. Acad. Sci. U. S. A. 101: 6570–6575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Muller J., Cacace A. M., Lyons W. E., McGill C. B., Morrison D. K. 2000. Identification of B-KSR1, a novel brain-specific isoform of KSR1 that functions in neuronal signaling. Mol. Cell. Biol. 20: 5529–5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Muller J., Ory S., Copeland T., Piwnica-Worms H., Morrison D. K. 2001. C-TAK1 regulates Ras signaling by phosphorylating the MAPK scaffold, KSR1. Mol. Cell 8: 983–993 [DOI] [PubMed] [Google Scholar]
- 47. Nguyen A., et al. 2002. Kinase suppressor of Ras (KSR) is a scaffold which facilitates mitogen-activated protein kinase activation in vivo. Mol. Cell. Biol. 22: 3035–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nicholls D. G. 2008. Oxidative stress and energy crises in neuronal dysfunction. Ann. N. Y. Acad. Sci. 1147: 53–60 [DOI] [PubMed] [Google Scholar]
- 49. Rabinovich A., Jin V. X., Rabinovich R., Xu X., Farnham P. J. 2008. E2F in vivo binding specificity: comparison of consensus versus nonconsensus binding sites. Genome Res. 18: 1763–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramanathan A., Wang C., Schreiber S. L. 2005. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc. Natl. Acad. Sci. U. S. A. 102: 5992–5997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roy F., Laberge G., Douziech M., Ferland-McCollough D., Therrien M. 2002. KSR is a scaffold required for activation of the ERK/MAPK module. Genes Dev. 16: 427–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schafer Z. T., et al. 2009. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 461: 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schreiber S. N., et al. 2004. The estrogen-related receptor α (ERRα) functions in PPARγ coactivator 1α (PGC-1α)-induced mitochondrial biogenesis. Proc. Natl. Acad. Sci. U. S. A. 101: 6472–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shaw R. J., Cantley L. C. 2006. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441: 424–430 [DOI] [PubMed] [Google Scholar]
- 55. Sladek R., Bader J., Giguere V. 1997. The orphan nuclear receptor estrogen-related receptor alpha is a transcriptional regulator of the human medium-chain acyl coenzyme A dehydrogenase gene. Mol. Cell. Biol. 17: 5400–5409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sodhi A., Montaner S., Miyazaki H., Gutkind J. S. 2001. MAPK and Akt act cooperatively but independently on hypoxia inducible factor-1α in rasV12 upregulation of VEGF. Biochem. Biophys. Res. Commun. 287: 292–300 [DOI] [PubMed] [Google Scholar]
- 57. Stein R. A., Gaillard S., McDonnell D. P. 2009. Estrogen-related receptor alpha induces the expression of vascular endothelial growth factor in breast cancer cells. J. Steroid Biochem. Mol. Biol. 114: 106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stewart S., et al. 1999. Kinase suppressor of Ras forms a multiprotein signaling complex and modulates MEK localization. Mol. Cell. Biol. 19: 5523–5534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Subramanian A., Kuehn H., Gould J., Tamayo P., Mesirov J. P. 2007. GSEA-P: a desktop application for gene set enrichment analysis. Bioinformatics 23: 3251–3253 [DOI] [PubMed] [Google Scholar]
- 60. Subramanian A., et al. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U. S. A. 102: 15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sundaram M., Han M. 1995. The C. elegans ksr-1 gene encodes a novel raf-related kinase involved in Ras-mediated signal transduction. Cell 83: 889–901 [DOI] [PubMed] [Google Scholar]
- 62. Telang S., Lane A., Nelson K., Arumugam S., Chesney J. 2007. The oncoprotein H-RasV12 increases mitochondrial metabolism. Mol. Cancer 6: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Therrien M., et al. 1995. KSR, a novel protein kinase required for RAS signal transduction. Cell 83: 879–888 [DOI] [PubMed] [Google Scholar]
- 64. Therrien M., Morrison D. K., Wong A. M., Rubin G. M. 2000. A genetic screen for modifiers of a kinase suppressor of Ras-dependent rough eye phenotype in Drosophila. Genetics 156: 1231–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vander Heiden M. G., Cantley L. C., Thompson C. B. 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang J., Wang Y., Wong C. 2010. Oestrogen-related receptor alpha inverse agonist XCT-790 arrests A549 lung cancer cell population growth by inducing mitochondrial reactive oxygen species production. Cell Prolif. 43: 103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Weinberg F., et al. 2010. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. U. S. A. 107: 8788–8793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wibbels T., Cowan J., LeBoeuf R. 1998. Temperature-dependent sex determination in the red-eared slider turtle, Trachemys scripta. J. Exp. Zool. 281: 409–416 [DOI] [PubMed] [Google Scholar]
- 69. Willy P. J., et al. 2004. Regulation of PPARγ coactivator 1α (PGC-1α) signaling by an estrogen-related receptor α (ERRα) ligand. Proc. Natl. Acad. Sci. U. S. A. 101: 8912–8917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wilson T. E., Fahrner T. J., Milbrandt J. 1993. The orphan receptors NGFI-B and steroidogenic factor 1 establish monomer binding as a third paradigm of nuclear receptor-DNA interaction. Mol. Cell. Biol. 13: 5794–5804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wise D. R., et al. 2008. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. U. S. A. 105: 18782–18787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wu F., et al. 2009. Estrogen-related receptor α (ERRα) inverse agonist XCT-790 induces cell death in chemotherapeutic resistant cancer cells. Chem. Biol. Interact. 181: 236–242 [DOI] [PubMed] [Google Scholar]
- 73. Wu M. 2007. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am. J. Physiol. Cell Physiol. 292: C125. [DOI] [PubMed] [Google Scholar]
- 74. Yang D., et al. 2010. Impairment of mitochondrial respiration in mouse fibroblasts by oncogenic H-RAS (Q61L). Cancer Biol. Ther. 9: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Young T. W., et al. 2004. Activation of antioxidant pathways in ras-mediated oncogenic transformation of human surface ovarian epithelial cells revealed by functional proteomics and mass spectrometry. Cancer Res. 64: 4577–4584 [DOI] [PubMed] [Google Scholar]
- 76. Yun J., et al. 2009. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science 325: 1555–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yuneva M., Zamboni N., Oefner P., Sachidanandam R., Lazebnik Y. 2007. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J. Cell Biol. 178: 93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang Y., et al. 2011. Kinase suppressor of Ras-1 protects against pulmonary Pseudomonas aeruginosa infections. Nat. Med. 17: 341–346 [DOI] [PubMed] [Google Scholar]