Abstract
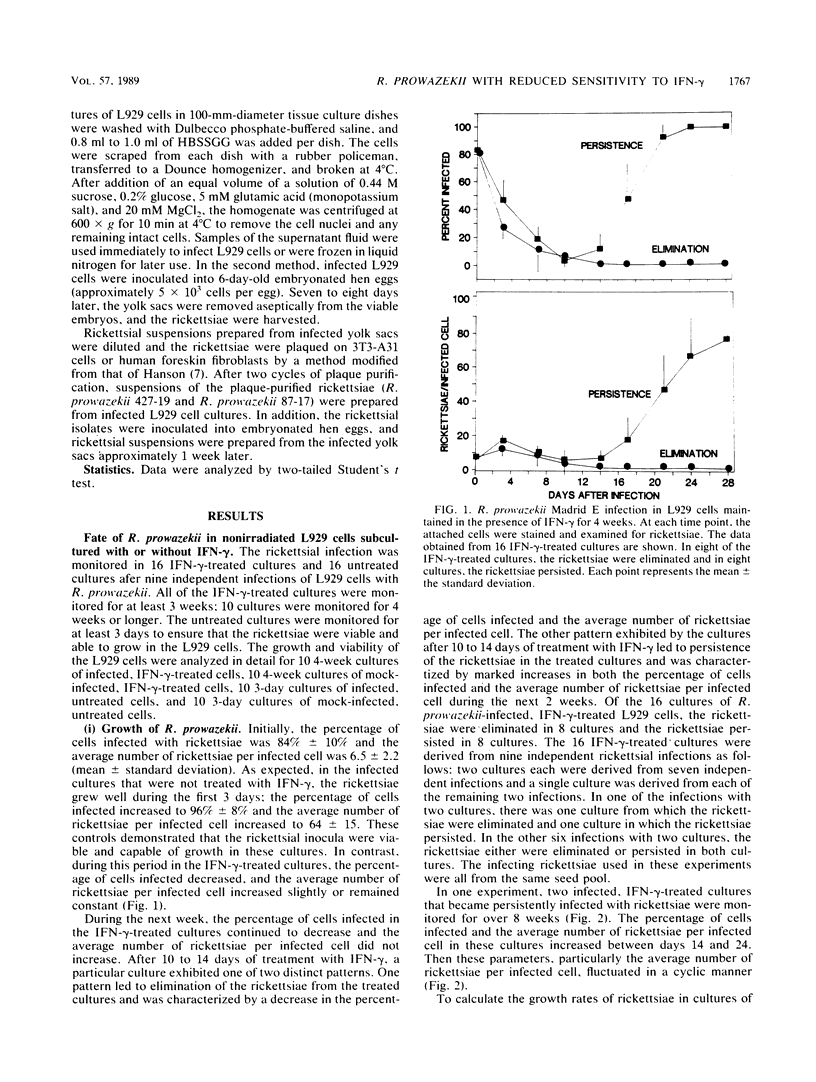
The growth of Rickettsia prowazekii Madrid E was monitored in mouse L929 cells subcultured for several weeks in the presence of gamma interferon (IFN-gamma) to determine whether the rickettsiae would be eliminated from or would persist in these cultures. R. prowazekii exhibited two distinct patterns in these IFN-gamma-treated cultures. In some cases, IFN-gamma-induced inhibition of rickettsial growth led to elimination of the rickettsiae from the L929 cell cultures; in other cases, the initial inhibition of rickettsial growth was followed by establishment of a persistent rickettsial infection in the IFN-gamma-treated L929 cells. During the first 3 days after infection, the growth rate of the L929 cells was significantly lower and higher percentages of the cells were killed in the IFN-gamma-treated, R. prowazekii-infected cultures than in the untreated, R. prowazekii-infected cultures or the mock-infected cultures, whether treated or untreated. This suppression of cell growth occurred in the infected, IFN-gamma-treated cultures that eventually exhibited the elimination pattern as well as the IFN-gamma-treated cultures that became persistently infected. It was not possible to predict the outcome of a particular infection from the early growth pattern of the culture. It was determined that the L929 cells in the persistently infected, IFN-gamma-treated cultures had not lost the ability to respond to IFN-gamma. These cells, after treatment with an antibiotic to eliminate the persistent rickettsiae, retained the ability to inhibit both the replication of vesicular stomatitis virus and the growth of R. prowazekii Madrid E after treatment with IFN-gamma. In contrast, rickettsiae isolated from two persistently infected, IFN-gamma-treated cultures were less sensitive than R. prowazekii Madrid E to the antirickettsial effects of IFN-gamma in standard L929 cells. The maintenance of the phenotype of these altered rickettsiae during plaque purification and passage in the absence of IFN-gamma suggests an alteration at the genetic level rather than phenotypic adaptation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balayera N. M., Nikdskaya V. N. Enhanced virulence of the vaccine strain E of Rickettsia prowazeke on passaging in white mice and guinea pigs. Acta Virol. 1972 Jun;16(1):80–82. [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. I. Multiplication of typhus rickettsiae in human macrophage cell cultures in the nonimmune system: influence of virulence of rickettsial strains and of chloramphenicol. Infect Immun. 1973 Oct;8(4):519–527. doi: 10.1128/iai.8.4.519-527.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson B. Improved plaque assay for Rickettsia tsutsugamushi. Am J Trop Med Hyg. 1987 May;36(3):631–638. doi: 10.4269/ajtmh.1987.36.631. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatovich V. F. Enhancement of the antigenic activity and virulence of the vaccine strain E of Rickettsia prow azeki by passages in cell culture. Acta Virol. 1975 Nov;19(6):481–485. [PubMed] [Google Scholar]
- Kazár J., Brezina R., Urvölgyi J. Studies on the E strain of Rickettsia prowazeki. Bull World Health Organ. 1973;49(3):257–265. [PMC free article] [PubMed] [Google Scholar]
- Li H., Jerrells T. R., Spitalny G. L., Walker D. H. Gamma interferon as a crucial host defense against Rickettsia conorii in vivo. Infect Immun. 1987 May;55(5):1252–1255. doi: 10.1128/iai.55.5.1252-1255.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Cloned mouse interferon-gamma inhibits the growth of Rickettsia prowazekii in cultured mouse fibroblasts. J Exp Med. 1983 Dec 1;158(6):2159–2164. doi: 10.1084/jem.158.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Comparison of the properties of antirickettsial activity and interferon in mouse lymphokines. Infect Immun. 1983 Oct;42(1):27–32. doi: 10.1128/iai.42.1.27-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Effect of mouse lymphokines and cloned mouse interferon-gamma on the interaction of Rickettsia prowazekii with mouse macrophage-like RAW264.7 cells. Infect Immun. 1984 Aug;45(2):303–308. doi: 10.1128/iai.45.2.303-308.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Gamma-interferon-induced inhibition of the growth of Rickettsia prowazekii in fibroblasts cannot be explained by the degradation of tryptophan or other amino acids. Infect Immun. 1986 Jul;53(1):38–46. doi: 10.1128/iai.53.1.38-46.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Inhibition of the growth of Rickettsia prowazekii in cultured fibroblasts by lymphokines. J Exp Med. 1983 Mar 1;157(3):974–986. doi: 10.1084/jem.157.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS E., DRESSLER H. R. Increased resistance to chloramphenicol in Rickettsia prowazekii with a note on failure to demonstrate genetic interaction among. J Bacteriol. 1962 Feb;83:409–414. doi: 10.1128/jb.83.2.409-414.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS E., DRESSLER H. R., SUITOR E. C., Jr Further studies of drug-resistant strains of Rickettsia prowazekii. J Bacteriol. 1959 Jan;77(1):91–100. doi: 10.1128/jb.77.1.91-100.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS E., DRESSLER H. R., SUITOR E. C., Jr Inhibition by acetylsalicylic acid of rickettsial strains resistant to p-aminobenzoic acid. J Bacteriol. 1959 Sep;78:432–440. doi: 10.1128/jb.78.3.432-440.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS E., DRESSLER H. R., SUITOR E. C., Jr Selection of mutant strain of Rickettsia prowazeki resistant to p-aminobenzoic acid. J Bacteriol. 1957 Mar;73(3):421–430. doi: 10.1128/jb.73.3.421-430.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS E., DRESSLER H. R. Selection of an erythromycin-resistant strain of Rickettsia prowazekii. Am J Hyg. 1960 May;71:292–298. doi: 10.1093/oxfordjournals.aje.a120113. [DOI] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Rickettsial hemolysis: rapid method for enumeration of metabolically active typhus rickettsiae. J Clin Microbiol. 1979 May;9(5):645–647. doi: 10.1128/jcm.9.5.645-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. The biology of rickettsiae. Annu Rev Microbiol. 1982;36:345–370. doi: 10.1146/annurev.mi.36.100182.002021. [DOI] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. Interferonlike factors from antigen- and mitogen-stimulated human leukocytes with antirickettsial and cytolytic actions on Rickettsia prowazekii. Infected human endothelial cells, fibroblasts, and macrophages. J Exp Med. 1983 Jun 1;157(6):1780–1793. doi: 10.1084/jem.157.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]


