Abstract
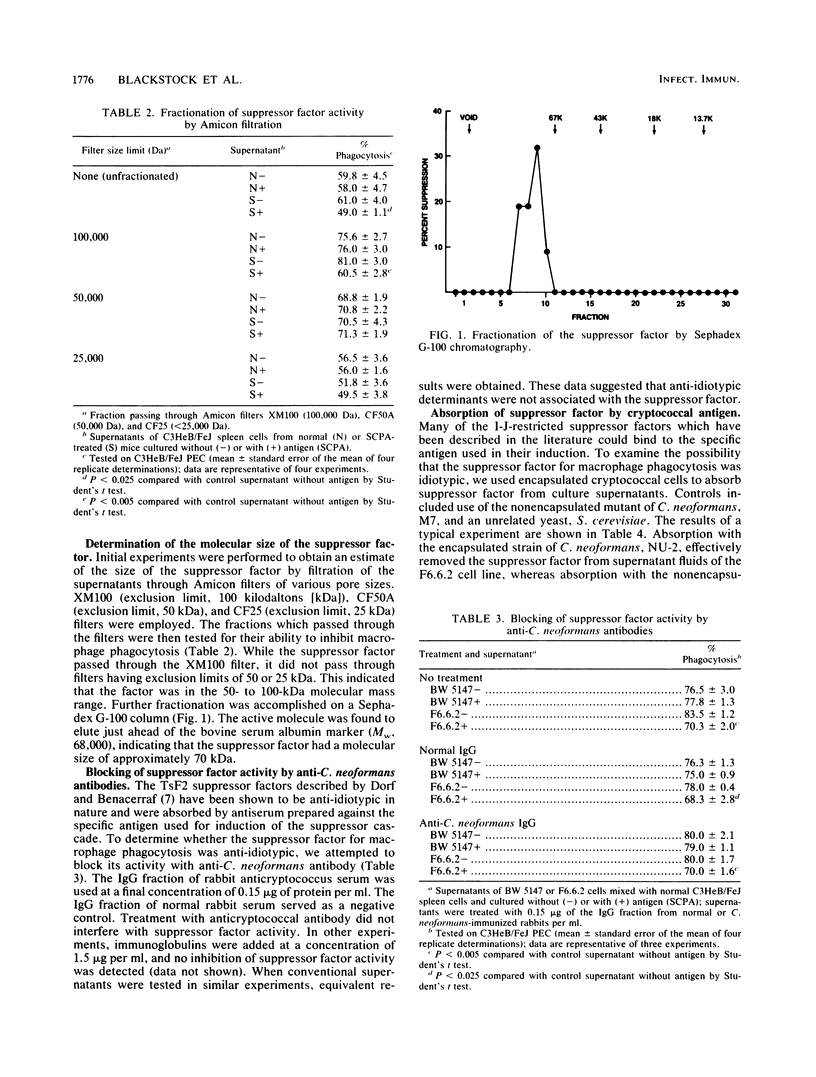
A T-suppressor factor which inhibits the phagocytic activity of a macrophage subset has been further characterized. This suppressor factor was first described for a murine model of cryptococcosis but was later found to be common to models of immunologic unresponsiveness. The suppressor factor was produced when suppressor cells were cultured in the presence of specific cryptococcal antigen. It could not be extracted from spleen cells and was not induced by antigen in cultures of lymph node cells. The suppressor factor was filtered through Amicon filters of 100-kilodalton (kDa) exclusion limit but was retained by filters excluding molecules of less than 50 kDa. By Sephadex G-100 chromatography, the factor eluted just ahead of bovine serum albumin (68 kDa). The activity of the suppressor factor could not be inhibited by anticryptococcal antibody, but it was inhibited by anti-I-J alloantiserum of the same genotype as the lymphocyte which produced the factor. Absorption with an encapsulated strain of Cryptococcus neoformans removed the suppressor factor from culture supernatants, while absorption with a nonencapsulated mutant or an unrelated yeast cell had not effect. On the basis of these observations, it was apparent that the suppressor factor was idiotypic in nature and that I-J and/or the I-J-interactive molecule played a role in the function of the suppressor factor. The requirement for antigenic stimulation for the production of suppressor factor in vitro distinguished it from the T-suppressor factor 3 described by others which regulates delayed-type hypersensitivity in cryptococcosis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Colizzi V., Zembala M. An overview of T-suppressor cell circuits. Annu Rev Immunol. 1986;4:37–68. doi: 10.1146/annurev.iy.04.040186.000345. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Dorf M. E., Colizzi V., Zembala M., James B. M. Equivalence of conventional anti-picryl T suppressor factor in the contact sensitivity system and monoclonal anti-NP TsF3: their final non-specific effect via the T acceptor cell. Immunology. 1984 Nov;53(3):491–497. [PMC free article] [PubMed] [Google Scholar]
- Blackstock R., Hernandez N. C. Inhibition of macrophage phagocytosis in cryptococcosis: phenotypic analysis of the suppressor cell. Cell Immunol. 1988 Jun;114(1):174–187. doi: 10.1016/0008-8749(88)90264-x. [DOI] [PubMed] [Google Scholar]
- Blackstock R., McCormack J. M., Hall N. K. Induction of a macrophage-suppressive lymphokine by soluble cryptococcal antigens and its association with models of immunologic tolerance. Infect Immun. 1987 Jan;55(1):233–239. doi: 10.1128/iai.55.1.233-239.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulmer G. S., Sans M. D. Cryptococcus neoformans. II. Phagocytosis by human leukocytes. J Bacteriol. 1967 Nov;94(5):1480–1483. doi: 10.1128/jb.94.5.1480-1483.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorf M. E., Benacerraf B. Suppressor cells and immunoregulation. Annu Rev Immunol. 1984;2:127–157. doi: 10.1146/annurev.iy.02.040184.001015. [DOI] [PubMed] [Google Scholar]
- Eckert T. F., Kozel T. R. Production and characterization of monoclonal antibodies specific for Cryptococcus neoformans capsular polysaccharide. Infect Immun. 1987 Aug;55(8):1895–1899. doi: 10.1128/iai.55.8.1895-1899.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidel P. L., Jr, Murphy J. W. Characterization of an in vitro-stimulated, Cryptococcus neoformans-specific second-order suppressor T cell and its precursor. Infect Immun. 1988 May;56(5):1267–1272. doi: 10.1128/iai.56.5.1267-1272.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaureguiberry B., Liao L., Kuchroo V., Dorf M. E., Diamond B. Monoclonal anti-idiotypic antibodies to an I-J interacting molecule inhibit suppression in an H-2 restricted way. J Immunol. 1988 May 15;140(10):3286–3289. [PubMed] [Google Scholar]
- Morgan M. A., Blackstock R. A., Bulmer G. S., Hall N. K. Modification of macrophage phagocytosis in murine cryptococcosis. Infect Immun. 1983 May;40(2):493–500. doi: 10.1128/iai.40.2.493-500.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W. Effects of first-order Cryptococcus-specific T-suppressor cells on induction of cells responsible for delayed-type hypersensitivity. Infect Immun. 1985 May;48(2):439–445. doi: 10.1128/iai.48.2.439-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. J Immunol. 1982 Jan;128(1):276–283. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. II. Characterization of first-order T suppressor cells (Ts1) and induction of second-order suppressor cells. J Immunol. 1983 Jun;130(6):2876–2881. [PubMed] [Google Scholar]
- Ptak W., Zembala M., Hanczakowski-Rewicka M., Asherson G. L. Nonspecific macrophage suppressor factor: its role in the inhibition of contact sensitivity to picryl chloride by specific T suppressor factor. Eur J Immunol. 1978 Sep;8(9):645–649. doi: 10.1002/eji.1830080908. [DOI] [PubMed] [Google Scholar]
- Robinson B. E., Hall N. K., Bulmer G. S., Blackstock R. Suppression of responses to cryptococcal antigen in murine cryptococcosis. Mycopathologia. 1982 Dec 27;80(3):157–163. doi: 10.1007/BF00437578. [DOI] [PubMed] [Google Scholar]
- Zembala M., Romano G. C., Colizzi V., Little J. A., Asherson G. L. Nonspecific T suppressor factor (nsTsF) cascade in contact sensitivity: nsTsF-1 causes an Ly-1+2- I-A+ immune T cell to produce a second, genetically restricted, nsTsF-2. J Immunol. 1986 Aug 15;137(4):1138–1143. [PubMed] [Google Scholar]


