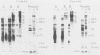Abstract
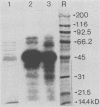
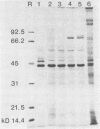
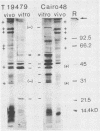
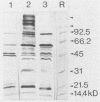
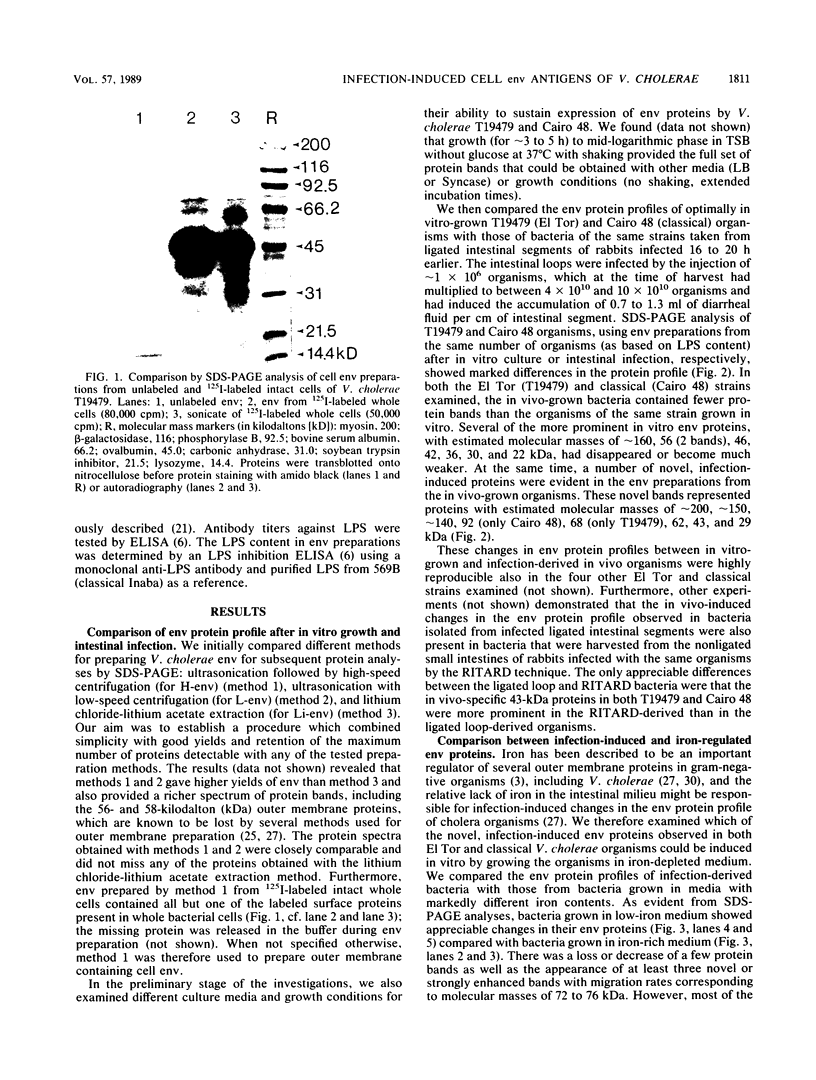
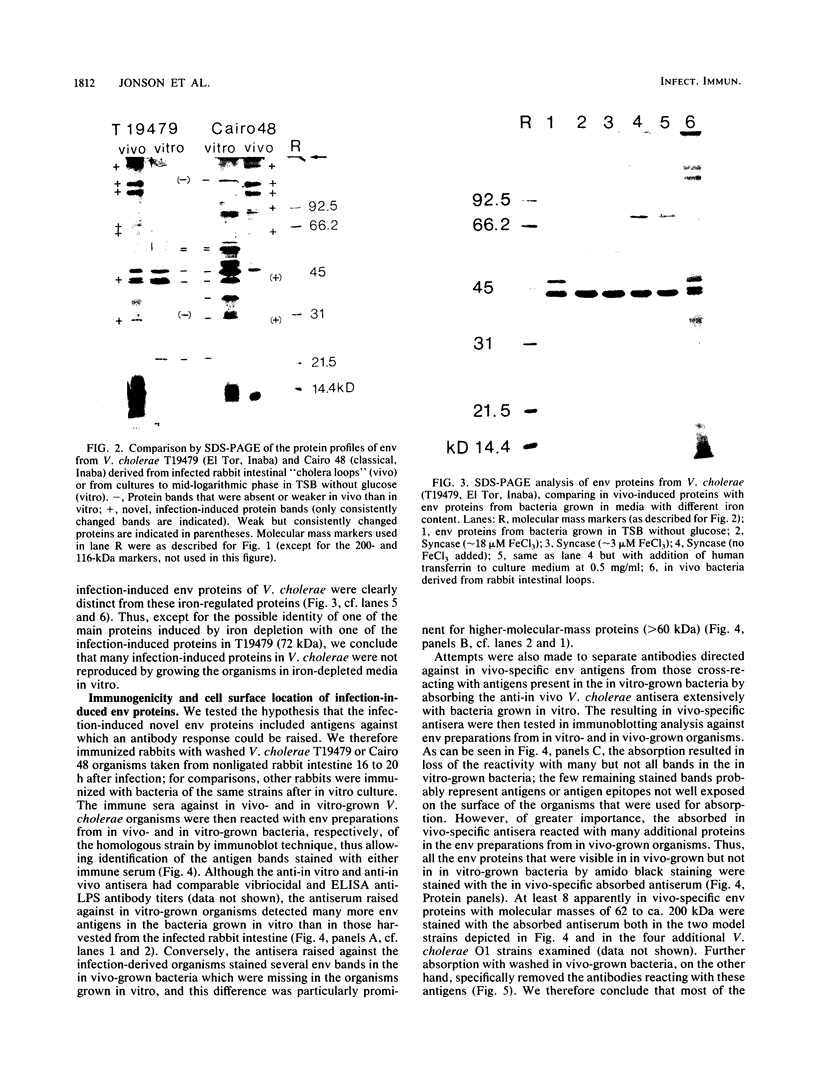
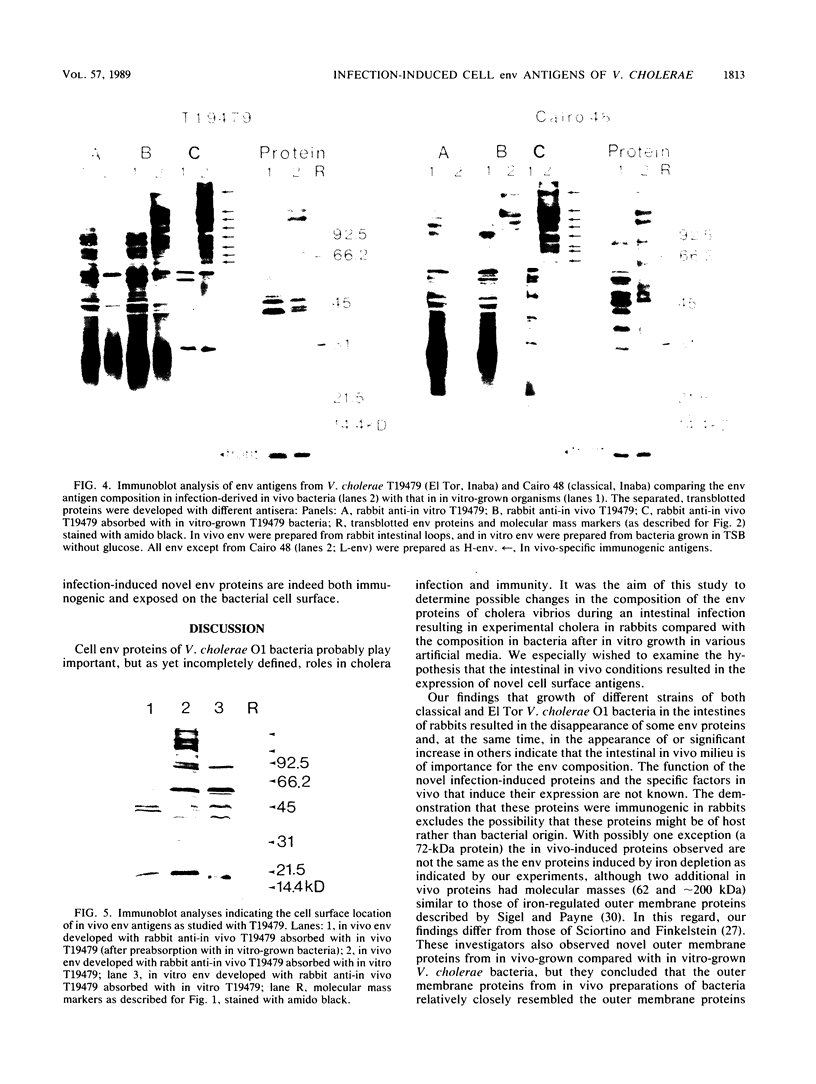
Vibrio cholerae O1 bacteria harvested directly from ligated or nonligated intestines of rabbits with experimental cholera expressed at least 7 to 8 novel, in vivo-specific cell envelope (env) proteins that were not found on vibrios after in vitro culture in various ordinary liquid media. At the same time, several of the env proteins ordinarily expressed in vitro had disappeared or become much reduced. The infection-induced novel env protein were immunogenic. In immunoblot analyses, antisera raised against in vivo-grown vibrios and then absorbed with in vitro-grown bacteria of the same strain specifically stained at least eight infection-induced antigens ranging from 62 to approximately 200 kilodaltons; absorption with washed in vivo-grown bacteria, on the other hand, removed the antibodies reacting with these antigens, indicating that the antigens were present on the bacterial cell surface. Conversely, antiserum against in vitro-grown bacteria reacted with several env antigens in in vitro-grown bacteria that were missing in the infection-derived vibrios. These adaptational changes were strikingly similar for different strains of cholera vibrios of both classical and El Tor biotypes. Most of the in vivo-specific proteins (with apparent molecular masses of approximately 200, approximately 150, approximately 140, 92, 68, 62, 43, and 29 kilodaltons) were not induced during cultivation of bacteria in iron-depleted medium and are probably not related to the iron-regulated env proteins known to be involved in iron transport systems.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Atthasampunna P., Chulasamaya M., Charunmethee P. Pathogenesis of experimental cholera: biologic ativities of purified procholeragen A. J Immunol. 1966 Mar;96(3):440–449. [PubMed] [Google Scholar]
- Hanne L. F., Finkelstein R. A. Characterization and distribution of the hemagglutinins produced by Vibrio cholerae. Infect Immun. 1982 Apr;36(1):209–214. doi: 10.1128/iai.36.1.209-214.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder I. A., Haidaris C. G. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: extracellular protease and elastase as in vivo virulence factors. Can J Microbiol. 1979 May;25(5):593–599. doi: 10.1139/m79-085. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Enzyme-linked immunosorbent assays for cholera serology. Infect Immun. 1973 May;7(5):759–763. doi: 10.1128/iai.7.5.759-763.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga M., Kuyyakanond T. Large production of cholera toxin by Vibrio cholerae O1 in yeast extract peptone water. J Clin Microbiol. 1987 Dec;25(12):2314–2316. doi: 10.1128/jcm.25.12.2314-2316.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonson G., Sanchez J., Svennerholm A. M. Expression and detection of different biotype-associated cell-bound haemagglutinins of Vibrio cholerae O1. J Gen Microbiol. 1989 Jan;135(1):111–120. doi: 10.1099/00221287-135-1-111. [DOI] [PubMed] [Google Scholar]
- Kelley J. T., Parker C. D. Identification and preliminary characterization of Vibrio cholerae outer membrane proteins. J Bacteriol. 1981 Feb;145(2):1018–1024. doi: 10.1128/jb.145.2.1018-1024.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusama H., Craig J. P. Production of Biologically Active Substances by Two Strains of Vibrio cholerae. Infect Immun. 1970 Jan;1(1):80–87. doi: 10.1128/iai.1.1.80-87.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lycke N., Svennerholm A. M., Holmgren J. Strong biotype and serotype cross-protective antibacterial and antitoxic immunity in rabbits after cholera infection. Microb Pathog. 1986 Aug;1(4):361–371. doi: 10.1016/0882-4010(86)90068-9. [DOI] [PubMed] [Google Scholar]
- Lång H. A., Palva E. T. A major outer membrane protein in Vibrio cholerae is maltose-inducible. Microb Pathog. 1987 Aug;3(2):143–147. doi: 10.1016/0882-4010(87)90072-6. [DOI] [PubMed] [Google Scholar]
- MCINTYRE O. R., FEELEY J. C. PASSIVE SERUM PROTECTION OF THE INFANT RABBIT AGAINST EXPERIMENTAL CHOLERA. J Infect Dis. 1964 Dec;114:468–475. doi: 10.1093/infdis/114.5.468. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Bartowsky E. J., Leavesly D. I., Hackett J. A., Heuzenroeder M. W. Molecular cloning using immune sera of a 22-kDal minor outer membrane protein of Vibrio cholerae. Gene. 1985;34(1):95–103. doi: 10.1016/0378-1119(85)90299-9. [DOI] [PubMed] [Google Scholar]
- Maurelli A. T., Blackmon B., Curtiss R., 3rd Temperature-dependent expression of virulence genes in Shigella species. Infect Immun. 1984 Jan;43(1):195–201. doi: 10.1128/iai.43.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver J., Grady G. F., Keusch G. T. Production and characterization of exotoxin(s) of Shigella dysenteriae type 1. J Infect Dis. 1975 May;131(5):559–566. doi: 10.1093/infdis/131.5.559. [DOI] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson K., Parker C. D. Identification and characterization of Vibrio cholerae surface proteins by radioiodination. Infect Immun. 1985 Apr;48(1):87–93. doi: 10.1128/iai.48.1.87-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S. H. Factors influencing in vitro skin permeability factor production by Vibrio cholerae. J Bacteriol. 1969 Oct;100(1):27–34. doi: 10.1128/jb.100.1.27-34.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciortino C. V., Finkelstein R. A. Vibrio cholerae expresses iron-regulated outer membrane proteins in vivo. Infect Immun. 1983 Dec;42(3):990–996. doi: 10.1128/iai.42.3.990-996.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciortino C. V., Yang Z. S., Finkelstein R. A. Monoclonal antibodies to outer membrane antigens of Vibrio cholerae. Infect Immun. 1985 Jul;49(1):122–131. doi: 10.1128/iai.49.1.122-131.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears S. D., Richardson K., Young C., Parker C. D., Levine M. M. Evaluation of the human immune response to outer membrane proteins of Vibrio cholerae. Infect Immun. 1984 May;44(2):439–444. doi: 10.1128/iai.44.2.439-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel S. P., Payne S. M. Effect of iron limitation on growth, siderophore production, and expression of outer membrane proteins of Vibrio cholerae. J Bacteriol. 1982 Apr;150(1):148–155. doi: 10.1128/jb.150.1.148-155.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples S. J., Asher S. E., Giannella R. A. Purification and characterization of heat-stable enterotoxin produced by a strain of E. coli pathogenic for man. J Biol Chem. 1980 May 25;255(10):4716–4721. [PubMed] [Google Scholar]
- Sullivan K. H., Williams R. P. Use of iodo-gen and iodine-125 to label the outer membrane proteins of whole cells of Neisseria gonorrhoeae. Anal Biochem. 1982 Mar 1;120(2):254–258. doi: 10.1016/0003-2697(82)90344-x. [DOI] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Broadbent D. A. Biochemical characterization of extracellular proteases from Vibrio cholerae. Infect Immun. 1982 Sep;37(3):875–883. doi: 10.1128/iai.37.3.875-883.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]