Abstract
The protein transporter, anthrax lethal toxin, is comprised of protective antigen (PA), a transmembrane translocase, and lethal factor (LF), a cytotoxic enzyme. Following assembly into holotoxin complexes, PA forms an oligomeric channel that unfolds LF and translocates it into the host cell. We report the crystal structure of the core of a lethal toxin complex to 3.1-Å resolution; the structure contains a PA octamer bound to four LF PA-binding domains (LFN). The first α helix and β strand of each LFN unfold and dock into a deep amphipathic cleft on the surface of the PA octamer, which we call the α clamp. The α clamp possesses nonspecific polypeptide binding activity and is functionally relevant to efficient holotoxin assembly, PA octamer formation, and LF unfolding and translocation. This structure provides insight on the mechanism of translocation-coupled protein unfolding.
Protein secretion and degradation are essential cellular processes that allow for protein trafficking, organelle biogenesis, protein quality control, and cell-cycle regulation1–3. Since folded proteins are thermodynamically stable under typical conditions, these processes often require complex, energy-consuming molecular machines3–6, which catalyze a series of unfolding and translocation reactions7–13. Anthrax toxin5,14, a three-protein virulence factor secreted by Bacillus anthracis, is an example of such a transmembrane protein delivery system (Supplementary Fig. 1 online). This bacterial toxin follows the classical two-component AB paradigm, where the A component is an active enzyme that localizes to and enters cells by forming complexes with the cell-binding, or B component. Anthrax toxin is composed of two A components, LF (91 kDa) and edema factor (EF, 89 kDa), and one B component, PA (83 kDa). Therefore, two different toxic complexes can form: lethal toxin (LT, PA plus LF) and edema toxin (ET, PA plus EF). LT (which we focus on herein) causes macrophage lysis15, immune system suppression16, and death14.
For LT to inflict its cytotoxic effects, PA and LF must assemble into active holotoxin complexes, which can translocate LF into host cells (Fig. 1a). Proteases present either on host-cell surfaces or in blood serum potentiate LT assembly by proteolytically nicking PA, yielding nPA17–19. Dissociation of a 20-kDa amino-terminal fragment from nPA exposes LF-binding sites, permitting assembly. The resulting LT complex contains multiple copies of LF bound to either a ring-shaped PA homoheptamer, PA7 (refs. 18–21), or homooctamer, PA8 (ref. 19). Octameric PA forms more robust LT complexes than heptameric PA under physiological conditions22. The crystal structures of the individual PA and LF monomers20,23 and the assembled PA heptamer24 and octamer19 are known. However, an atomic-resolution X-ray crystal structure of a lethal toxin co-complex has not been described.
Figure 1. The structure of LF’s PA-binding domain in complex with the PA octamer.
(a) An overview of LT assembly and LF translocation. LF [1J7N23 (pink) with LFN (red)] and a PA63 subunit [3HVD19 (light blue) with D1′ (blue)]. LF and PA63 co-assemble into either heptameric (PA7LF3) or octameric (PA8LF4) LT complexes. The PA8LF4 complex depicted is verified by mass spectrometry (Supplementary Fig. 2a online) and based upon structural data presented herein. LT is endocytosed; the endosome is acidified, causing PA to form a β-barrel channel25; LF translocates through the channel under a ΔpH/Δψ-driving force to enter the cytosol; and LF then disrupts normal cellular physiology by cleaving mitogen-activated kinase kinases50. (The channel depicted is a model intended for illustration purposes only.) (b) (Left) Ribbons depiction of the PA2LFN ternary complex. PAC (chain A, blue), PAN (chain B, green), LFN (chain C, red), and calcium ions (gray spheres). (Right) Slices through a surface rendering of the two LFN-binding subsites: (top) the carboxy-terminal binding subsite and (bottom) the α-clamp subsite. (c) Axial rendering of the biological unit, the PA8(LFN)4 complex, colored as in (b). The PA octamer is a molecular surface; and LFN’s helices and strands are cylinders and planks, respectively. The structure is produced from chains A, B and C, using the C4 symmetry axis, which is parallel to the c edge of the unit cell at (−½a, 0b). (d) LFN α1/β1 binds the α-clamp subsite formed at the interface of two PA subunits, driving the assembly of dimeric and tetrameric PA intermediates19, which in turn form PA8 complexes.
After the LT complex is endocytosed, the PA oligomer transforms into a transmembrane, β-barrel channel25 through which LF translocates to enter the cytosol. Due to the narrowness of the channel, LF unfolds during translocation. The acidic endosomal pH conditions required for toxin action15 not only aid in the destabilization of LF26 but also drive further LF unfolding9 and translocation by means of a proton motive driving force7. This driving force is comprised of a proton gradient (ΔpH) and membrane potential (Δψ). Efficient coupling of the ΔpH requires a catalytic active site in the channel, called the ϕ clamp, composed of a narrow ring of phenylalanine residues7,8. The ϕ clamp forms a narrowly apposed substrate clamping site in the central lumen of the PA channel8, and it allows the channel to catalyze unfolding9 and translocation8 presumably by forming transient interactions with the unfolded translocating chain8.
Many, but not all, protein processing machines that translocate, unfold and/or refold proteins utilize analogous polypeptide clamping features to denature a protein and engage with its unfolded structure. The features that bind to unstructured or unfolded polypeptides include hydrophobic/aromatic pore loops8,11,27–29, polypeptide clamping sites8,30, and other substrate binding clefts or adapters31–33. Some of these machines utilize tandem polypeptide binding sites8,9,31: one site is a substrate docking site, which feeds into a second hydrophobic site found deeper within the pore. Questions surround the mechanism of these clamping sites and their interactions with unfolded substrates. How do these sites unfold proteins? How do they process the wide chemical complexity and configurational flexibility contained in an unfolding substrate? These questions have remained unanswered, in part because atomic resolution structures of unfolding intermediates in complex with these clamps have not been described. Here we report a structure of a partially unfolded substrate, the PA-binding domain of LF, in complex with its unfolding machine, the PA oligomer.
RESULTS
Crystal structure of the PA8(LFN)4 complex
For these crystallographic studies, we focused on the PA8 oligomer, considering its enhanced thermostability as well as its advantageous fourfold, square-planar symmetry19. By mass spectrometry (MS), we find that the PA8LF4 complex is physiologically relevant, as it assembles from the full-length, wild-type (WT) PA and LF subunits (Supplementary Fig. 2a online). Our best diffracting crystals contain LFN (LF residues 1-263) and a PA construct lacking its membrane-insertion loop19, which is superfluous to the known PA-LFN interaction34. LFN, the minimal portion of LF that specifically binds PA35, can translocate heterologous domains as amino- or carboxy-terminal fusions into cells36,37. EF contains a homologous PA-binding domain, and the PA-LFN interaction is likely general to LT and ET complexes38. Homogenous PA8(LFN)4 complexes (Supplementary Fig. 2b online) form crystals in the P4212 space group that diffract X-rays to 3.1 Å (Table 1). Molecular replacement solutions indentify two PA2 complexes and significant (2.7σ) unassigned electron density (Fo–Fc) for α helices located proximal to the domain 1′ (D1′) surface of each PA2 complex. Rounds of polyalanine-helix modeling and refinement reveal that the novel helical density aligns well with α2, α4, α9, and α10 of LFN. The two occurrences of the PA2LFN ternary complex (Fig. 1b) in the asymmetric unit are structurally identical; its PA subunits are structurally similar to the full-length PA monomer20 and the PA subunits observed in the PA7 and PA8 prechannel oligomers19,24. Thus the biological unit—the PA8(LFN)4 prechannel complex (Fig. 1c)—is comprised of four PA2LFN ternary complexes (Fig. 1d).
Table 1.
Data collection and refinement statistics
| PA8(LFN)4a | |
|---|---|
| Data collection | |
| Space group | P4212 |
| Cell dimensions | |
| a, b, c (Å) | 178.38, 178.38, 240.36 |
| α, β, γ (°) | 90, 90, 90 |
| Resolution (Å) | 49.8-3.1(3.2-3.1)b |
| Rp.i.m. | 6.9(46.0) |
| I / σI | 11.4(2.2) |
| Completeness (%) | 92.0(78.0) |
| Redundancy | 7.9(8.0) |
| Refinement | |
| Resolution (Å) | 49.8-3.1 |
| No. reflections | 65, 165 |
| Rwork / Rfree | 24.9/ 28.1 |
| No. atoms | |
| Protein | 20,397 |
| Ligand/ion | 8 |
| Water | 4 |
| B-factors | |
| Protein | 100.7 |
| Ligand/ion | 53.3 |
| Water | 56.7 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.005 |
| Bond angles (°) | 0.610 |
Data for this complex were collected from a single crystal.
Values in parentheses are for the highest-resolution shell.
Interestingly, LFN α1/β1 (residues 29–50) unfolds and adopts a novel conformation relative to free LF (1J7N23). LFN α1/β1 docks in the cleft formed between adjacent PA subunits and aligns well with the experimental electron density (Fig. 2a,b). We can assign this unique conformation of α1/β1 since it extends from LFN α2 as a contiguous stretch of electron density contoured at σ=1 (Supplementary Fig. 3a online). LFN’s carboxy terminus also reveals well-defined electron density (Fig. 2c). Overall, LFN excludes 1900 Å2 of solvent accessible surface area (SASA) on the PA dimer. This surface is comprised of two discontinuous LFN-binding subsites (Fig. 1b) formed by adjacent PA subunits, termed PAN and PAC (to reflect whether the PA subunit interacts primarily with the amino terminus or carboxy terminus of LFN, respectively). The details of these respective subsites, called the α-clamp binding subsite and the carboxy-terminal binding subsite, are depicted in Figure 3a,b. Thus upon binding the PA oligomer, LFN partially unfolds, whereby its first α helix and β strand (i) separate from the main body of the protein, (ii) dock into the cleft between two adjacent PA subunits (Fig. 1b), and (iii) orient toward the center of the PA oligomer lumen (Fig. 1c).
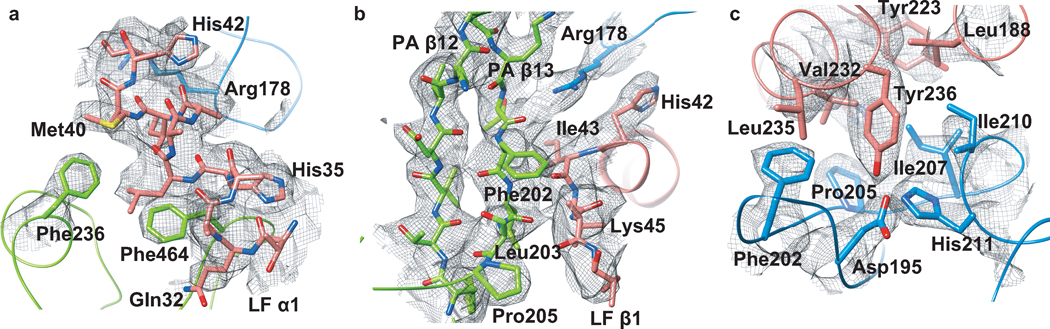
Figure 2. LFN electron density in the PA8(LFN)4 complex.
A composite simulated-annealing (SA) omit map calculated in PHENIX51 to 3.1 Å contoured at σ=1 (gray mesh). The models of PAN, PAC and LFN are rendered in green, blue, and red, respectively. Secondary structure elements and individual residues are labeled. (a) LFN α1 (residues 31–42) in complex with PAN. Lysine and glutamate residues are truncated to Cβ for clarity. (b) LFN α1 in complex with PAN β12–13. LFN Lys45 is truncated to Cβ for clarity. (c) LFN’s carboxy-terminal binding subsite interaction with PAC. Additional stereo-pair images of LFN omit maps following SA refinement are depicted in Supplementary Figure 3 online.
Figure 3. The PA octamer binds LFN in two distinct subsites.
Detailed views of (a) the α-clamp binding subsite and (b) the carboxy-terminal binding subsite are depicted. Highlighted non-covalent interactions are indicated with red dashed lines. Chains and Ca2+ ions are colored as in Figure 1b. Changes in equilibrium binding free energy (ΔΔG) for PA channel complexes, comparing (c) site-directed mutants of PA, (d) site-directed mutants of LFN, and (e) Δn LFN amino-terminal truncation mutants. In (c–e), the reference state is WT LFN:WT PA. (f) (Left) LFN α1/β1-replacement mutant binding to WT PA; ΔΔG values are referenced to WT LFN. (Right) LF1–20-DTA, LF1–60-DTA, Δ47 LFN and LFN α1/β1-replacement mutant binding to PA R178A; ΔΔG values are referenced to WT PA. LFN α1/β1-replacement mutants either include multiple point mutations in the α1/β1 sequence (32QEEHLKEIMKHIVK46I) or replacements of the α1/β1 sequence with other sequences from LF or EF. The name, replacement sequence, and sequence identity (%) are listed for each: LFα14, SEEGRGLLKKLQI (23%); LFα28, NSKKFIDIFKEEG (23%); EFα1, EKEKFKDSINNLV (31%); hydrophilic sequence 1 (HS1), QEEHSKEISKHSVKS (73%); aromatic sequence 1 (ArS1), QEEHFKEIFKHFVKF (73%). See Supplementary Figure 7 online for alignments and helical-wheel depictions of the α1/β1-replacement sequences. In (c–f), ΔΔG = RT ln KdMUT / KdWT, where the equilibrium dissociation constants (Kd) were measured for the mutant (MUT) and WT proteins at pH 7.4, Δψ = 0 mV (Supplementary Fig. 6 online); R is the gas constant; and T is the temperature. The error bars are the mean ±s.d. (n = 2–6).
The carboxy-terminal binding subsite
At the carboxy-terminal subsite, LFN’s carboxy-terminal subdomain excludes ~900 Å2 on PAC (Fig. 3b). The structure reveals a hydrophobic interface, involving PAC Phe202, Pro205, Ile207, and Ile210 and LF Val232, Leu235, His229, Tyr223, Leu188, and Tyr236. In particular, LF Tyr236 is well packed against PAC Ile210 (Fig. 2c) and its phenol hydroxyl forms a hydrogen-bonding network with PAC His211 and Asp195 near the center of the hydrophobic interface (Fig. 3b). Additional electrostatic interactions surround this hydrophobic core. The carboxyl side chain of PAC Glu190 forms a pair of hydrogen bonds with both the γ hydroxyl and amide nitrogen of LF Thr141; PAC Lys197, Lys213, Lys214 and Lys218 form salt bridges with LF Asp182, Asp187, Asp184, and Glu142, respectively; and PAN Arg200 forms a salt bridge with LF Glu139. PA and LF residues localized in this binding subsite are corroborated by mutagenesis studies, probing binding (Fig. 3c,d), assembly/binding (Supplementary Fig. 4a online),34,38–41 and cytotoxicity41 (Supplementary Fig. 4b,c online).
The α-clamp binding subsite
At the α-clamp subsite, PAN and PAC interact with LFN’s unfolded α1 and β1 structures (Fig. 3a). Remarkably, hydrogen bonds lost upon LFN unfolding are reformed on the surface of PA: LFN α1 maintains a similar helical conformation; and LFN β1 (Ile43 and Lys45) forms parallel β-sheet hydrogen bonds with Leu203 in PAN β13 (Fig. 2b). PAN Pro205, which is positioned at the end of PAN β13, terminates the parallel-sheet interactions with LFN β1. Overall, LFN α1/β1 excludes 1000 Å2 of SASA on PA. LFN α1 is docked deep into the α-clamp cleft at the interface of adjacent PA subunits (Figs. 1b and 3a). Reminiscent of calmodulin complexes with peptide helices42,43, PA’s twin Ca2+-binding sites scaffold the cleft and define its distinct shape and chemical character, including: (i) a delocalized anionic potential created by the excess of negatively-charged PA residues chelating the two Ca2+ ions and (ii) a large proportion of SASA contributed by PA backbone atoms. LFN’s side chains are not well-packed with side chains in the α-clamp cleft, in contrast to the carboxy-terminal binding subsite (Fig. 3a,b). Interestingly, PA contacts the side chains of LF Met40 and His35 through backbone interactions. PAC Arg178 contacts the hydrophilic face of α1 at LF His42 while maintaining a hydrogen bond with the backbone carbonyl of PAN Thr201. Aromatic residues, PAN Phe236 and Phe464, and aliphatic residues, PAN Leu187 and Leu203, line the cleft face opposite of PAC Arg178. Upon binding LFN, PAN Phe202 repositions its phenyl group toward LFN β1, shielding β1’s backbone hydrogen bonds with PAN Leu203. The chemical nature of the α-clamp cleft suggests that it is well-suited to bind an unfolded β strand and an amphipathic helix with a positively-charged face.
Both LF-binding subsites are critical for cytotoxicity activity
We initially characterized the PA-LF binding interaction using cytotoxicity assays. Site-directed mutagenesis studies on PA and LF residues involved in either binding subsite reveal defects in LT-induced macrophage cytolysis (Supplementary Fig. 4b,c online). To further address the interaction between LFN’s α1/β1 sequence and the α clamp, we created fusions of the first 20 or 60 residues of LF and the A fragment from diphtheria toxin (DTA), called LF1–20-DTA and LF1–60-DTA, respectively. When co-administered with PA, we find LF1–60-DTA is 100-fold more cytotoxic than LF1–20-DTA or hexahistadine-tagged DTA (His6-DTA, DTA with an amino-terminal, 18-residue leader containing the hexahistidine sequence, Supplementary Fig. 4d online). Interestingly, despite lacking the α1/β1 sequence, His6-DTA44 and LF1–20-DTA are cytotoxic when co-treated with WT PA (Supplementary Fig. 4d online); however, all of these DTA constructs are ~1000-fold less cytotoxic when co-treated with the α-clamp mutant, PA R178A (Supplementary Fig. 4e online). Thus the α clamp has broad substrate specificity. However, the role of the α1/β1-α-clamp interaction in toxin function is difficult to surmise from cytotoxicity assays alone, since toxin uptake involves multiple steps (e.g., PA assembly, LF binding, unfolding and translocation).
The role of the α clamp in LT assembly
To determine the role of the α clamp in LT assembly, we performed multiple in vitro PA-LFN assembly assays. By native PAGE, we find that PA mutations introduced into the LFN-PA-binding interface disrupt PA co-assembly with LFN (Supplementary Fig. 4a online). To focus on the role of LFN α1/β1 in PA assembly, we labeled PA K563C with two different fluorescent probes. A 1:1 ratiometric mixture of these labeled nPA K563C constructs (nPA*) produces an increase in fluorescence resonance energy transfer (FRET) upon assembly with LFN45. Using this FRET assay, we find that 5-fold more nPA* assembles with WT LFN than with the Δ47 LFN amino-terminal truncation (which lacks both α1 and β1, Supplementary Fig. 5a online). The circular dichroism (CD) spectra of Δ47 and WT LFN are comparable, demonstrating that the assembly defect is not due to the misfolding of Δ47 LFN (Supplementary Fig. 5b online). Using electron microscopy (EM), native PAGE, and MS, we find that the percentage of octameric PA oligomers is greatly reduced for Δ47 LFN relative to WT LFN (Supplementary Fig. 5c–e online). By EM, we estimate that ~3% of the PA oligomers produced with Δ47 LFN are octameric (10-fold less than that observed with WT LFN, Supplementary Fig. 5d online). Thus LFN’s α1 and β1 structures not only drive PA oligomerization, but also they are critical to the mechanism of PA octamer formation (Fig. 1d).
Mapping the LFN-binding interaction with the PA channel
Using electrophysiology, we measure LFN binding by observing kinetic and equilibrium changes in channel conductance8 (Supplementary Fig. 6a–c online); i.e., when LFN binds to the PA channel, it inserts its amino-terminal end into the channel and blocks conductance. We monitor binding in the absence of an applied Δψ to eliminate its influence on the channel-substrate interaction. Since PA7 and PA8 have similar translocation19 and cell cytotoxicity22 activities, we use the PA7 oligomer to maintain consistency with prior reports.7–9 To determine the overall thermodynamic contribution of LFN α1/β1, we made a series of additional Δn LFN amino-terminal truncations (where n is the number of deleted residues). These Δn LFN do not block PA channel conductance, as they lack sufficient unfolded/unstructured sequence on their amino termini. We use a competition assay to measure Δn LFN binding: first we block PA channel conductance with WT LFN (~100 pM); then we add the competitor Δn LFN and monitor the restoration of the conductance (Supplementary Fig. 6d,e online). We find that Δ42 and Δ47 LFN reduce WT PA-channel-binding affinity by 3.6–3.8 kcal mol−1 relative to WT LFN (Fig. 3e). However, since Δ27, Δ32, and Δ39 LFN destabilize the complex by about 1.2–1.4 kcal mol−1, the α1/β1 interaction is worth ~2.5 kcal mol−1. We assume that downstream interactions within the channel provide the additional ~1 kcal mol−1 of stabilization. We conclude that LFN α1/β1 binds to the PA channel and provides substantial stabilization of the PA-LFN complex.
To investigate the details of the interaction between the PA channel and LFN, we engineered point mutations into residues localized in either LFN binding subsite and estimated their relative energetic contribution to channel binding (Fig. 3c,d). Several mutations localized in the carboxy-terminal binding subsite, PA R200S, I207S, and H211A, disrupt LFN binding by 1–1.5 kcal mol−1. These residues form two binding “hotspots”, i.e., locations where point mutations disrupt binding most severely46. By contrast, the mutations, F202S and P205S, located between these two carboxy-terminal-site hotspots have minimal effects on LFN binding, reflecting that LFN’s carboxy terminus does not make substantial contact with these residues (Fig. 3b). The LFN Y236A mutant most appreciably perturbs PA-channel binding and represents the LFN hotspot in the carboxy-terminal subsite interaction. Other adjacent LFN residues in the carboxy-terminal subsite interaction have minimal effects on PA channel binding.
We then investigated the relative energetic contribution of residues localized in the α-clamp binding subsite (Fig. 3c,d). We find that PA Arg178 comprises the major hotspot site in PA’s α clamp, where the R178A mutation destabilizes the complex by 2.9 kcal mol−1. While the aromatic PA mutant, F464S, destabilizes LFN binding at the α-clamp site by 0.7 kcal mol−1, the PA F236S mutant does not. Additionally, we find that none of 23 point mutations introduced into LFN α1 and β1 destabilizes the LFN-PA channel complex. Interestingly, the mutation, LFN M40A, stabilizes the complex 1.3 kcal mol−1 (Fig. 3d). These results indicate contrasting binding energetic behaviors for the two different LFN-binding subsites. At the carboxy-terminal subsite, a classical interface is observed, where specific LFN and PA side chains comprise the respective hotspots on either interface. At the α-clamp subsite, while we identify PA Arg178 as a major hotspot residue, no clear hotspot can be identified on LFN α1/β1. These observations suggest that the stabilizing interactions in the α-clamp subsite do not involve specific LFN side chains, but rather the ~2.5 kcal mol−1 of binding stabilization is due to the formation of nonspecific contacts and the more general exclusion of SASA.
The PA α clamp possesses nonspecific binding activity
The robustness of the binding interaction is intriguing given the paucity of specific α-clamp interactions. To test the specificity of the α-clamp interaction, we either replaced the entire LFN α1/β1 sequence with other non-homologous sequences from LF and EF or introduced multiple mutations into α1/β1 (Supplementary Fig. 7 online). Interestingly, we find that these LFN α1/β1 replacements bind with similar affinities as WT LFN (differing by 0.2 to 1.0 kcal mol−1, Fig. 3f). Furthermore, multisite LFN mutants in which the buried hydrophobic face of α1/β1 is replaced with either four Ser residues (LFN HS1) or four Phe residues (LFN Ar1) bind PA with similar affinity as WT LFN (Fig. 3f), indicating that the α clamp also binds non-amphipathic helices. Finally, we find that these LFN α1/β1-replacement constructs bind 1.3–2.4 kcal mol−1 less tightly to PA R178A relative to WT PA (Fig. 3f), thereby confirming that this nonspecific-binding activity is localized to the α-clamp subsite. Thus the α clamp binds a broad array of sequences, providing 1.5–4 kcal mol−1 of stabilization (depending upon the identity of the α1/β1 sequence).
LFN must unfold to bind the α-clamp subsite
Our crystal structure and thermodynamic binding data indicate that the α-clamp subsite binds nonspecifically to unfolded protein substrates. This model is well supported by several additional lines of evidence. First, the thermodynamic comparison of WT LFN and the truncated Δn LFN mutants is appropriate because these mutants have similar folded secondary structure content as WT LFN (Supplementary Fig. 5b online). Moreover, the Δ47 LFN construct binds similarly to PA R178A as WT PA (Fig. 3f), confirming that the Δ47 LFN truncation does not bind at the α-clamp site, as implied by the structure (Fig. 1b). Second, fusions of LF’s amino terminus and DTA (LF1–60-DTA and LF1–20-DTA) are sufficient to bind to the α-clamp site, since their affinity for the PA channel is disrupted by the PA R178A mutation (Fig. 3f and Supplementary Fig. 8 online). This result indicates that the α clamp is an independent binding site capable of binding to unstructured sequences at the amino-terminus of a substrate. Third, knowing that LFN α1/β1 unfolds upon binding PA (Fig. 4a), we engineered the double mutant, LFN I39C E72C (LFNC39-C72, which forms a disulfide bond that prevents α1/β1 unfolding). Interestingly, LFNC39-C72 has 104-fold reduced affinity for PA channels under non-reducing conditions (Fig. 4b); however, under reducing conditions (in the presence of dithiothreitol, DTT), LFNC39-C72 binds with the same affinity as WT LFN (Fig. 4b). We also kinetically observe a DTT-dependent LFNC39-C72 blockade of PA channels (Supplementary Fig. 9a online). Therefore, LFN must unfold α1 and β1 to properly bind the α clamp and interact stably with PA oligomers.
Figure 4. Dynamics and thermodynamics of the pre-translocation unfolding of LFN.
(a) Rendering of LFN's unfolding transition on the surface of the PANPAC dimer (green and blue, respectively). Free LFN (gold) (PDB 1J7N23) is Cα-aligned to the LFN in the PA8(LFN)4 complex (red). (b) LFNC39–C72 binding to WT PA channels (pH 7.4, 0 mV) in the presence of 5 mM DTT (red ▲) and in the absence of DTT (black ▲). A WT LFN binding curve (○) is also shown. Normalized equilibrium currents were fit to single-site binding model to obtain Kd values: WT LFN, Kd = 120 (±30) pM; LFNC39–C72, Kd = 1.2 (±0.1) µM; and LFNC39–C72 + 5 mM DTT, Kd = 240 (±60) pM. (c) Equilibrium stability measurements (pH 7.5, 20 °C) of amino-terminal deletions of LFN (Δn LFN). Equilibrium free energy differences (ΔΔGNU) were obtained from denaturant titration data fit to a four-state equilibrium unfolding model26 (Supplementary Fig. 9b online), where ΔΔGNU = ΔGNU(Δn) – ΔGNU(WT). Error bars are the mean ±s.d. (n = 3–4). Fit parameters are listed in Supplementary Table 1 online. (d) Residues in LFN are colored by their differences in normalized B factor (ΔBnorm), which is obtained by comparing the model of free LFN (1J7N, structure 1) and LFN in complex with PA (structure 2) using ΔBnorm = B1,i / <B1> - B2,i / <B2>. The <B> is the average B factor for the entire chain. ΔBnorm values indicating increasing and decreasing disorder upon binding to PA are colored red and blue, respectively. (e) ΔBnorm is plotted against the normalized fluorescence anisotropy (FA) change (ΔFAnorm) for 7 different site-specifically-labeled residues (37, 48, 72, 126, 164, 199, and 242) in LFN. ΔFAnorm = FA1,i / <FA1> - FA2,i / <FA2>, where free LFN and the LFN-PA oligomer complex are state 1 and state 2, respectively. The linear fit is significant (p = 0.04). Raw anisotropy changes upon binding the PA oligomer for these labeled LFN are shown in Supplementary Figure 10 online.
Binding to PA induces strain and disorder into LFN
We then asked how the unfolding of LFN α1/β1 on the surface of PA affects the remaining folded structure of LFN. First, we measured the stability of the Δn LFN mutants using chemical denaturant titrations probed by CD at 222 nm (CD222). The Δn mutants’ stabilities are estimated by fitting the CD222-probed titration data to a four-state equilibrium unfolding model (N⇄I⇄J⇄U)26 (Supplementary Fig. 9b and Supplementary Table 1 online). We find the truncation mutants possess native (N), intermediate (I and J), and unfolded (U) states. The truncations, however, destabilize the N state by ~1.2 kcal mol−1, where the deletion of the α1 helix is more destabilizing than the deletion of the β1 strand (Fig. 4c). Second, we compared the crystallographic atomic displacement parameters (B factors) of bound LFN with free LFN (1J7N23). In this analysis, we calculate the relative change in normalized B factor (ΔBnorm) for each LFN residue upon binding PA (Fig. 4d). The β2–β4 sheet and surrounding helices increase in Bnorm upon binding PA, whereas α1/β1 decrease in Bnorm (Fig. 4d). To corroborate these ΔBnorm values, we measure changes in backbone and side chain mobility using fluorescence anisotropy (FA). LFN mutants with unique Cys substitutions were labeled with thiol-reactive fluorescent probes. Upon binding WT PA7 oligomers, the fluorescent probes attached to LFN’s α1/β1 structures show gains in normalized relative FA (FAnorm), and conversely, probes in the β2–β4 sheet show losses in FAnorm (Supplementary Fig. 10a online). Overall, these ΔFAnorm values inversely correlate with ΔBnorm values (p value of 0.04, Fig. 4e), confirming that the more dynamic regions in the crystal are also dynamic in solution. Therefore, we conclude that the ~2.5 kcal mol−1 of stabilization gained when α1/β1 binds to the α-clamp site not only offsets the ~1.2 kcal mol−1 of thermodynamic destabilization imparted by the unfolding of α1/β1 but also accounts for the observed entropic increases in strain and disorder throughout LFN’s remaining folded structure.
The role of the α clamp in protein translocation
To determine the role of the α clamp during protein translocation, we use planar lipid bilayer electrophysiology, which records changes in PA conductance as substrate-blocked channels translocate their substrates and reopen7–9. We examined 37 point mutations in PA and LFN. Of the 13 PA mutants tested, we find that the α-clamp mutant, PA F202S, slows LFN translocation 20-fold, or 1.7 kcal mol−1 (Fig. 5a). A subset of the LFN point mutations (H35A, M40A, and H42A), which point toward either face of the α-clamp cleft (Fig. 3a), inhibit translocation 0.8–1.7 kcal mol−1 (Fig. 5a). These translocation defects are observed for both PA7 and PA8 channels (Supplementary Fig. 11a online). Conversely, other buried α1 sites (LFN Leu36, Ile39, and Ile43) are tolerant to substitution and do not affect protein translocation (Fig. 5a). Interestingly, we find that the observed positional translocation defects are restored when a bulky group is placed at position 40 (ref. 9) and a positively-charged residue is placed at positions 35 and 42 (Fig. 5a). All of the LFN α1/β1 replacements translocate similarly to WT LFN (Fig. 5a). We conclude, therefore, that efficient LFN unfolding and translocation are catalyzed by the aromatic α-clamp residue (PA Phe202); however, the LFN α1/β1 sequence itself has rather minimal charge and steric requirements.
Figure 5. The role of the α clamp in LFN and LF translocation.
Planar lipid bilayer translocation results for various mutant channels and substrates. (a) Differences in translocation activation energy (ΔΔG‡) for (top) LFN mutants, (bottom left) LFN α1/β1-replacement mutants, and (bottom right) PA mutants are shown. The reference state is WT LFN:WT PA. ΔΔG‡ = ΔG‡(WT) – ΔG‡(MUT), and ΔG‡ = RT ln t1/2 / c. The t1/2 value is the time for half of the protein to translocate, and c is a 1-sec reference constant. All LFN translocation rates were measured at symmetrical pH 5.6, Δψ = 40 mV. A negative value indicates the rate of translocation slowed upon mutation. The relative translocation efficiencies for these LFN translocations are given in Supplementary Figure 11b online. (b) Full-length LF translocation at pHcis = 6.1, pHtrans = 7.4, ΔpH = 1.3, Δψ = 20 mV. (left) ΔΔG‡ values and (right) relative translocation efficiencies (εMUT/εWT) for mutant PA channels. Individual LF translocation records are shown in Supplementary Figure 12 online. Error bars in (a–b) are the mean ±s.d. (n = 2–12). (c) (left) LFN α1/β1 (red ribbon) unfolds from the structured carboxy-terminal subdomain (red surface) by binding into the α-clamp site (cyan surface) on the PA oligomer (gray surface). The interaction is comprised of nonspecific interactions. The α-clamp sites orient the unfolded structure toward the central pore, where the protein is translocated. (right) Residues in PA’s α-clamp site (cyan) that affect LFN and/or LF translocation are rendered as sticks. LFN α1/β1 (red ribbon) and parallel β-sheet hydrogen bonds (black dotted lines) between LFN β1 and PA β13 are shown.
The broad substrate specificity of the α clamp led us to ask which PA residues facilitate translocation of full-length LF, a more complex, multidomain substrate. LF has a different rate-limiting step than LFN and requires a greater driving force7; therefore, we measure its translocation kinetics under a ΔpH and Δψ. We find the PA α-clamp mutants, F202S and P205S, reduce LF translocation efficiency, ε, by ~60% (where ε = Aobs/Aexp, Aexp and Aobs are the expected and observed amplitudes, respectively, Supplementary Fig. 12 online). The PA mutants F236S and F202S inhibit the rate of LF translocation (Fig. 5b). Interestingly, these PA mutants do not appreciably affect LFN binding (Fig. 3c), and only PA F202S inhibits LFN translocation (Fig. 5a). Finally, we find PA R178A is defective in LFN binding but not defective in translocation. We conclude that hydrophobic and aromatic residues surrounding the α clamp (Fig. 5c) catalyze the translocation of LF.
DISCUSSION
Some models8,30 propose that nonspecific clamping sites are critical features of unfolding machines. In general, unfoldases are thought to denature proteins by applying mechanical forces9 and transiently trapping partially unfolded conformations in nonspecific binding sites8. Unfolded protein, however, is inherently more complex than folded protein, especially in terms of its configurational flexibility and combinatorial chemical complexity. Therefore, a translocase channel would have to accommodate an ever-changing array of possible chemistries and configurations as the unfolded chain is translocated. An elegant solution to this problem may be that unfolded sequences adopt a more rigid and uniform α-helical or β-strand conformation upon binding to an unfoldase, as we observe in the PA-LFN complex (Fig. 3a). Indeed we find that PA’s α clamp can bind to a broad array of amino acid sequences (Fig. 3f). This nonspecific binding activity likely reflects the general helical shape complementarity of the α-clamp site, which excludes ~1000 Å2 on PA without making specific side-chain-side-chain interactions. Additionally, backbone hydrogen bonds, which are ubiquitous features of polypeptides, can provide nonspecific contact points between the translocase and substrate, as we observe between LFN β1 and PAN β13 (Figs. 3a and 5c).
Broad peptide-binding specificity has been observed in other systems, including calmodulin42,43; the ClpXP adapter, SspB32,33; the chaperone, GroEL/ES47–49; and the unfoldase, ClpA/Hsp100 (ref. 31). For calmodulin, which is analogous structurally to the PA oligomer’s α-clamp cleft, multiple peptide helices are recognized by the cleft formed by its twin Ca2+-ion binding sites. The ClpXP adapter, SspB, binds multiple unstructured carboxy-terminal degradation signal tags in various conformations in a cleft. The chaperone complex, GroEL/ES, can bind to various amphipathic helices and strands. A substrate binding site, identified in the unfolding machine ClpA/Hsp100, is located above the ϕ-clamp-type site and may be analogous to the α-clamp site on the PA oligomer.
Our structure provides new insight on how a nonspecific polypeptide clamp can unfold its substrate. By binding to LFN in multiple locations using nonspecific interactions [i.e., in the α clamp (Fig. 3a) and ϕ clamp8], LFN can be partially unfolded (Fig. 4a) and maintained in a more strained (Fig. 4d,e) and less stable conformation (Fig. 4c–e). The region of LFN that is most destabilized upon binding PA (Fig. 4d,e) coincides with LFN’s β2–β4 sheet, which was previously reported as the mechanical breakpoint, or structure that is rate-limiting to the unfolding step translocation9. Therefore, we infer the α-clamp site stabilizes unfolding intermediates, introduces strain into the mechanical breakpoint, and feeds unfolded structure into the central ϕ-clamp site.
We estimate that the costs associated with binding to the α-clamp site (Fig. 3c–e) may be offset by orienting the substrate toward the central lumen (Fig. 5c), reducing the stability of the substrate (Fig. 4c), and minimizing the diffusional mobility of unstructured regions before (Fig. 4d,e) or during translocation8. We expect that nonspecific-clamping sites should lessen the counterproductive diffusive motions expected for large sections of unfolded polypeptide chain by maintaining contact with the unfolded chain and further reducing backbone conformational entropy, thus allowing the Δψ/ΔpH driving force to efficiently unfold9 and translocate proteins7 (Fig. 5a,b). Although the α clamp forms a stable complex with unfolded structure, this intermediate does not represent a thermodynamic trap. Rather populating partially unfolded translocation intermediates would lower a much greater overall rate-limiting barrier expected in the absence of such intermediates, thereby allowing translocation to proceed on a biologically reasonable timescale.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/nsmb/.
Supplementary Material
ACKNOWLEDGMENTS
We thank H. Gong, E. Haddadian, T. Sosnick, and K. Freed for assistance in refining the backbone torsional angles, using their unpublished TOP algorithm; J. Colby for assistance in purifying constructs; M. Brown for constructing the pET15-LFN-SalI vector; J. Berger and N. Echols for advice on crystallography; R. Zalpuri at the Robert D. Ogg Electron Microscope Laboratory; J. Holton and G. Meigs at the 8.3.1 beamline of the Advanced Light Source; and T. Sosnick, J. Collier, J. Berger, and J. Kuriyan for helpful advice. This work was supported by University of California start-up funds (B.A.K) and NIH research grants R01-AI077703 (B.A.K.) and R01-GM064712 (E.R.W.)
Footnotes
Accession codes. The structure factors and coordinates for the PA8(LFN)4 complex have been deposited in the PDB (accession code 3KWV).
Note: Supplementary information is available on the Nature Structural & Molecular Biology website.
AUTHOR CONTRIBUTIONS
G.K.F. crystallized, solved, and refined the PA8(LFN)4 structure. G.K.F., K.L.T., H.J.S, A.F.K., S.G.G., and I.I.T. obtained functional data. G.K.F., K.L.T., H.J.S, A.F.K., E.R.W., and B.A.K. prepared the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
References
- 1.Wickner W, Schekman R. Protein translocation across biological membranes. Science. 2005;310:1452–1456. doi: 10.1126/science.1113752. [DOI] [PubMed] [Google Scholar]
- 2.Navon A, Ciechanover A. The 26 S proteasome: from basic mechanisms to drug targeting. J. Biol. Chem. 2009;284:33713–33718. doi: 10.1074/jbc.R109.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauer RT, et al. Sculpting the proteome with AAA(+) proteases and disassembly machines. Cell. 2004;119:9–18. doi: 10.1016/j.cell.2004.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Y. Toward an atomic model of the 26S proteasome. Curr. Opin. Struct. Biol. 2009;19:203–208. doi: 10.1016/j.sbi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 6.Matouschek A. Protein unfolding--an important process in vivo? Curr. Opin. Struct. Biol. 2003;13:98–109. doi: 10.1016/s0959-440x(03)00010-1. [DOI] [PubMed] [Google Scholar]
- 7.Krantz BA, Finkelstein A, Collier RJ. Protein translocation through the anthrax toxin transmembrane pore is driven by a proton gradient. J. Mol. Biol. 2006;355:968–979. doi: 10.1016/j.jmb.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Krantz BA, et al. A phenylalanine clamp catalyzes protein translocation through the anthrax toxin pore. Science. 2005;309:777–781. doi: 10.1126/science.1113380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thoren KL, Worden EJ, Yassif JM, Krantz BA. Lethal factor unfolding is the most force-dependent step of anthrax toxin translocation. Proc. Natl Acad. Sci. U.S.A. 2009;106:21555–21560. doi: 10.1073/pnas.0905880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenniston JA, Baker TA, Fernandez JM, Sauer RT. Linkage between ATP consumption and mechanical unfolding during the protein processing reactions of an AAA+ degradation machine. Cell. 2003;114:511–520. doi: 10.1016/s0092-8674(03)00612-3. [DOI] [PubMed] [Google Scholar]
- 11.Martin A, Baker TA, Sauer RT. Pore loops of the AAA+ ClpX machine grip substrates to drive translocation and unfolding. Nat. Struct. Mol. Biol. 2008;15:1147–1151. doi: 10.1038/nsmb.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S, Ratliff KS, Matouschek A. Protein unfolding by the mitochondrial membrane potential. Nat. Struct. Biol. 2002;9:301–307. doi: 10.1038/nsb772. [DOI] [PubMed] [Google Scholar]
- 13.Huang S, Ratliff KS, Schwartz MP, Spenner JM, Matouschek A. Mitochondria unfold precursor proteins by unraveling them from their N-termini. Nat. Struct. Biol. 1999;6:1132–1138. doi: 10.1038/70073. [DOI] [PubMed] [Google Scholar]
- 14.Smith H, Keppie J. Observations on experimental anthrax: demonstration of a specific lethal factor produced in vivo by Bacillus anthracis. Nature. 1954;173:689. doi: 10.1038/173869a0. [DOI] [PubMed] [Google Scholar]
- 15.Friedlander AM. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J. Biol. Chem. 1986;261:7123–7126. [PubMed] [Google Scholar]
- 16.Agrawal A, Pulendran B. Anthrax lethal toxin: a weapon of multisystem destruction. Cell Mol. Life Sci. 2004;61:2859–2865. doi: 10.1007/s00018-004-4251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ezzell JW, Abshire TG. Serum protease cleavage of Bacillus anthracis protective antigen. J. Gen. Microbiol. 1992;138:543–549. doi: 10.1099/00221287-138-3-543. [DOI] [PubMed] [Google Scholar]
- 18.Milne JC, Furlong D, Hanna PC, Wall JS, Collier RJ. Anthrax protective antigen forms oligomers during intoxication of mammalian cells. J. Biol. Chem. 1994;269:20607–20612. [PubMed] [Google Scholar]
- 19.Kintzer AF, et al. The protective antigen component of anthrax toxin forms functional octameric complexes. J. Mol. Biol. 2009;392:614–629. doi: 10.1016/j.jmb.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petosa C, Collier RJ, Klimpel KR, Leppla SH, Liddington RC. Crystal structure of the anthrax toxin protective antigen. Nature. 1997;385:833–838. doi: 10.1038/385833a0. [DOI] [PubMed] [Google Scholar]
- 21.Katayama H, et al. GroEL as a molecular scaffold for structural analysis of the anthrax toxin pore. Nature Struct. Mol. Biol. 2008;15:754–760. doi: 10.1038/nsmb.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kintzer AF, et al. Role of the protective antigen octamer in the molecular mechanism of anthrax lethal toxin stabilization in plasma. J. Mol. Biol. 2010;399:741–758. doi: 10.1016/j.jmb.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pannifer AD, et al. Crystal structure of the anthrax lethal factor. Nature. 2001;414:229–233. doi: 10.1038/n35101998. [DOI] [PubMed] [Google Scholar]
- 24.Lacy DB, Wigelsworth DJ, Melnyk RA, Harrison SC, Collier RJ. Structure of heptameric protective antigen bound to an anthrax toxin receptor: a role for receptor in pH-dependent pore formation. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13147–13151. doi: 10.1073/pnas.0405405101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benson EL, Huynh PD, Finkelstein A, Collier RJ. Identification of residues lining the anthrax protective antigen channel. Biochemistry. 1998;37:3941–3948. doi: 10.1021/bi972657b. [DOI] [PubMed] [Google Scholar]
- 26.Krantz BA, Trivedi AD, Cunningham K, Christensen KA, Collier RJ. Acid-induced unfolding of the amino-terminal domains of the lethal and edema factors of anthrax toxin. J. Mol. Biol. 2004;344:739–756. doi: 10.1016/j.jmb.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 27.Van den Berg B, et al. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- 28.Lum R, Niggemann M, Glover JR. Peptide and protein binding in the axial channel of Hsp104. Insights into the mechanism of protein unfolding. J. Biol. Chem. 2008;283:30139–30150. doi: 10.1074/jbc.M804849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, et al. Crystal structures of the HslVU peptidase-ATPase complex reveal an ATP-dependent proteolysis mechanism. Structure. 2001;9:177–184. doi: 10.1016/s0969-2126(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 30.Zimmer J, Nam Y, Rapoport TA. Structure of a complex of the ATPase SecA and the protein-translocation channel. Nature. 2008;455:936–943. doi: 10.1038/nature07335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinnerwisch J, Fenton WA, Furtak KJ, Farr GW, Horwich AL. Loops in the central channel of ClpA chaperone mediate protein binding, unfolding, and translocation. Cell. 2005;121:1029–1041. doi: 10.1016/j.cell.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Levchenko I, Grant RA, Flynn JM, Sauer RT, Baker TA. Versatile modes of peptide recognition by the AAA+ adaptor protein SspB. Nat. Struct. Mol. Biol. 2005;12:520–525. doi: 10.1038/nsmb934. [DOI] [PubMed] [Google Scholar]
- 33.Levchenko I, Grant RA, Wah DA, Sauer RT, Baker TA. Structure of a delivery protein for an AAA+ protease in complex with a peptide degradation tag. Mol. Cell. 2003;12:365–372. doi: 10.1016/j.molcel.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham K, Lacy DB, Mogridge J, Collier RJ. Mapping the lethal factor and edema factor binding sites on oligomeric anthrax protective antigen. Proc. Natl Acad. Sci. U.S.A. 2002;99:7049–7053. doi: 10.1073/pnas.062160399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arora N, Leppla SH. Residues 1-254 of anthrax toxin lethal factor are sufficient to cause cellular uptake of fused polypeptides. J. Biol. Chem. 1993;268:3334–3341. [PubMed] [Google Scholar]
- 36.Arora N, Leppla SH. Fusions of anthrax toxin lethal factor with shiga toxin and diphtheria toxin enzymatic domains are toxic to mammalian cells. Infect. Immun. 1994;62:4955–4961. doi: 10.1128/iai.62.11.4955-4961.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milne JC, Blanke SR, Hanna PC, Collier RJ. Protective antigen-binding domain of anthrax lethal factor mediates translocation of a heterologous protein fused to its amino- or carboxy-terminus. Mol. Microbiol. 1995;15:661–666. doi: 10.1111/j.1365-2958.1995.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 38.Lacy DB, Mourez M, Fouassier A, Collier RJ. Mapping the anthrax protective antigen binding site on the lethal and edema factors. J. Biol. Chem. 2002;277:3006–3010. doi: 10.1074/jbc.M109997200. [DOI] [PubMed] [Google Scholar]
- 39.Lacy DB, et al. A model of anthrax toxin lethal factor bound to protective antigen. Proc. Natl Acad. Sci. U.S.A. 2005;102:16409–16414. doi: 10.1073/pnas.0508259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melnyk RA, et al. Structural determinants for the binding of anthrax lethal factor to oligomeric protective antigen. J. Biol. Chem. 2006;281:1630–1635. doi: 10.1074/jbc.M511164200. [DOI] [PubMed] [Google Scholar]
- 41.Chauhan V, Bhatnagar R. Identification of amino acid residues of anthrax protective antigen involved in binding with lethal factor. Infect. Immun. 2002;70:4477–4484. doi: 10.1128/IAI.70.8.4477-4484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meador WE, Means AR, Quiocho FA. Target enzyme recognition by calmodulin: 2.4 A structure of a calmodulin-peptide complex. Science. 1992;257:1251–1255. doi: 10.1126/science.1519061. [DOI] [PubMed] [Google Scholar]
- 43.Meador WE, Means AR, Quiocho FA. Modulation of calmodulin plasticity in molecular recognition on the basis of x-ray structures. Science. 1993;262:1718–1721. doi: 10.1126/science.8259515. [DOI] [PubMed] [Google Scholar]
- 44.Blanke SR, Milne JC, Benson EL, Collier RJ. Fused polycationic peptide mediates delivery of diphtheria toxin A chain to the cytosol in the presence of anthrax protective antigen. Proc. Natl Acad. Sci. U.S.A. 1996;93:8437–8442. doi: 10.1073/pnas.93.16.8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christensen KA, Krantz BA, Collier RJ. Assembly and disassembly kinetics of anthrax toxin complexes. Biochemistry. 2006;45:2380–2386. doi: 10.1021/bi051830y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clackson T, Wells JA. A hot spot of binding energy in a hormone-receptor interface. Science. 1995;267:383–386. doi: 10.1126/science.7529940. [DOI] [PubMed] [Google Scholar]
- 47.Landry SJ, Gierasch LM. The chaperonin GroEL binds a polypeptide in an alpha-helical conformation. Biochemistry. 1991;30:7359–7362. doi: 10.1021/bi00244a001. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Gao X, Chen L. GroEL Recognizes an Amphipathic Helix and Binds to the Hydrophobic Side. J. Biol. Chem. 2009;284:4324–4331. doi: 10.1074/jbc.M804818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Z, Feng H, Landry SJ, Maxwell J, Gierasch LM. Basis of substrate binding by the chaperonin GroEL. Biochemistry. 1999;38:12537–12546. doi: 10.1021/bi991070p. [DOI] [PubMed] [Google Scholar]
- 50.Duesbery NS, et al. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 51.Adams PD, et al. Recent developments in the PHENIX software for automated crystallographic structure determination. J. Synchrotron Rad. 2004;11:53–55. doi: 10.1107/s0909049503024130. [DOI] [PubMed] [Google Scholar]
- 52.MacDowell AA, et al. Suite of three protein crystallography beamlines with single superconducting bend magnet as the source. J. Synchrotron Rad. 2004;11:447–455. doi: 10.1107/S0909049504024835. [DOI] [PubMed] [Google Scholar]
- 53.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. In: Carter CW Jr, Sweet RM, editors. Methods in Enzymology Vol. 276: Macromolecular Crystallography, part A. New York: Academic Press, Inc.; 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- 54.Storoni LC, McCoy AJ, Read RJ. Likelihood-enhanced fast rotation functions. Acta Crystallogr. D Biol. Crystallogr. 2004;60:432–438. doi: 10.1107/S0907444903028956. [DOI] [PubMed] [Google Scholar]
- 55.Emsley P, Cowtan K. COOT: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 56.Davis IW, et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375–W383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 58.Mogridge J, Cunningham K, Collier RJ. Stoichiometry of anthrax toxin complexes. Biochemistry. 2002;41:1079–1082. doi: 10.1021/bi015860m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.