Abstract
The leading cause of infertility in men and women is quantitative and qualitative defects in human germ cell (oocyte and sperm) development. Yet, it has not been possible to examine the unique developmental genetics of human germ cell formation and differentiation due to inaccessibility of germ cells during fetal development. Although several studies have shown that germ cells can be differentiated from mouse and human embryonic stem cells, human germ cells differentiated in these studies generally did not develop beyond the earliest stages1-8. Here we used a germ cell reporter to quantitate and isolate primordial germ cells derived from both male and female hESCs. Then, by silencing and overexpressing genes that encode germ cell-specific cytoplasmic RNA-binding proteins (not transcription factors), we modulated human germ cell formation and developmental progression. We observed that human DAZL (Deleted in AZoospermia-Like) functions in primordial germ cell formation, whereas closely-related genes, DAZ and BOULE, promote later stages of meiosis and development of haploid gametes. These results are significant to the generation of gametes for future basic science and potential clinical applications.
Historically, human germ cell development has been intractable to direct analysis; yet, infertility is unusually common in both men and women, with genetic requirements that differ from those of other commonly-studied species9,10. Here, we sought to develop a system for direct experimental examination of landmark events and genetic requirements in human germ cell formation, maintenance of pluripotency, epigenetic reprogramming and progression through meiosis (Supplementary Fig. 1). Although previous studies had demonstrated that bone morphogenetic proteins (BMPs) promote differentiation of hESCs to germ cells in embryoid bodies (EBs), the process was inefficient5. Thus, we explored adherent differentiation of hESCs and observed the induction of a variety of morphological changes (Supplementary Fig. 2). Furthermore, differentiation was accompanied by increased expression of the germ cell-specific proteins, VASA and DAZL, in all hESC lines tested (two female (XX) and two male (XY) lines from four independent derivations; Fig. 1a, Supplementary Fig.3).
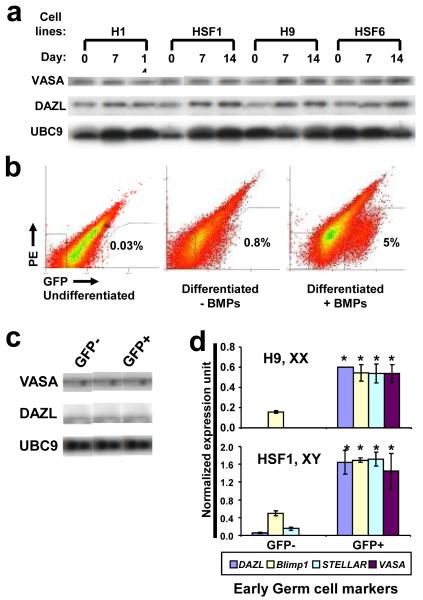
Figure 1. Enrichment of human germ cells by BMPs and VASA:GFP reporter.
a, Western analysis of hESCs after differentiation with BMPs at 7 and 14 days. Equal amounts of cell lysates were loaded in each lane. UBC9 was used as a loading control. b, FACS analysis of GFP populations. c, Western analysis of VASA and DAZL after FACS. d, Expression of early germ cell markers via qPCR Taqman probes. 20,000 GFP+ and GFP- cells (Day 7 of differentiation with BMPs) were subjected to qPCR analysis. Error bar = standard deviation; asterisk = significant difference by t-test (p<0.05), n=2.
Based on this data, and previous studies indicating that VASA is germ cell specific4,5,11-12, we constructed a VASA reporter in order to purify germ cells from the complex cell mixture resulting from hESC differentiation (Supplementary Fig.4). We introduced the reporter into undifferentiated hESCs, and then following differentiation, isolated GFP+ cells (putative primordial germ cells (PGC)) via fluorescence-activated cell sorting (FACS) (Fig. 1b). We observed that both XY- and XX-bearing hESCs reproducibly gave rise to a GFP+ population after 7 and 14 days of differentiation, and that the percentage of GFP+ cells reached approximately 5% with addition of BMPs which are required for mouse PGC formation13 (Supplementary Fig. 5). Protein analysis confirmed that the GFP+ cells are enriched for endogenous VASA and DAZL proteins (Fig. 1c). VASA protein was localized specifically to the cytoplasm of the GFP+ cells and was not detected in GFP- cells (Supplementary Fig. 6a). Further analysis showed that, as expected, OCT4 protein was expressed most highly in undifferentiated hESCs but also in both GFP+ and GFP- populations at lower levels due to differentiation (Supplementary Fig. 6b).
Gene expression profiling was carried out on the GFP+ and GFP- populations. Early germ cell markers such as DAZL, BLIMP1, STELLAR, and VASA were significantly enriched in the GFP+ populations (Fig. 1d), whereas those typically expressed during later stages of germ cell development were either not detected or not enriched, with the exception of low levels of Synaptonemal Complex Protein 3 (SCP3) in the GFP+ population (Supplementary Fig. 7). γH2AX and SCP3 immunostaining was used to examine meiotic progression throughout our experiments; γH2AX is an indicator of meiotic recombination based on binding to double strand breaks14,15 and SCP3 is indicative of synaptonemal complex (SC) formation in meiotic prophase I16. When the cells were stained for SCP3 and γH2AX, we observed that the GFP+ cells showed only low levels of scattered, punctate SCP3 staining in rare cells and there was no staining of γH2AX (Supplementary Fig. 8). These results indicated that the GFP+ cells are likely at a pre-meiotic stage (with rare cells entering meiosis). We also observed GFP+ cells were enriched for expression of a subset of pluripotency genes, LIN28, NANOG, OCT4, and TERT, consistent with previous reports of their expression in human germ cells17,18.
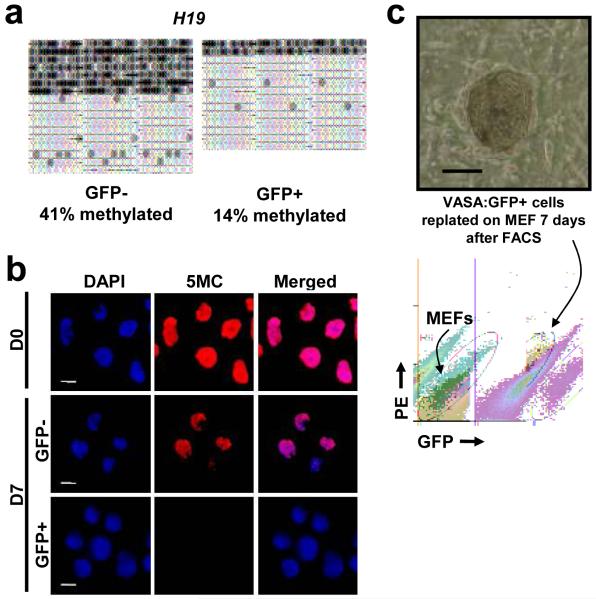
Epigenetic reprogramming is diagnostic of germ cell development19. Thus, we characterized erasure of methylation (hypomethylation) globally and at the DMRs (differently methylated regions) of imprinted loci. We found that the H19 locus was hypomethylated in GFP+ cells relative to GFP- cells (Fig. 2a). Results from other imprinted loci (PEG1, SNRPN, KCNQ) confirmed that the GFP+ cells also showed significantly lower levels of methylation at these DMRs relative to other cell types (Supplementary Fig. 9). Further, examination of global DNA methylation levels (5MC; Fig. 2b) provided strong evidence that the VASA:GFP+ population is in the process of erasing methylation globally. Identity of the populations of GFP- and GFP+ cells was verified by staining for VASA and OCT4 (Supplementary Fig. 6).
Figure 2. Germ cell properties of VASA:GFP+ cells.
a, GFP+ population was hypomethylated at H19 locus. b, 5-methyl cytosine staining of the VASA:GFP+ population to detect global methylation. Cells were immunostained using monoclonal 5-methyl cytosine antibody. Images are taken at the same exposure time to show different levels of staining. c, Phase contrast pictures showing representative colony from GFP+ cells after 7 days of replating. No colonies were observed from plating of GFP- population. FACS plot demonstrates that GFP+ cells maintained GFP expression after 7 day of replating on MEFs. Scale bar = 100 micron in c.
PGCs possess the ability to establish embryonic germ (EG) cell lines with diagnostic gene expression and morphology3,20. Thus, we tested whether GFP+ cells form EG lines on inactivated feeder cells in media lacking the growth factor bFGF. We found that the GFP+ cells gave rise to colonies that resembled EGs21 after seven days (Fig. 2c, Supplementary Fig. 10a), whereas the GFP- cells did not give rise to any colonies. Similar to EGs22, replated GFP+ cells had intense alkaline phosphatase activity (Supplementary Fig. 10b) and remained GFP+ after extensive culture (Fig. 2c). Gene expression profiles of replated cells (after 20 days) were similar to those of freshly-isolated GFP+ cells, with a few exceptions (Supplementary Fig. 11). We noted, however, that DMRs of replated cells had significantly more methylation after replating (Supplementary Fig. 9). This is similar to previous reports with hESCs23 but had not been examined in human EGs.
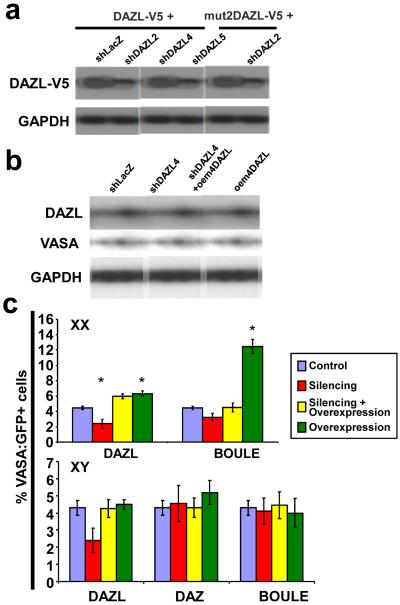
Since gene expression, immunostaining, epigenetic status, and ability to give rise to colonies resembling EGs strongly suggested that the GFP+ cells are PGCs, we next examined genetic requirements for formation and differentiation of human PGCs. We focused on the human DAZ gene family which contains three members: four human DAZ genes which are commonly deleted from the Y chromosome of infertile men who lack germ cells24,25 and autosomal DAZL and BOULE homologs which are conserved from invertebrates to humans10. We first silenced expression of the autosomal DAZL gene by shRNA technology (Supplementary Fig. 12a, b) and observed reduced protein levels with three silencing constructs (Fig. 3a); specificity of silencing was confirmed with synonymous mutations (Supplementary Fig. 12c). Changing just three nucleotides in the shDAZL targeting region created a mutated DAZL-V5 resistant to silencing (Fig. 3a). When hESCs were stably integrated with DAZL silencing vectors and differentiated for 14 days with BMPs, expression of DAZL and VASA was significantly reduced in cells carrying the shDAZL4 or shDAZL2 vectors (Fig. 3b; Supplementary Fig. 13a). Overexpression of mutated DAZL resulted in rescue as observed by elevated DAZL and VASA protein expression. To our surprise, overexpression of DAZL alone elevated endogenous VASA levels relative to controls, suggesting VASA is regulated by DAZL.
Figure 3. Silencing of DAZ family members and germ cell numbers.
a, Western analysis of DAZL-V5 silenced by independent shDAZL constructs. DAZL with V5 epitope was cotransfected with control (shLacZ) or shDAZLs in 293T cells. Mut2DAZL-V5 was resistant to shDAZL2. b, Western analysis of DAZL and VASA after silencing with shDAZL4 or rescue in hESCs (H9). c, FACS results using H9 (XX line) and HSF1 (XY line) for silencing of DAZL, BOULE, and DAZ. Error bars = standard deviation; asterisk = significant difference in percentage of VASA:GFP+ cells by one way ANOVA, (p<0.05), n=2 (Averages from two independently differentiated samples at 14 days).
To examine whether silencing of BOULE or DAZ affects PGC differentiation, we identified multiple shBOULE and shDAZ constructs that significantly reduced expression of these proteins (Supplementary Fig. 13b, c). We introduced the silencing constructs into hESCs carrying the VASA:GFP reporter with individual shRNAs: shDAZL and shBOULE separately into H9(XX), and shDAZL, shBOULE, and shDAZ into HSF1(XY). Corresponding target genes were also co-transduced to rescue silencing effects. We observed that the VASA:GFP+ population was reduced to almost half by silencing DAZL in both XX and XY lines (Fig. 3c, Supplementary Fig. 14; statistically significant in XX and marginal in XY cells). In contrast, silencing of BOULE reduced the GFP+ population slightly in the XX line but not in the XY line and the number of GFP+ cells was unaffected when DAZ was silenced. Finally, overexpression of BOULE alone increased the VASA:GFP+ population to nearly 12% in XX but not in XY cells, suggesting that BOULE plays a more important role in directing human female PGC differentiation than male. Overexpression of combinations of DAZL, BOULE, and DAZ did not result in synergistic enhancement of PGC formation (data not shown).
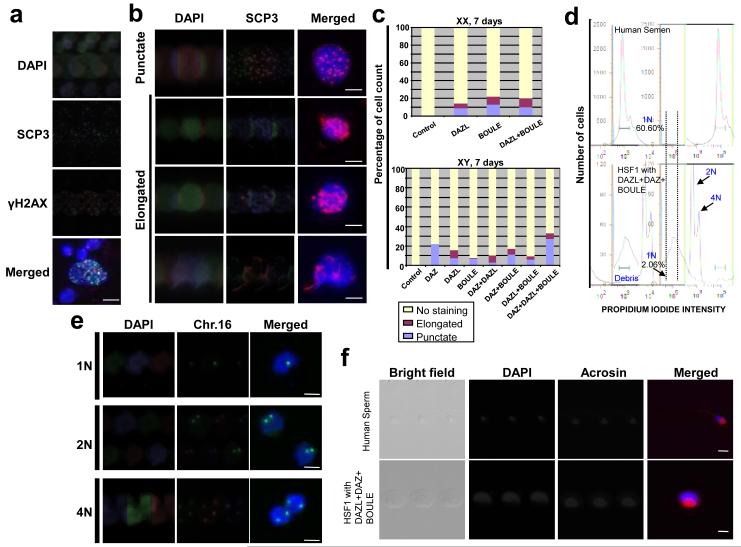
The data above suggested that overexpression of DAZL, and BOULE (in XX lines), promotes PGC formation. We next examined if overexpression of these genes promotes germ cell differentiation beyond the PGC stage. Thus, combinations of vectors that overexpressed DAZ gene family members were introduced into XX and XY hESCs. hESCs were differentiated for 7 days in the absence of BMPs (to test whether internal factors alone can induce late germ cell differentiation) and examined for meiotic progression. The majority of nuclei from differentiated hESCs showed no obvious γH2AX staining (including nuclei that did not enter meiosis and those that might have completed meiosis) (Fig. 4a). When γH2AX was detected in the nuclei, it was accompanied by more than 10 punctate SCP3 foci, indicating that the nuclei had entered meiotic prophase I. Elongated SCP3 localization with different intensities and lengths was also detected but was not accompanied by γH2AX staining (Fig. 4b). This staining pattern likely corresponds to that of SCs at zygotene, pachytene, or diplotene stages. To quantify SC formation, we categorized SCP3 staining as punctate or elongated, and counted the percentage of nuclei in each category and those negative for SCP3. Overexpression of DAZL, BOULE, or a combination of both gave similar results at Day 7 in the XX line (Fig. 4c). In XY cells, overexpression of DAZ+DAZL+BOULE resulted in the highest percentage of cells with SCP3 staining (Fig. 4c; Supplementary Fig. 15). Indeed, overexpression of DAZ alone gave rise to more than 20% of cells with punctate SCP3, much more than DAZL or BOULE alone. We infer that overexpression of DAZL or BOULE was sufficient to induce elongated SC formation in the XY line, but DAZ was required to achieve the highest level of SC formation.
Figure 4. Overexpression of DAZL, DAZ and BOULE induces meiotic progression and haploid formation.
a, Meiotic spread from Day 7 differentiated cells; immunofluorescence staining with SCP3 and γH2AX. b, Meiotic cells overexpressing DAZ family proteins and stained for SCP3. c, Percentage of cells showing punctate or elongated SCP3 staining at Day 7. 200 meiotic spreads were counted and categorized for each sample. Scale = 10 micron. d, FACS of DNA content of human semen and cells overexpressing DAZL, DAZ, and BOULE. e, Fluorescent in situ hybridization of chromosome 16 in cells sorted as 1N, 2N, 4N. f, ACROSIN staining of 1N population from human semen and HSF1 with 3 overexpression vectors. All cells were from whole cultures without GFP FACS.
We next determined if haploid cells were produced. We found that mRNA expression of the mature sperm markers, TEKT1 and ACROSIN, was highly elevated in cells that overexpressed all three family members at Day 14 (Supplementary Fig. 16), suggesting potential formation of haploid gametes. Moreover, when cells were sorted by DNA content (using parameters developed to sort 1N cells from a human semen sample obtained from the Stanford IVF Clinic), ~2% of cells had 1N content on Day 14 following overexpression of DAZL, BOULE and DAZ. No corresponding haploid cells were isolated from control cells that lacked overexpression of DAZ gene family members (Fig. 4d; Supplementary Fig. 17). DNA content was confirmed by fluorescent in situ hybridization (FISH) using a probe for Chromosome 16. As expected, sorted 1N cells possessed a single chromosome 16, whereas 2N and 4N cells carried 2 and 4 chromosomes, respectively (Fig. 4e; Supplementary Fig. 18a, 19). In addition, the majority of 1N cells also expressed the mature sperm protein, ACROSIN, which is present from spermatid to spermatozoan stage26 (Fig. 4f; Supplementary Figs. 18b, 20). In contrast, 2N cells differentiated in the same culture were negative for ACROSIN. We also observed that the H19 DMR was hypermethylated in 1N cells, whereas, SNRPN and KCNQ DMRs were hypomethylated with patterns similar to those detected in human semen (Supplementary Fig. 21). Finally, we observed that expression of the genes, MIS, FSHR, LHR, SOX9, was greater in cultures that produced the highest number of germ cells (overexpressed DAZ, DAZL and BOULE proteins; in whole culture without FACS) suggesting increased numbers of Sertoli and Leydig cells7 in the same differentiated cultures to support maturation of male germ cells (Supplementary Fig. 22).
In summary, our results indicate that human germ cells can be differentiated and isolated from pluripotent hESCs and they possess the ability to enter and progress through meiosis. Moreover, we observed that members of the human DAZ gene family that encode translational regulators modulate both germ cell formation and differentiation. The human DAZL gene functions primarily in PGC formation, whereas DAZ and BOULE function primarily to promote germ cell progression to meiosis, and formation of haploid germ cells that resemble round spermatids in cellular and molecular characteristics.
METHODS SUMMARY
VASA:GFP reporter, transduction and FACS
2.5 kb of human VASA upstream of the first codon was cloned into pENTR 5′-TOPO. eGFP was fused 1kb downstream of the last codon of human VASA, and cloned into pENTR/D-TOPO. Cloned plasmids were recombined27 to create pLVGV. Lentiviral supernatant was produced, hESCs were transduced overnight on matrigel in conditioned medium and subsequently selected with geneticin (200ng/ml) for 7 days. Selected hESCs were differentiated for times indicated and harvested by brief treatment with Collagenase IV and then TrypLEExpress (Invitrogen). The cell suspension was prepared in differentiation medium for FACS with a MoFlow or BD cell sorter.
Supplementary Material
Acknowledgements
We thank Dr. David Suter for the p2k7 vectors, Dr. Barry Behr for procurement of clinical samples, Cory Nicholas for assistance with SCP3 staining, Shuwei Jiang and Patty Lovelace for FACS expertise. K Kee was supported by a California TRDRP postdoctoral fellowship, V Angeles was supported by an NIH fellowship. This research was supported by funds from the NIH NICHD, the TRDRP and the CIRM (to RARP).
References
- 1.Hubner K, et al. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251–1256. doi: 10.1126/science.1083452. [DOI] [PubMed] [Google Scholar]
- 2.Toyooka Y, Tsunekawa N, Akasu R, Noce T. Embryonic stem cells can form germ cells in vitro. Proc. Natl. Acad. Sci. 2003;100:11457–11462. doi: 10.1073/pnas.1932826100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geijsen N, et al. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–54. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- 4.Clark AT, et al. Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum. Mol. Genet. 2004;13:727–39. doi: 10.1093/hmg/ddh088. [DOI] [PubMed] [Google Scholar]
- 5.Kee K, Gonsalves JM, Clark AT, Reijo Pera RA. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem Cells Dev. 2006;15:831–837. doi: 10.1089/scd.2006.15.831. [DOI] [PubMed] [Google Scholar]
- 6.Tilgner K, et al. Isolation of primoridal germ cells from differentiating human embryonic stem cells. Stem Cells. 2008;26:3075–3085. doi: 10.1634/stemcells.2008-0289. [DOI] [PubMed] [Google Scholar]
- 7.Bucay N, et al. A novel approach for the derivation of putative primordial germ cells and Sertoli cells from human embryonic stem cells. Stem Cells. 2008;27:68–77. doi: 10.1634/stemcells.2007-1018. [DOI] [PubMed] [Google Scholar]
- 8.Park TS, et al. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells. 2009;27:783–795. doi: 10.1002/stem.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hull MGR, et al. Population study of causes, treatment, and outcome of infertility. Brit. Med. J. 1985;291:1693–1697. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu EY, Moore FL, Reijo Pera RA. A gene family required for human germ cell development evolved from an ancient meiotic gene conserved in metazoans. Proc. Natl. Acad. Sci. 2001;98:7414–7519. doi: 10.1073/pnas.131090498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujiwara Y, et al. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc. Natl. Acad. Sci. 1994;91:12258–12262. doi: 10.1073/pnas.91.25.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castrillon DH, Quade BJ, Wang TY, Quigley C, Crum CP. The human VASA gene is specifically expressed in the germ cell lineage. Proc. Natl. Acad. Sci. 2000;97:9585–9590. doi: 10.1073/pnas.160274797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao GQ. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
- 14.Mahadevaiah SK, et al. Recombinational DNA double-strand breaks in mice precede synapsis. Nature Genet. 2001;27:271–276. doi: 10.1038/85830. [DOI] [PubMed] [Google Scholar]
- 15.Lenzi ML, et al. Extreme heterogeneity in the molecular events leading to the establishment of chiasmata during meiosis i in human oocytes. Am. J. Hum. Genet. 2005;76:112–127. doi: 10.1086/427268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heyting C. Synaptonemal complexes: structure and function. Curr. Opin. Cell Biol. 1996;8:389–396. doi: 10.1016/s0955-0674(96)80015-9. [DOI] [PubMed] [Google Scholar]
- 17.Perrett RM, et al. The early human germ cell lineage does not express SOX2 during in vivo development or upon in vitro culture. Biol. Reprod. 2008;78:852–858. doi: 10.1095/biolreprod.107.066175. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto T, et al. Isolation and expression analysis of the testis-specific gene, STRA8, stimulated by retinoic acid gene 8. J. Assist. Reprod. Genet. 2002;19:531–535. doi: 10.1023/A:1020963919596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hajkova P, et al. Epigenetic reprogramming in mouse primordial germ cells. Mech. Dev. 2002;117:15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- 20.West JA, et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature. 2009;460:909–13. doi: 10.1038/nature08210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shamblott MJ, et al. Derivation of pluirpotent stem cells from cultured human primordial germ cells. Proc. Natl. Acad. Sci. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaren A. Primordial germ cells in the mouse. Dev. Biol. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 23.Rugg-Gunn PJ, Ferguson-Smith AC, Pedersen RA. Epigenetic status of human embryonic stem cells. Nature Genet. 2005;37:585–587. doi: 10.1038/ng1556. [DOI] [PubMed] [Google Scholar]
- 24.Reijo R, et al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nature Genet. 1995;10:383–393. doi: 10.1038/ng0895-383. [DOI] [PubMed] [Google Scholar]
- 25.Reijo R, Alagappan RK, Patrizio P, Page DC. Severe oligospermia resulting from deletions of the Azoospermia Factor gene on the Y chromosome. Lancet. 1996;347:1290–1293. doi: 10.1016/s0140-6736(96)90938-1. [DOI] [PubMed] [Google Scholar]
- 26.Florke-Gerloff S, Topfer-Petersen E, Muller-Esterl W, Schill WB, Engel W. Acrosin and the acrosome in human spermatogenesis. Hum. Genet. 1983;65:61–67. doi: 10.1007/BF00285030. [DOI] [PubMed] [Google Scholar]
- 27.Suter DM, et al. Rapid generation of stable transgenic embryonic stem cell lines using modular lentivectors. Stem Cells. 2006;24:615–623. doi: 10.1634/stemcells.2005-0226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.