Abstract
Background: researchers and sponsors increasingly confront the issue of whether participants in a clinical trial should have post-trial access (PTA) to the trial drug. Legislation and guidelines are inconsistent, ambiguous or silent about many aspects of PTA. Recent research highlights the potential importance of systematic reviews (SRs) of reason-based literatures in informing decision-making in medicine, medical research and health policy. Purpose: to systematically review reasons why drug trial participants should, or need not be ensured PTA to the trial drug and the uses of such reasons. Data sources: databases in science/medicine, law and ethics, thesis databases, bibliographies, research ethics books and included publications’ notes/bibliographies. Publication selection: a publication was included if it included a reason as above. See article for detailed inclusion conditions. Data extraction and analysis: two reviewers extracted and analyzed data on publications and reasons. Results: of 2060 publications identified, 75 were included. These mentioned reasons based on morality, legality, interests/incentives, or practicality, comprising 36 broad (235 narrow) types of reason. None of the included publications, which included informal reviews and reports by official bodies, mentioned more than 22 broad (59 narrow) types. For many reasons, publications differed about the reason’s interpretation, implications and/or persuasiveness. Publications differed also regarding costs, feasibility and legality of PTA. Limitations: reason types could be applied differently. The quality of reasons was not measured. Conclusion: this review captured a greater variety of reasons and of their uses than any included publication. Decisions based on informal reviews or sub-sets of literature are likely to be biased. Research is needed on PTA ethics, costs, feasibility and legality and on assessing the quality of reason-based literature.
Introduction
Special arrangements to ensure that research participants have post-trial access (PTA) to the trial drug can be crucial for participants worldwide who help test drugs unavailable through government-funded services (Kolata and Eichenwald, 1999; National Bioethics Advisory Commission Group, 2001; Macklin, 2004; BBC staff, 2007). With the increasing globalization of research, sponsors and researchers increasingly confront the issue of whether drug trial participants and/or their communities should, or need not be ensured PTA to the trial drug or other possible benefits. A spectrum of views has been expressed by academics, participants, oversight agencies and industry organizations on this issue (Busse, 1997; National Bioethics Advisory Commission Group, 2001; PhRMA Group, 2001; Nuffield Council on Bioethics Group, 2002; Berkley, 2003; Fernandez et al., 2003; Greenwood and Hausdorff, 2003; Macklin, 2004; Slack et al., 2005; Shaffer et al., 2006; Lavery, 2008; Sofaer et al., 2009). International and national guidelines require PTA to the trial drug in at least some circumstances (CIOMS, 2002; The European Group on Ethics in Science and New Technologies to the European Commission, 2003; India Council of Medical Research, 2006; World Medical Association, 2008) and impose various procedural obligations, such as inclusion of information about PTA in literature for participants and in registries (UNAIDS Group, 2000; National Bioethics Advisory Commission Group, 2001; CIOMS, 2002; Nuffield Council on Bioethics Group, 2002; World Medical Association, 2004; World Medical Association, 2008); a recent literature assesses compliance. (Cohen et al., 2008; Ciaranello et al., 2009; Shah et al., 2009). However, the guidelines conflict, change their stance on PTA (World Medical Association, 2004; Wolinsky, 2006; World Medical Association, 2008), and give little detail about when PTA to trial drugs should be ensured, for how long, and by whom. The US Code of Federal Regulations does not mention PTA (45CFR46, revised 2009). Some major sponsors of research are prohibited from funding PTA (NIH Group, 2005) or state that their remit excludes this (Wellcome Trust Group, 2004).
A systematic review involves an (as far as possible) exhaustive and reproducible search and evaluation of literature that meets pre-specified inclusion criteria to answer a focused question. The genre was developed in the late 1970s in social science, and later spread to medicine, where it was used with the aim of enabling maximally informed, minimally biased decisions. It was transferred, with similar intent, to non-clinical empirical research, including empirical bioethics (which uses qualitative or quantitative research to address empirical questions relevant to bioethics), and philosophical or reason-based bioethics (which employs reasoning to address bioethical questions) (Lemmer et al., 1999; McCullough et al., 2007; Strech et al., 2008). While systematic reviews of the ‘vast literature [on research ethics]’ (Emanuel et al., 2008: 4) have been advocated to help clinical researchers grasp its current status, and to improve decision-making in health care, there is only one systematic review of philosophical bioethics (McCullough et al., 2007). It covers just seven articles and addresses an ethical question: the question of whether a specific clinical intervention is ethically justified. As we explain and argue elsewhere, systematic reviews that answer an ethical question are likely to mislead decision-makers, and more sweeping revisions of systematic review methodology than McCullough et al. propose are needed for systematic reviews of reason-based literature to be of use. In particular, a systematic review of philosophical bioethics should address the factual question of which reasons have been given when discussing the ethical question, and it should present detailed information on reasons. As we also argue, decision-makers will still need literature that discusses the quality of reasons and their implications for the ethical question: a systematic review of reasons and literature assessing quality are each necessary parts of decision-makers’ dossier (Sofaer and Strech).
This systematic review addresses the question: ‘which reasons have been given for the views that former participants in a drug trial should, or need not, be ensured PTA to the trial drug?’ (Ensuring PTA here implies special arrangements when participants will otherwise lack PTA, but implies no verbal or written assurances to potential participants or participants.) A secondary question is: ‘how have these reasons been used to argue that post-trial access should, or need not, be ensured?’ In particular, what were the reasons taken to imply? Were the reasons endorsed? Were uses of reasons informed by previously published uses of the same reason? It is the first systematic review of reasons, and first systematic review of a large philosophical bioethics literature. This systematic review does not settle the question of whether former participants should, or need not be ensured PTA to the trial drug. However, as explained later and elsewhere, its usefulness to decision-makers and philosophers lies in aiding the identification of the strong reasons and their implications for ensuring PTA, and in setting the agenda for empirical and conceptual research to improve the information-base of decision-making (Sofaer and Strech, 2011).
Methods
We included a publication, e.g. article, if and only if:
It included a reason why PTA should or need not be provided.
The PTA was for former participants in a drug trial.
The PTA was to a drug tested in the trial; and
The publication was a peer-reviewed, published academic article or book; national-level report or working paper; or PhD thesis.
Reason in condition (I) covers reasons that support ensuring (PTA to the drug or other possible benefit), and reasons that were simply mentioned without being explicitly endorsed or rejected. PTA provision mentioned under condition (I) includes PTA-promoting actions e.g. the funding or affordable pricing of the drug post-trial, pre-trial planning about PTA and requiring such actions. We employed conditions (II) and (III) because of possible differences between reasons for ensuring PTA to participants versus non-participants, and for providing the trial drug versus e.g. trial devices (Millum, 2009). Condition (IV) excluded laws, legal cases, case commentaries and guidelines because of the literature’s extent and the difficulty of describing a reproducible search. Publications were not excluded explicitly based on language: we are together able to read in a number of languages and planned to employ translators if the search yielded promising publications that we could not adequately understand.
Reference librarians helped to select databases in science/medicine (Medline, LocatorPlus), law (Westlaw International) and ethics (ETHXWeb, JSTOR, Euroethics, Endebit), as well as thesis databases (Ethos-Beta Electronic Theses Online Service, WorldCat Dissertations; the latter is not limited to PhD theses). No database’s index terms included post-trial access or synonym. For each database, we first searched with Boolean operations of keywords and of the database’s relevant index terms, if any. On retrieving publications known to meet the inclusion conditions, we identified their index terms and keywords (including but not limited to ones referring to PTA), and sorted these into five content classes: access, clinical trials, drugs, ethics and research subjects. For our database-specific search terms and strings, please see table A1. Searches had no start-date but, where specifiable, a 2 October 2009 end-date. We next used bibliographic review and recommendations from authors of included publications to identify reports and books on research ethics, and hand-searched their tables of contents and indexes. Last, we searched in-text references, notes and bibliographies of included publications for promising titles (a ‘snow-ball search’) (Greenhalgh and Peacock, 2005).
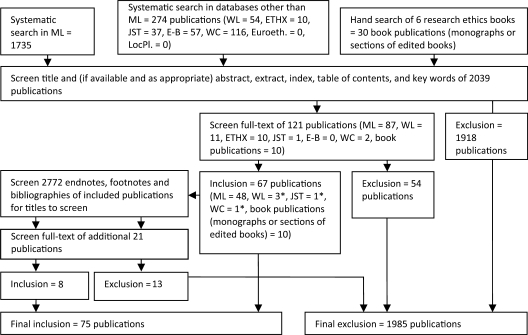
Next, we worked independently through the resulting publication list to exclude publications based on their title, any abstract/extract and any index terms. Each author thus produced a list of publications that were candidates for meeting the inclusion conditions. One list had 134 publications, the other had 95; 93 were on both lists. After jointly resolving discrepancies to create one list of 121 publications, we each read the full text of each and selected those that met the above inclusion criteria. We repeated the process on the results of the snow-ball search until no further qualifying publications emerged. Figure 1 documents the search.
Figure 1.
Selection of publications. ML, Medline; WL, Westlaw; ETHX, ETHXWeb; JST, JSTOR; E-B, Ethos-Beta Electronic Theses Online Service; WC, Worldcat; Euroeth., Euroethics; LocPl., LocatorPlus; asterisks indicate number of publications that were not also retrieved by the systematic search in ML. Reasons for excluding 1985 publications: no content on research conducted on humans; or no content on clinical trials of drugs conducted on humans; or no content on whether PTA the trial drug should; or need not, be ensured to participants, their host community or country; or no reason for the view that PTA the trial drug should (or need not) be ensured to participants.
We distinguish here between the mention of a reason expressed by a specific passage in a publication (Reason Mention) from a Type of Reason, which may have different Reason Mentions in different publications. We distinguish also between broad Types of Reason, e.g. avoid exploitation, and narrow Types of Reason, e.g. avoid exploiting research participants, or avoid exploiting the host country. In our analysis, each broad Type of Reason comprises several narrow types, and each narrow type falls under one broad type. Bold text indicates Types of Reason.
As we found no useful precedent for the systematic extraction and analysis of Reason Mentions, we developed a framework of narrow and broad Types of Reasons that best accommodated the reasons mentioned in the included publications. (For the development, details and limitations of our model for systematic reviews, see (Strech and Sofaer, How to write a systematic review of reasons, manuscript.) In brief, one author (NS) commenced extraction by identifying and numbering 10 publications already known to meet the inclusion conditions, and which illustrated a range of reasons and challenges to analysis. Both authors then independently extracted Reason Mentions from publication 1 and assigned each Reason Mention to a broad and a narrow type, assigning Reason Mentions to different narrow Types of Reason whenever it was suspected that the Reason Mentions might express different Types of Reason. (The narrow Types of Reason were thus very narrow.) Discrepancies between the resulting spreadsheets were identified, discussed and resolved. The process was repeated, for a total of nine publications, until coding additional publications resulted in no further changes to data types, and discrepancies between corresponding cells’ contents were easily resolvable. N.S. then extracted and analyzed data from the remaining publications. D.S. checked the analysis. N.S. then identified and removed repeated Reason Mentions within each publication.
Last, N.S. used Excel to derive/calculate the results, which D.S. checked. We also conducted a sensitivity analysis, which aimed to see whether the difference in the number and variety of reasons captured by our systematic review versus the most comprehensive included publication was preserved when Types of Reason were more broadly individuated: we merged similar narrow Types of Reason, and recounted the numbers of reasons identified by our systematic review versus the most comprehensive publication. Table 1 shows which Types of Reason were merged (see the last note under the table).
Table 1.
Broad and narrow Types of Reasons
 |
 |
 |
 |
 |
 |
 |
 |
Notes: • This table lists all the mentioned reasons why PTA should or need not be ensured, including those that were rejected, or neither rejected nor endorsed. Given that reasons will in any case need to be appraised, it better aids the decision-maker to err on the side of comprehensiveness. • Finer-grained data is available on request. • No colour background: reason used just for the view that PTA should be ensured; number in square brackets with no colour background is number of reason mentions used for the view that PTA should be ensured. • Grey background: reason used just for the view that PTA need not be ensured or with unclear implications; number in square brackets with grey colour background is number of reason mentions used for the view that PTA need not be ensured. • Red background: some reason mentions were for the view that PTA should be ensured and some for the view that it need not be ensured, or implications for PTA were left unspecified. • Note that, for many broad Types of Reason, the number after the broad type (e.g. Avoid exploitation) is more than the sum of numbers after the narrow types because, for colourless or grey reasons the number given excludes the few reason mentions with unclear implications. • Round parentheses: reasons followed by the same number in parentheses were merged in the sensitivity analysis.
Disagreements and problems that arose in the selection of publications and the data extraction and analysis were resolved by frequent discussion and not by deference to the second author; we did not need to appeal to a third person. Details of the types of data extracted and types of results are given in Strech and Sofaer, How to write a systematic review of reasons, manuscript).
Results
Publication Characteristics
Seventy-five publications were included (Supplementary Figure 1), published between 1991 and September 2009 inclusive. Nearly three-quarters (55, 73%) were published between 2002 and September 2009 inclusive. There was no book focusing on PTA. Publications used many different terms to refer to PTA.1 Table 2 describes further characteristics of the included publications.
Table 2.
Characteristics of publications included in this systematic review
| Features of publication | n (%) of publications |
|---|---|
| Content typea | |
| Philosophy discussionb | 62 (83) |
| Policy critiquec | 26 (35) |
| Informal philosophical reviewd | 18 (24) |
| Policy reviewe | 18 (24) |
| Opinion piecef | 4 (5) |
| Case study of PTAg | 2 (3) |
| Commentaryh | 2 (3) |
| Empirical studyi | 2 (3) |
| Discussion of PTA legal casej | 1 (1) |
| Publication type | |
| Article | 55 (73) |
| Sections with distinct author groups in edited books | 9 (12)k |
| Report | 5 (7) |
| Journal-published letters | 3 (4) |
| Journal-published news article | 1 (1) |
| Monograph | 1 (1) |
| PhD thesis | 1 (1) |
| PTA discussed in relation to which trials? | |
| All | 57 (76) |
| HIV/AIDSl | 18 (24) |
| Mental health | 1 (1) |
| Genetics | 1 (1) |
| Published in 2004 (the most prolific year) | 13 (17) |
| Publications whose main topic was not PTA | 47 (63) |
| Publications concerning only research conducted by resource-rich in resource-poor countries | 58 (77) |
| English-language publicationsm | 75 (100) |
| Characteristics of 55 articles included | |
| Field of journal | |
| Medicine | 18 (33) |
| Bioethics | 17 (31) |
| Law | 12 (22) |
| Public health | 4 (7) |
| Science | 2 (4) |
| Articles published online at www.scidev.net | 2 (4) |
aPublications were assigned more than one content type where appropriate.
bDiscussion of whether there are PTA obligations.
cAssessment of PTA regulations, guidelines and/or policies.
dPublication that mentioned >12 narrow types of reasons that met the inclusion conditions.
eReview of regulations, guidelines and/or policies regarding PTA.
fExpression of mere opinion about whether there are PTA obligations.
gDescription of trial in which PTA was an issue.
hCommentary on another publication included in this review.
iPublication reporting empirical study, not necessarily of PTA.
jDiscussion of legal case in which PTA was an issue.
kThe 9 sections occurred in a total of three edited books.
lThe HIV/AIDS publications concerned AZT, ART or vaccine research.
mSee ‘Limitations’ section.
Identity, Incidence and Implications of Reasons
The 75 publications together included 781 Reason Mentions, which we grouped under 36 broad and 235 narrow Types of Reason; when similar narrow types were merged to conduct the sensitivity analysis, there were 202 narrow Types of Reason (Table 1). To generalize over the broad Types of Reason, reasons were based on considerations of morality, legality, interests and incentives, and/or practicality. Moral reasons belonged to two overlapping families: based on justice, or the roles of and relationships between stake-holders in research. Reasons classed under legality included reasons claiming that guidelines do (or do not) require ensuring PTA.
The most frequently mentioned broad Types of Reason were avoid exploitation (which had 97 Reason Mentions) and stake-holder interests: the interests of stake-holders in research such as participants, sponsors, governments of countries hosting research and society (86 Reason Mentions). A total of 14 (39%) broad Types of Reason had 5 or fewer Reason Mentions. The most-mentioned narrow Types of Reason were avoid exploiting the host country (23 Reason Mentions; mentioned only for ensuring PTA), and if PTA is required, this will reduce the incentive to conduct research with consequent loss of potential benefits to potential research hosts (23 Reason Mentions; mentioned only for the view that PTA need not be ensured), followed by avoid exploiting participants (19 Reason Mentions; mentioned only for ensuring PTA), and avoid exploiting the host community (18; mentioned only for ensuring PTA). Most (210, 89%) narrow Types of Reason had five or fewer Reason Mentions.
Reason Mentions classed under avoidance of exploitation concerned the exploitation of the host country (23 Reason Mentions2 (Crouch and Arras, 1998; Glantz et al., 1998; Benatar, 2000; Lie, 2000; Cooley, 2001; National Bioethics Advisory Commission Group, 2001; Chang, 2002; Kottow, 2002; Orentlicher, 2002; Page, 2002; Participants in the Conference on Ethical Aspects of Research in Developing Countries, 2002; Lo and Bayer, 2003; Lavery, 2004; Macklin, 2004; Page, 2004; Participants in the 2001 conference on ethical aspects of research in developing countries, 2004; Emanuel, 2008; Wertheimer, 2008)), participants (19 Reason Mentions in 15 publications (Cleaton-Jones, 1997; McLean, 1997; Crouch and Arras, 1998; Emanuel, 1998; National Bioethics Advisory Commission Group, 2001; Brody, 2002; Nuffield Council on Bioethics Group, 2002; Orentlicher, 2002; Macklin, 2004; Ballantyne, 2005; Grady, 2005; Benatar and Fleischer, 2007; Hawkins and Emanuel, 2008; Lavery, 2008; Zong, 2008)), the host community (18 Reason Mentions in 13 publications, (Gostin, 1991; Annas and Grodin, 1998; Glantz et al., 1998; Page, 2002; Participants in the Conference on Ethical Aspects of Research in Developing Countries, 2002; Macklin, 2004; Participants in the 2001 conference on ethical aspects of research in developing countries, 2004; Yearby, 2004; Basu et al., 2006; Gbadegesin and Wendler, 2006; Emanuel, 2008; Lavery, 2008; Siegel, 2008)), or an unspecified group (16 Reason Mentions in 8 publications, (Crouch and Arras, 1998; Participants in the Conference on Ethical Aspects of Research in Developing Countries, 2002; Macklin, 2004; Ashcroft, 2005; Ballantyne, 2006b; Lavery, 2008; Siegel, 2008; Sachs, 2009)). Publications agreeing that avoiding exploitation is sometimes or always a reason for ensuring PTA differed as to whether avoiding exploitation requires providing PTA in particular, (McLean, 1997; Annas and Grodin, 1998; Crouch and Arras, 1998; Glantz et al., 1998; Benatar, 2000; Chang, 2002; Kottow, 2002; Page, 2002; Lo and Bayer, 2003; Basu et al., 2006; Benatar and Fleischer, 2007) or a fair level of benefits that may but need not include PTA3 (Brody, 2002; Orentlicher, 2002; Participants in the Conference on Ethical Aspects of Research in Developing Countries, 2002; Lavery, 2004; Participants in the 2001 conference on ethical aspects of research in developing countries, 2004; Ballantyne, 2005; Ballantyne, 2006a; Carse and Little, 2008; Emanuel, 2008; Siegel, 2008).
Reciprocity is the notion that, because one party (X) benefits another (Y), Y is obliged to benefit X in return. Reason Mentions classed under reciprocity differed as to the identity of X (participants or the host community) and of Y (the sponsor, researchers, society, the world, any host country non-participant or host country non-participants who have the same medical condition as participants). Eight of the 12 possible combinations of X and Y were mentioned. Some reciprocity Reason Mentions differed also regarding why participants/communities may be entitled to benefit, e.g. because they assumed risk (Lie, 2000; Chang, 2002; Macpherson, 2004; Ashcroft, 2005; Merritt, 2007; Carse and Little, 2008; Sachs, 2009; Shah et al., 2009) or were used to create benefit for mankind (Gostin, 1991).
Role Reason Mentions collectively reflected conflicting views regarding various role-related obligations, powers, and limits to the powers of researchers, sponsors and governments. For example, many reasons for the view that PTA need not be ensured appealed to researchers’ or sponsors’ lack of influence on health policy or on the drug approval process. A key conflict regarded whether researchers have the same role as doctors (implying that researchers should ensure PTA) or different role (implying that researchers need not ensure PTA); reasons appealing to the purpose of research or to the relationship between researchers and participants/communities were similarly polarized.
Although logistical obstacles were most often left unspecified, a broad range was mentioned. Such obstacles were almost without exception taken to imply that PTA need not be ensured. Concerns about the safety and/or efficacy of the trial drug were only used to argue for the view that PTA need not be ensured in specific cases or against the view that PTA should always be required.
Fourteen non-maleficence Reason Mentions appealed to the view that participants should not be worse off after the trial, but completed ‘not be worse off after the trial than … with respect to … ’ differently. For most such Reason Mentions (11, 76%), participants should not be worse off than during the trial; for two (14%), than before it; for one (7%), than if they had not participated. For most (13, 92%), the relevant respect was health, whereas for one (7%) it was health care.
Publications endorsing4 a narrow reason for a conclusion agreed about whether the reason was for ensuring PTA or for the view that PTA need not be ensured. The most frequently endorsed reasons5 included ones used just for ensuring PTA (avoid exploiting participants, participants’ health need), and others used just to argue that PTA need not be ensured (host community’s interests may be better served by receiving benefit other than PTA).
Attitudes Taken to Reasons
Most reasons were clearly accepted or rejected at most or all Reason Mentions. However, several common6 broad reasons were exceptions, including cost (clear attitude expressed in 20 of 30 Reason Mentions, 66%) and distributive justice (18 of 23, 78%).
Reasons were rejected on various moral and empirical bases. For example, publications mentioning if PTA is required, this will reduce the incentive to conduct research with consequent loss of potential benefits to potential research hosts differed as to whether requiring PTA would reduce the research conducted (Cooley, 2001; Nuffield Council on Bioethics Group, 2002; Macklin, 2004). Those who thought it would disagreed about whether this was a reason why PTA need not be ensured. One publication denied this on the grounds that a reduction in research would not mean a loss of benefits for communities who would have hosted the research without receiving its benefits (Glantz et al., 1998). Another denied it because it is good to prevent exploitative research (Chang, 2002).
Spectrum and Incidence of Conclusions
Three-quarters of the publications (56, 75%) took a stance on whether PTA should, or need not, be ensured, and a quarter (19, 25%) on whether ensuring PTA should be required (Supplementary Table 1). Of all the conclusions drawn by publications, the most common conclusion was that PTA should sometimes be provided (45 publications, 60%), although some publications (10, 13%) concluded PTA should always be provided and one (1%) that there is no obligation. Of those concluding PTA should sometimes be provided, some thought that products/services likely to be beneficial should be provided, but not necessarily PTA to the trial intervention, e.g. (Participants in the Conference on Ethical Aspects of Research in Developing Countries, 2002).
Among publications considering whether PTA should be required (19 publications, 25%), the most common conclusion was that it should not (16, 84%). Sixteen (21%) expressed conclusions about pre-trial obligations to discuss, plan or enter into agreements regarding PTA; 13 (17%) endorsed such an obligation. No publication distinguished these obligations’ scope, and publications differed about whether such actions should be required. Six (8%) endorsed an obligation to advocate for ensuring PTA or to refer participants to treatment or other trials (Crouch and Arras, 1998; National Bioethics Advisory Commission Group, 2001; Nuffield Council on Bioethics Group, 2002, 2005; Pace et al., 2003; Carse and Little, 2008). Four (5%) claimed Research Ethics Committees (Institutional Review Boards) should approve only research with appropriate PTA arrangements, though without defining appropriateness.
Nearly half (25, 45%) of publications claiming that PTA should be provided sometimes or always did not specify an agent obliged. Publications concluding there is a PTA-related obligation identified, collectively and individually, various agents who have the obligation; exceptions were obligations to advocate for ensuring PTA or to refer participants to other care or research, collectively attributed only to researchers and sponsors, and to approve research only if it has appropriate PTA arrangements, attributed only to RECs. It is our opinion that some but not all publications referencing several agents claimed that responsible agents should collaborate, but we did not systematically collect data on responsibilities to collaborate.
Many conclusions that PTA should be provided did not contain all of the following information: who should fund, provide or receive the trial drug, and/or for how long.
Reasons Endorsed and Conclusions Drawn by Individual Publications
Supplementary Table 2 gives, for each publication, the reasons that were clearly for ensuring PTA or for the view that PTA need not be ensured, and that were clearly endorsed or rejected by the author, and the publication’s conclusion. Publications varied widely in the number of narrow reasons mentioned, including those presented in a third party’s voice (range: 1–67) (table available on request from the corresponding author). The average number of narrow Types of Reason mentioned by informal philosophy reviews (Glantz et al., 1998; Hutt, 1998; Lie, 2000; National Bioethics Advisory Commission Group, 2001; Chang, 2002; Nuffield Council on Bioethics, 2002; Orentlicher, 2002; Page, 2002; Macklin, 2004; Ashcroft, 2005; Grady, 2005; Ballantyne, 2006a; Carse and Little, 2008; Emanuel, 2008; Lavery, 2008; Zong, 2008; Millum, 2009) was 24, compared to this review’s 235. The most comprehensive included publication, an informal review, (Lavery, 2008) reported 22 broad and 59 narrow Types of Reason (58, when narrow types were broadened in the sensitivity analysis). Among the reasons it did not mention were, for example, host community's interests may be better served by receiving benefit other than PTA.
Use of Relevant Literature
Publications collectively referenced7 20 regulations, guidelines or recommendation-containing reports (instruments), most commonly CIOMS’s guidelines (12 or 16% of publications mentioned a version of CIOMS’s guidelines (CIOMS, 1982; CIOMS, 1983; CIOMS, 1993; CIOMS, 2002)), NBAC’s (National Bioethics Advisory Commission Group, 2001) (11 or 15% of publications) and NCOB’s reports (Nuffield Council on Bioethics Group, 2002) (9 or 12% of publications). Over half of instrument-referencing publications (28/51, 55%) referenced one or more of these three. Over two-thirds (14, 67%) of instruments were referenced by just one publication.
Of the 55 articles included in this systematic review, the most-referenced articles were the early Glantz et al.8 (10 publications, 13%), (Cooley, 2001; National Bioethics Advisory Commission Group, 2001; Nuffield Council on Bioethics Group, 2002; Orentlicher, 2002; Page, 2002; Participants in the Conference on Ethical Aspects of Research in Developing Countries, 2002; Macklin, 2004; Participants in the 2001 conference on ethical aspects of research in developing countries, 2004; Gbadegesin and Wendler, 2006; Emanuel, 2008) and Crouch and Arras (Crouch and Arras, 1998) (9, 12%) (Cooley, 2001; Kottow, 2002; Orentlicher, 2002; Page, 2002; Macklin, 2004; Ballantyne, 2006b; Gbadegesin and Wendler, 2006; Emanuel, 2008; Siegel, 2008). Both these early papers argue that avoiding exploitation requires ensuring PTA and neither reference any theory of exploitation or reflect on the nature of exploitation. Many later authors citing these publications accepted that it was important to avoid exploitation but argued that this did not require ensuring PTA. These all cited Wertheimer, whose theory of exploitation implies their view (Wertheimer, 1999; Wertheimer, 2008); most reflected on the nature of exploitation.
Nearly two-thirds of publications mentioning a reciprocity reason (13/21, 62%) mentioned just one combination and either did not reference any publication mentioning a reciprocity reason (7/21, 33%) (Gostin, 1991; Harth and Thong, 1995; Hutt, 1998; Lie, 2000; Berkley, 2003; Macpherson, 2004; Carse and Little, 2008) or referenced just one (6/21, 29%) (Chang, 2002; Grady, 2005; Ballantyne, 2006b; Zong, 2008; Sachs, 2009; Shah et al., 2009). Few of these publications (5/21, 24%, 2 of which shared an author) discussed the formulation and persuasiveness of reciprocity reasons (Cooley, 2001; National Bioethics Advisory Commission Group, 2001; Merritt and Grady, 2006; Merritt, 2007; Millum, 2009).
Authors mentioning reasons based on nonmaleficence, rights, autonomy or (with few exceptions) the role of sponsors or researchers, relationships, the purpose of research (Nuffield Council on Bioethics Group, 2002; London, 2005), or distributive justice (Macklin, 2004) failed to acknowledge alternative formulations of the reason or reflect on the reason. None of the 30 cost Reason Mentions distinguished the (very different) marginal cost of producing a drug, the cost of discovery/development and gaining approval, and this cost combined with that of failed discovery/development; few distinguished the various cost-components of PTA such as drug, skilled labor, and equipment, (e.g. Gbadegesin and Wendler, 2006). Few publications distinguished the implications of the reasons they mentioned for PTA for participants in the trial versus for non-participants, (e.g. Millum, 2009).
Discussion
Our systematic review found many (36) broad Types of Reason, based on considerations of morality, legality, interests and incentives, and/or practicality, for the views that PTA to the trial drug should, or need not be ensured for participants. It identified a greater variety of reasons compared to all publications including informal philosophy reviews, as confirmed by sensitivity analysis.
While this systematic review does not settle the question of whether PTA to the trial drug should, or need not be ensured to trial participants, it has various direct and indirect uses for decision-makers such as policymakers, regulators, Institutional Review Boards (Research Ethics Committees) and designers of research protocols. Its list of reasons (Table 1) is the best guard currently available against failing to identify infrequently published or only inadequately presented reasons that are nonetheless strong. On the one hand, if the publishing world is working well, infrequently published reasons will be a distraction. On the other hand, the most frequently published reasons may simply be the best publicized, perhaps due to conflicts of interest that induce authors to endorse weak reasons and to ignore or reject strong reasons. In the absence of evidence either way, a systematic review is crucial in that it gives even such reasons an equal voice, prior to identification of the strong ones.
Our finding that the most-endorsed reasons included ones used just for ensuring PTA and others just for the view that PTA need not be ensured provides a salutary warning against considering only reasons that PTA should be ensured, or only reasons that PTA need not be ensured. Given the challenges to locating this particular literature, the list of publications included (Supplementary Figure 1) obviates the need for decision-makers to hire hundreds of hours of skilled labor to locate relevant publications. Furthermore, because our review describes the search and selection process in detail, decision-makers can check that the list is complete with much less effort than we exerted. Our summary of positions taken by individual publications (Supplementary Table 2) may aid decision-makers who are interested in specific publications but who cannot access them or lack the time or analysis skills to extract the reasons endorsed, attitudes taken to these and conclusions drawn.
This systematic review may also aid decision-making indirectly, by identifying research whose results should enable better-grounded decisions. Unlike the included informal reviews, (Glantz et al., 1998; Hutt, 1998; Lie, 2000; National Bioethics Advisory Commission Group, 2001; Chang, 2002; Nuffield Council on Bioethics, 2002; Orentlicher, 2002; Page, 2002; Macklin, 2004; Ashcroft, 2005; Grady, 2005; Ballantyne, 2006a; Carse and Little, 2008; Emanuel, 2008; Lavery, 2008; Zong, 2008; Millum, 2009) this systematic review identifies all the empirical or ethical points of dissent. Its identification of stark differences between publications on matters of fact shows that multi-disciplinary research is urgently needed to understand the costs,9 feasibility, legality and effects of requiring PTA, and any tendency of PTA offers to potential participants to boost recruitment or to reduce the quality of informed consent.
We consider decision-makers’ need for our systematic review strong, on the assumptions that the need for a systematic review increases with the literature’s size, the variety of fields or literary genres, the barriers to retrieving and assessing the literature, the differences between the perspectives of decision-makers and those affected by their decisions; and the inconsistency and incompleteness of guidance. Admittedly, a systematic review makes more demands on decision-makers than an informal review, but its advantages justify these demands. The first systematic review of a large reason-based literature and the first of how reasons were used, this systematic review comprises a starting-point for future systematic reviews of reasons relevant to decision-makers.
This systematic review has further implications for policymaking. It suggests the need to scrutinize key guidance documents that assume that avoiding exploitation requires ensuring PTA, (CIOMS, 2002) because it shows that the mature literature rejected the view that avoiding exploitation requires PTA, e.g. (Brody, 2002; Orentlicher, 2002; Participants in the Conference on Ethical Aspects of Research in Developing Countries, 2002; Lavery, 2004; Participants in the 2001 conference on ethical aspects of research in developing countries, 2004; Ballantyne, 2005; Ballantyne, 2006a; Carse and Little, 2008; Emanuel, 2008; Siegel, 2008). Moreover, various findings suggest that, for practical purposes, decision-procedures will be needed to set guidelines on PTA (Daniels, 2008), because authors may fail to reach agreement: (i) The most-endorsed reasons included ones used just for ensuring PTA, and others used just for the view that PTA need not be ensured, suggesting that disagreement will persist; further discussion is needed, however, to determine if disagreement is intractable. (ii) Some publications (14, 19%) expressed no view about any PTA-related obligation. (iii) Like some prominent instruments, (UNAIDS Group, 2000; National Bioethics Advisory Commission Group, 2001; CIOMS, 2002; World Medical Association, 2008; 45CFR46, revised 2009) some publications claiming PTA should sometimes or always be provided (25, 45%) did not specify an agent obliged.
The systematic review is of interest also to philosophers who seek to understand whether PTA should, or need not be ensured. First, it reduces the chance that they, as well as decision-makers, will fail to identify the strong reasons and their implications for ensuring PTA. Second, the systematic review aids the identification of reasons relevant to determining whether there are obligations, such as ensuring PTA to trial results (Fernandez et al., 2003), providing ART to participants who seroconvert in HIV vaccine trials (Berkley, 2003; Slack et al., 2005), or providing participants with care for non-trial conditions10 (Richardson and Belsky, 2004; Richardson, 2007). Third, its identification of inter-publication differences in the naming, formulation and implications of moral reasons suggests that conceptual research is needed. For example, the differences identified in authors’ use of terms for concepts such as exploitation and reciprocity suggests that research is needed on how to distinguish such concepts. This systematic review should facilitate this task, in that it presents a list of very finely individuated reasons, and so simplifies the task into one of deciding which of these to merge. Last, it raises the question of whether publications’ focus on exploitation is justified or is a serendipitous result of the focus of much-referenced publications, guidelines and reports (CIOMS, 1982; CIOMS, 1983; CIOMS, 1991; CIOMS, 1993; Crouch and Arras, 1998; Glantz et al., 1998; National Bioethics Advisory Commission Group, 2001; CIOMS, 2002). This focus differs from that of participants whose views are reported in the limited empirical literature (Kass and Hyder, 2002; Shaffer et al., 2006; Sofaer et al., 2009). Such conceptual research may also inform policy.
Quality
Decision-makers will need a distillation of the best reasons and their implications, and guidance on how to weight reasons, neither of which this systematic review provides. There is need for research to construct a suitable measure of quality and determine how best to distil. For the reasons given above, distillation should occur only after a systematic review such as this one has captured all the published reasons and how they have been used. The systematic review remains an essential ingredient of decision-makers’ brief for the reasons above and because of decision-makers’ need to check the distillation and its legitimacy.
While we did not collect data on the quality of reasons, our impressions suggest scope for improvement. A small number of publications seemed excellent e.g. (Merritt and Grady, 2006; Lavery, 2008). However, the authors of many interpretations of reasons did not express awareness of some or any interpretations. Some reasons were presented unclearly and many were discussed only briefly or not at all. There has been minimal or no influence on the PTA literature of analyses of social or distributive justice, autonomy or just health care (Dworkin, 2000; Daniels, 2008) or distinctions in economics between types of cost. Some publications appear unaware of theories of exploitation such as (Wertheimer, 1999; Wertheimer, 2008). The three most frequently-referenced instruments require PTA sometimes, (CIOMS, 1982; CIOMS, 1983; CIOMS, 1993; National Bioethics Advisory Commission Group, 2001; CIOMS, 2002; Nuffield Council on Bioethics Group, 2002) yet others impose more demanding obligations (The European Group on Ethics in Science and New Technologies to the European Commission, 2003). Differences in instruments referenced may explain why some (11) legality Reason Mentions were used for the view that PTA should be ensured and others (18) for the view that PTA need not be ensured. While some inter-publication differences regarding the interpretation, implications or persuasiveness of exploitation, reciprocity, role and responsiveness appeared grounded in awareness of different interpretations of reasons or their implications, we cannot rule out that other differences are due to lack of imagination compounded by ignorance of relevant literature. We believe that, in general, systematic review of reasons will improve authors’ awareness of the need to consider reasons’ alternative interpretations and implications.
Furthermore, many publications omitted to indicate the strength of reasons relative to that of other reasons or to establishing the conclusion in the absence of defeating reasons. Some reasoning appeared invalid. For example, some reasons why PTA need not be ensured showed, at most, why specific agents, e.g. sponsors, need not ensure PTA.
No included publication described its search, let alone a reproducible, exhaustive one (McCullough et al., 2004). Some variation in publications’ number of narrow reasons mentioned (range: 1–67) was justified: one excellent publication scrutinized one reason, explicitly stating there may be other reasons it would not consider (Merritt and Grady, 2006). Yet, other publications asserted conclusions based on inadequate discussion of fewer reasons than had at the relevant time been published, e.g. (King, 1997; Brody, 2002; Kottow, 2002; Basu et al., 2006; Benatar and Fleischer, 2007).
Recommendations
We recommend that authors be aware of a broader range of relevant literature, including regulations, guidelines and recommendation-containing reports, and that they acknowledge when insufficient evidence limits the strength of factual reasons regarding e.g. cost (Guyatt et al., 2008). Authors should carefully consider and state each reason’s implications, if any, for participants versus non-participants (Millum, 2009) and for whether it requires PTA in particular or, rather, a package of possible benefits that need not include PTA, (Participants in the 2001 conference on ethical aspects of research in developing countries, 2004) and also any all-things considered conclusion. Further research should address which inter-publication differences are legitimate. As existing comparative legal analyses are of small samples of instruments, (Nuffield Council on Bioethics Group, 1999; National Bioethics Advisory Commission Group, 2001; Macklin, 2004; Lavery, 2008; Zong, 2008) we recommend the examination of a larger sample. The field would benefit from a book-length, interdisciplinary discussion. Implementing these recommendations may increase inter-publication agreement and improve PTA policy.
Limitations
We did not assess quality because the methodology of quality assessment poses substantial challenges and has yet to be developed. However, in order to answer our secondary research question on how reasons have been used, we did collect data on whether Reason Mentions referenced appropriately. Unfortunately, such data is inadequate even as a partial indicator of quality: possibly, lack of an appropriate reference is in some genres not an indicator of low quality, as our finding that some informal reviews written by prominent authors give few or no references (Ashcroft, 2005) may suggest. Furthermore, it was conceptually and practically difficult to decide when a reason was original (and thus to conclude that no reference was necessary) and, in some cases, also to decide when a reference was appropriate. The data nonetheless gave some indication of authors’ breadth of awareness of relevant literature and guidelines.
A further limitation of this systematic review is that it may fail to capture the perspectives of participants, industry, regulators and some lawyers: our particular inclusion conditions were not met by empirical literature reporting participants’ views, industry grey literature, laws, guidelines, cases or commentaries. Nonetheless, some of their reasons were captured vicariously through publications’ attribution of reasons to other parties or when publications reasoned e.g. that PTA should be provided because guidelines so require. Our systematic review also gives some indication of instruments’ influence on the literature.
We may have missed some qualifying publications. Databases’ index terms did not include post-trial access or synonym, and were applied inconsistently. Although we searched for and within books on research ethics, we did not search systematically for these. Databases did not provide abstracts for some publications, which we screened based on title and keywords alone. Although we did not exclude publications based on language, our database selection created bias in favor of English-language publications and this may explain why all the publications included were written in English. We contacted some, but not all authors of qualifying publications for help in identifying others. Neither author was blinded as to publications’ identity: one (N.S.) knew much literature too well to be blinded. Selection of publications was consensus-based; possibly, independent scoring, or coding by others, might select different publications (McCullough et al., 2007). Our operational definition of informal philosophy review did not capture some publications that seemed to be informal philosophy reviews. However, an operational definition was necessary; ours was justified (the operational definition and justification are available on request from the corresponding author); and we rest our finding about the relative comprehensiveness of our systematic review on the maximum number of reasons mentioned by any publication, as calculated in the sensitivity analysis, not on the average number of reasons given by informal philosophy reviews.
There were also limitations to our assignment of Reason Mentions to Reason Types. (1) Because we decided to assign Reason Mentions to Reason Types based not just on the words used to express the reasons, but also on the meaning of those words, it is possible that different reviewers would make different assignments. Our analysis is nonetheless valid because reviewers assigned types independently, checked for discrepancies, and were able to resolve all discrepancies. (2) Differences in referencing conventions and with establishing originality made it difficult to decide when an appropriate reference was (not) given where one was necessary.
Our sum of Types of Reasons had additional limitations. (1) Types could be made narrower or broader; some broad types cover diverse narrow types; and there may be similarly good reasons to class a narrow Type of Reason under different broad types. In response: (i) our sensitivity analysis shows that our systematic review mentions a much greater variety of reasons than any publication included, whether Types of Reason are individuated narrowly or broadly. (ii) Narrowly-individuated narrow Types of Reason are meaningful and homogenous and their assignment to broad types was obvious and well-justified. (iii) When deciding how broad the broad Types of Reason should be, we faced a trade-off between having few but heterogeneous broad types versus impractically many, but more natural broad types. Given the practical aims of this article, we allowed some heterogeneity. (2) We summed the number of Reason Mentions that were used to support slightly different types of conclusions. However, we gave qualitative results where more appropriate. We note also that classical systematic reviews face the analogous problem that the studies reviewed addressed slightly different research questions. (3) We did not exclude, from the sum of Reason Mentions, reasons mentioned by the same author in different included publications. However, removal of such repeated reasons would make little difference to the sum. (4) The difference between the number of narrow Types of Reason identified by our systematic review and the most comprehensive informal review is slightly exaggerated because our systematic review used some narrow types such as unspecified logistical obstacles. However, there were only 12 unspecified types (0.5% of the narrow Types of Reason).
This systematic review presented data on the frequency with which Types of Reason were mentioned in order to identify reasons on which attention has focused, and neglected reasons. However, this data should be presented with caution. Research is needed to determine if decision-makers assume that the more common reasons are the stronger ones; if so, authors of future systematic reviews targeted at decision-makers should consider not presenting data on how frequently (or infrequently) reasons are mentioned.11
Supplementary Data
Supplementary data are available at PHE Online.
Funding
This work was supported by the Wellcome Trust (grant number 088360) to N.S.; and the German Research Society (Deutsche Forschungsgemeinschaft; grant number STR 1070/2-1) to D.S.
Conflict of Interest
N.S. is currently collaborating with the UK’s National Research Ethics Service to write its guidance on post-trial access. N.S. owns shares in pharmaceutical companies with a total value of under $600 on 13 July 2010.
Supplementary Material
Acknowledgements
The authors would like to thank Andrea Ciaranello, Penney Lewis, Leif Wenar and anonymous reviewers for comments on an earlier version, and reference librarians Vanessa Gamet and Carol Ann Mita for help selecting databases and constructing search strings. Select data from this article were presented at the German Network of Evidence-based Medicine in Berlin in December 2009; at King’s College London (UK), Centre for Bioethics and Society, in London in February 2010; at the German Academy of Medical Ethics’ working group called ‘Empirical Ethics’ in Hannover in April 2010; at the 10th World Congress of Bioethics, in Singapore in July 2010; and at the EACME conference, in Oslo in September 2010.
Appendix A
Table A1.
Search terms and strings
| Database | Search string |
|---|---|
| Medline | Focus on ethics: |
| (("Ethics"[Mesh] OR "Human Rights"[Mesh] OR "ethics"[Subheading])) AND ((((((((((((("Health Services Accessibility"[Mesh])) OR (("Continuity of Patient Care"[Mesh]))) OR (("Drugs, Investigational/supply and distribution"[Mesh]))) OR ((post-trial provision))) OR ((post-trial obligations))) OR ((post-trial access))) OR ((post-trial benefits))) OR ((post-trial responsibility))) OR ((follow-up)))) AND ((((("Clinical Trials as Topic"[Mesh])) OR (("Biomedical research"[Mesh]))) OR (("Human experimentation"[Mesh])))))) | |
| Focus on developing countries: | |
| ((((("Vulnerable Populations"[MeSH Terms])) OR (("Developing Countries"[Mesh])))) AND (((("Patient Advocacy"[MeSH Terms])) OR (("Ethics"[Mesh] OR "Human Rights"[Mesh] OR "ethics"[Subheading]))))) AND (((((((("clinical trials as topic"[Mesh])) OR (("Human Experimentation"[Mesh]))) OR (("Biomedical Research"[MeSH Terms]))) OR (("Drugs, Investigational/supply and distribution"[Mesh])))) OR (("Research/organization and administration"[Mesh])))) | |
| Disjunction of terms used to refer to PTA in included publications: a | |
| “post-trial provision” or “post-trial obligation” or “post-trial obligations” or “post-trial access” or “posttrial access” or “post-trial benefit” or “post-trial benefits” or “post-trial responsibility” or “post-trial responsibilities” or “prior agreements” | |
| Westlaw | “post-trial access” |
| JSTOR | “post-trial obligation” |
| Scidev.net | “post-trial responsibility” |
| LocatorPlus | “post-trial obligations” |
| Euroethics | “reasonable availability” |
| “post-trial provision” | |
| “Post-trial follow-up” | |
| “after the trial" and research and drug | |
| ETHXweb | post-trial |
| Ethos-Beta Electronic Theses Online Service | post-trial (any field) and (ethics (any field) or bioethics (any field)) |
| WorldCat Dissertations | “post-trial access” (keyword) |
| “after research” (keyword) | |
| “post-trial” (keyword) | |
| exploitation and “clinical research” |
aThese terms also included post-trial followup, after participation or aftercare, but we excluded them from the search string because the resulting searches were insufficiently specific.
Notes
1. The terms are listed in a table available from the corresponding author.
2. In this sentence as elsewhere, counts of reasons exclude repeats of reasons in the same publication.
3. Publications agreeing, that a fair level of benefits may but need not include PTA, with the latter view were on average more recent.
4. Sometimes or always.
5. Endorsed >10 times and endorsed by >95% of commentators that mentioned the reason.
6. >22 Reason Mentions.
7. References were counted only if they were references given when mentioning reasons why PTA should, or need not be ensured.
8. Publications that referenced Glantz et al., but not when mentioning a qualifying reason, are excluded from this count.
9. This will require the identification an appropriate, measurable concept of cost, as well as empirical research.
10. A result tangential to this article is that, for the above reasons, systematic reviews may be an important research tool for philosophical bioethicists. However, systematic reviews of philosophical literature pose various conceptual and methodological challenges (McCullough et al., 2007) (see also p. [complete once paper has been type-set]).
11. The issue of whether to include frequency data is discussed in greater length in (Sofaer and Strech, 2011; Strech and Sofaer, How to write a systematic review of reasons, manuscript.)
References
- 45CFR46 revised (2009). Code of federal regulations title 45. Public welfare. Department of health and human services. National Institutes of Health Office for Protection from Research Risks. 45.
- Annas G J, Grodin M A. Human Rights and Maternal-Fetal HIV Transmission Prevention Trials in Africa. American Journal of Public Health. 1998;88:560–563. doi: 10.2105/ajph.88.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft R. After the Trial is Over: What are the Sponsor’s Obligations? 2005 http://www.scidev.net/en/policy-briefs/after-the-trial-is-over-what-are-the-sponsor-s-obl.html [accessed 27 May 2011] [Google Scholar]
- Ballantyne A. HIV International Clinical Research: Exploitation and Risk. Bioethics. 2005;19:476–491. doi: 10.1111/j.1467-8519.2005.00459.x. [DOI] [PubMed] [Google Scholar]
- Ballantyne A J. 2006a. Exploitation in HIV/AIDS International Clinical Research. PhD Dissertation, Monash University. [Google Scholar]
- Ballantyne A J. 2006b. Exploitation in HIV/AIDS International Clinical Research. PhD Dissertation, Monash University. [Google Scholar]
- Basu S, Andrews J, Smith-Rohrberg D. Populations Who Test Drugs Should Benefit from them. Nature. 2006;440:605. doi: 10.1038/440605d. [DOI] [PubMed] [Google Scholar]
- BBC Staff. Trial Volunteers ‘Left in the Lurch’. BBC News Online. 2007 24 December. [Google Scholar]
- Benatar S R. Justice and International Research: A Response to Reidar K. Lie. In: Levine R J, Gorovitz S, editors. Biomedical Research Ethics: Updating International Guidelines. A Consultation. 2000. pp. 41–50. [Google Scholar]
- Benatar S R, Fleischer T E. Ethical Issues in Research in Low-Income Countries. The International Journal of Tuberculosis and Lung Disease. 2007;11:617–623. [PubMed] [Google Scholar]
- Berkley S. Thorny Issues in the Ethics of AIDS Vaccine Trials. Lancet. 2003;362:992. doi: 10.1016/S0140-6736(03)14371-1. [DOI] [PubMed] [Google Scholar]
- Brody B A. Ethical Issues in Clinical Trials in Developing Countries. Statistics in Medicine. 2002;21:2853–2858. doi: 10.1002/sim.1289. [DOI] [PubMed] [Google Scholar]
- Busse P. Strident, but Essential: The Voices of People with AIDS. British Medical Journal. 1997;314:888–889. doi: 10.1136/bmj.314.7084.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carse A L, Little M O. Exploitation and the Enterprise of Medical Research. In: Hawkins J S, Emanuel E J, editors. Exploitation and Developing Countries: The Ethics of Clinical Research. Princeton and Oxford: Princeton University Press; 2008. pp. 206–245. [Google Scholar]
- Chang E. Fitting a Square Peg into a Round Hole? Imposing Informed Consent and Post-Trial Obligations on United States Sponsored Clinical Trials in Developing Countries. Southern California Interdisciplinary Law Journal. 2002;11:339–360. [PubMed] [Google Scholar]
- Ciaranello A L, Walensky R P, Sax P E, Chang Y, Freedberg K A, Weissman J S. Access to Medications and Medical Care after Participation in HIV Clinical Trials: A Systematic Review of Trial Protocols and Informed Consent Documents. HIV Clinical Trials. 2009;10:13–24. doi: 10.1310/hct1001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIOMS. Proposed International Guidelines for Biomedical Research Involving Human Subjects. Geneva: World Health Organization and Council for International Organizations of Medical Sciences; 1982. [Google Scholar]
- CIOMS. International Ethical Guidelines for Biomedical Research Involving Human Subjects. Proposed Guidelines. Geneva: CIOMS (Council for International Organisations of Medical Sciences) and WHO (World Health Organization); 1983. [PubMed] [Google Scholar]
- CIOMS. International Guidelines for Ethical Review of Epidemiological Studies. Geneva: Council of International Organizations of Medical Sciences (CIOMS); 1991. [Google Scholar]
- CIOMS. International Ethical Guidelines for Biomedical Research Involving Human Subjects. Geneva: Council for International Organizations of Medical Sciences; 1993. [PubMed] [Google Scholar]
- CIOMS. International Ethical Guidelines for Biomedical Research Involving Human Subjects. Geneva: Council for International Organizations of Medical Sciences; 2002. [PubMed] [Google Scholar]
- Cleaton-Jones P E. An Ethical Dilemma. Availability of Antiretroviral Therapy After Clinical Trials with HIV Infected Patients are Ended. British Medical Journal. 1997;314:887–888. doi: 10.1136/bmj.314.7084.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, O'Neill J, Joffres M, Upshur R, Mills E. Reporting of Informed Consent, Standard of Care and Post-Trial Obligations in Global Randomized Intervention Trials: A Systematic Survey of Registered Trials. Developing World Bioethics. 2008;9:74–80. doi: 10.1111/j.1471-8847.2008.00233.x. [DOI] [PubMed] [Google Scholar]
- Cooley D R. Distributive Justice and Clinical Trials in the Third World. Theoretical Medicine and Bioethics. 2001;22:151–167. doi: 10.1023/a:1011452716028. [DOI] [PubMed] [Google Scholar]
- Crouch R A, Arras J D. Azt Trials and Tribulations. Hastings Center Report. 1998;28:26–34. [PubMed] [Google Scholar]
- Daniels N. Just Health: Meeting Health Needs Fairly. Cambridge; New York: Cambridge University Press; 2008. [Google Scholar]
- Dworkin R. Sovereign Virtue: The Theory and Practice of Equality. Cambridge, MA: Harvard University Press; 2000. [Google Scholar]
- Emanuel E J. A World of Research Subjects. Hastings Center Report. 1998;28:25. [PubMed] [Google Scholar]
- Emanuel E J. Benefits to Host Countries. In: Emanuel E J, Grady C, Crouch R A, Lie R K, Miller F G, Wendler D, editors. The Oxford Textbook of Clinical Research Ethics. Princeton; Oxford: Princeton University Press; 2008. pp. 719–728. [Google Scholar]
- Emanuel E J, Grady C, Crouch R A, Lie R K, Miller F G, Wendler D. Introduction. In: Emanuel E J, Grady C, Crouch R A, Lie R K, Miller F G, Wendler D, editors. The Oxford Textbook of Clinical Research Ethics. New York: Oxford University Press; 2008. pp. 3–6. [Google Scholar]
- Fernandez C V, Kodish E, Weijer C. Informing Study Participants of Research Results: An Ethical Imperative. IRB: Ethics and Human Research. 2003;25:12–19. [PubMed] [Google Scholar]
- Gbadegesin S, Wendler D. Protecting Communities in Health Research from Exploitation. Bioethics. 2006;20:248–253. doi: 10.1111/j.1467-8519.2006.00501.x. [DOI] [PubMed] [Google Scholar]
- Glantz L H, Annas G J, Grodin M A, Mariner W K. Research in Developing Countries: Taking “Benefit” Seriously. Hastings Center Report. 1998;28:38–42. [PubMed] [Google Scholar]
- Gostin L. Ethical Principles for the Conduct of Human Subject Research: Population-based Research and Ethics. Law, Medicine and Health Care. 1991;19:191–201. doi: 10.1111/j.1748-720x.1991.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Grady C. The Challenge of Assuring Continued Post-Trial Access to Beneficial Treatment. Yale Journal of Health Policy, Law, and Ethics. 2005;5:425–435. [PubMed] [Google Scholar]
- Greenhalgh T, Peacock R. Effectiveness and Efficiency of Search Methods in Systematic Reviews of Complex Evidence: Audit of Primary Sources. British Medical Journal. 2005;331:1064–1065. doi: 10.1136/bmj.38636.593461.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B, Hausdorff W P. After a Trial is Over: The Ethical Issues. 2003. [Internet]. Available from: http://www.scidev.net/en/policy-briefs/after-a-trial-is-over-the-ethical-issues-1.html [accessed 27 May 2011] [Google Scholar]
- Guyatt G H, Oxman A D, Vist G E, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann H J. Grade: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. British Medical Journal. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harth S C, Thong Y H. Aftercare for Participants in Clinical Research: Ethical Considerations in an Asthma Drug Trial. Journal of Medical Ethics. 1995;21:225–228. doi: 10.1136/jme.21.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J S, Emanuel E J. Introduction: Why exploitation? In: Hawkins J S, Emanuel E J, editors. Exploitation and Developing Countries: The Ethics of Clinical Research. Princeton; Oxford: Princeton University Press; 2008. pp. 1–20. [Google Scholar]
- Hutt L E. Freebies for Subject 641: A Discussion of the Ethical Prospect of Providing Drug Trial Subjects with Post-Trial Access to the Drug Tested–A Canadian Perspective. Health Law Journal. 1998;6:169–187. [PubMed] [Google Scholar]
- India Council of Medical Research. Ethical Guidelines for Biomedical Research on Human Participants. New Delhi: Indian Council of Medical Research; 2006. [Google Scholar]
- Kass N, Hyder A. Attitudes and Experiences of U.S. and Developing Country Investigators Regarding U.S. HUMAN Subjects Regulations. In: National Bioethics Advisory Commission Group, editor. Ethical and Policy Issues in International Research: Clinical Trials in Developing Countries II: Commissioned Papers. Bethesda, MD: National Bioethics Advisory Commission; 2002. pp. B1–B220. [Google Scholar]
- King P. A Partnership to Resolve the Conundrum. British Medical Journal. 1997;314:890–891. doi: 10.1136/bmj.314.7084.890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolata G, Eichenwald K. Stopgap Medicine, a Special Report: For the Uninsured, Drug Trials are Health Care. New York Times. 1999 22 June. [PubMed] [Google Scholar]
- Kottow M H. Who is My Brother’s Keeper? Journal of Medical Ethics. 2002;28:24–27. doi: 10.1136/jme.28.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery J V. Putting International Research Ethics Guidelines to Work for the Benefit of Developing Countries. Yale Journal of Health Policy, Law and Ethics. 2004;4:319–336. [PubMed] [Google Scholar]
- Lavery J V. The Obligation to Ensure Access to Beneficial Treatments for Research Participants at the Conclusion of Clinical Trials. In: Emanuel E J, Grady C, Crouch R A, Lie R K, Miller F G, Wendler D, editors. The Oxford Textbook of Clinical Research Ethics. New York: Oxford University Press; 2008. pp. 697–710. [Google Scholar]
- Lemmer B, Grellier R, Steven J. Systematic Review of Nonrandom and Qualitative Research Literature: Exploring and Uncovering an Evidence Base for Health Visiting and Decision Making. Qualitative Health Research. 1999;9:315–328. [Google Scholar]
- Lie R. Justice and International Research. In: Levine R, Gorovitz S, Gallagher J, editors. Biomedical Research Ethics: Updating International Guidelines. Geneva: CIOMS; 2000. pp. 27–40. [Google Scholar]
- Lo B, Bayer R. Establishing Ethical Trials for Treatment and Prevention of AIDS in Developing Countries. British Medical Journal. 2003;327:337–339. doi: 10.1136/bmj.327.7410.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London A J. Justice and the Human Development Approach to International Research. Hastings Center Report. 2005;35:24–37. [PubMed] [Google Scholar]
- Macklin R. Double Standards in Medical Research in Developing Countries. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- Macpherson C C. Research Sponsors Duties to Developing World Host Nations: The Ongoing WMA Discussion of Possible Revisions to the 2000 Declaration of Helsinki (paragraph 30) Developing World Bioethics. 2004;4:173–175. doi: 10.1111/j.1471-8731.2004.00092.x. [DOI] [PubMed] [Google Scholar]
- McCullough L B, Coverdale J H, Chervenak F A. Argument-based Medical Ethics: A Formal Tool for Critically Appraising the Normative Medical Ethics Literature. American Journal of Obstetrics and Gynecology. 2004;191:1097–1102. doi: 10.1016/j.ajog.2004.06.060. [DOI] [PubMed] [Google Scholar]
- McCullough L B, Coverdale J H, Chervenak F A. Constructing a Systematic Review for Argument-Based Clinical Ethics Literature: The Example of Concealed Medications. Journal of Medicine and Philosophy. 2007;32:65–76. doi: 10.1080/03605310601152206. [DOI] [PubMed] [Google Scholar]
- McLean G R. A Case for Goodwill. British Medical Journal. 1997;314:890. doi: 10.1136/bmj.314.7084.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt M. Bioethics, Philosophy and Global Health. Yale Journal of Health Policy, Law, and Ethics. 2007;7:273–317. [PubMed] [Google Scholar]
- Merritt M, Grady C. Reciprocity and Post-Trial Access for Participants in Antiretroviral Therapy Trials. AIDS. 2006;20:1791–1794. doi: 10.1097/01.aids.0000244197.88134.45. [DOI] [PubMed] [Google Scholar]
- Millum J. Post-trial Access to Antiretrovirals: Who Owes What to Whom? Bioethics. 2009 doi: 10.1111/j.1467-8519.2009.01736.x. [Internet]. Available from: http://onlinelibrary.wiley.com/doi/10.1111/j.1467-8519.2009.01736.x/pdf [accessed 7 July 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Bioethics Advisory Commission Group. Ethical and Policy Issues in International Research: Clinical Trials in Developing Countries. Bethesda, MD: National Bioethics Advisory Commission; 2001. [PubMed] [Google Scholar]
- NIH Group. 2005. Guidance for Addressing the Provision of Antiretroviral Treatment for Trial Participants Following their Completion of NIH-Funded HIV Antiretroviral Treatment Trials in Developing Countries. In National Institutes of Health (ed.) [Google Scholar]
- Nuffield Council on Bioethics. The Ethics of Research Related to Healthcare in Developing Countries. London: Nuffield Council on Bioethics; 2002. [Google Scholar]
- Nuffield Council on Bioethics Group. The Ethics of Clinical Research in Developing Countries: A Discussion Paper. London: Nuffield Council on Bioethics; 1999. [Google Scholar]
- Nuffield Council on Bioethics Group. The Ethics of Research Related to Healthcare in Developing Countries. London: Nuffield Council on Bioethics; 2002. [Google Scholar]
- Orentlicher D. Universality and its Limits: When Research Ethics can Reflect Local Circumstances. The Journal of Law, Medicine and Ethics. 2002;30:403–410. doi: 10.1111/j.1748-720x.2002.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Pace C, Miller F G, Danis M. Enrolling the Uninsured in Clinical Trials: An Ethical Perspective. Critical Care Medicine. 2003;31:S121–S125. doi: 10.1097/01.CCM.0000054907.33928.48. [DOI] [PubMed] [Google Scholar]
- Page A K. Prior Agreements in International Clinical Trials: Ensuring the Benefits of Research to Developing Countries. Yale Journal of Health Policy, Law, and Ethics. 2002;3:35–66. [PubMed] [Google Scholar]
- Page A K. Ethical Issues in International Biomedical Research: An Overview. Journal of Health Law. 2004;37:629–665. [PubMed] [Google Scholar]
- Participants in the 2001 Conference on Ethical Aspects of Research in Developing Countries. Moral Standards for Research in Developing Countries: From “reasonable availability” to “fair benefits”. Hastings Center Report. 2004;34:17–27. [PubMed] [Google Scholar]
- Participants in the Conference on Ethical Aspects of Research in Developing Countries. Ethics. Fair benefits for research in developing countries. Science. 2002;298(5601):2133–2134. doi: 10.1126/science.1076899. [DOI] [PubMed] [Google Scholar]
- PhRMA Group. PhRMA Discussion Paper on the Declaration of Helsinki as Revised in October 2000. Washington, DC, USA: Pharmaceutical Research and Manufacturers of America; 2001. [Google Scholar]
- Richardson H S. Gradations of Researchers’ Obligation to Provide Ancillary Care for HIV/AIDS in Developing Countries. American Journal of Public Health. 2007;97:1956–1961. doi: 10.2105/AJPH.2006.093658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H S, Belsky L. The Ancillary-Care Responsibilities of Medical Researchers: An Ethical Framework for Thinking about the Clinical Care that Researchers Owe their Subjects. Hastings Center Report. 2004;34:25–33. [PubMed] [Google Scholar]
- Sachs B. Going from Principles to Rules in Research Ethics. Bioethics. 2009 doi: 10.1111/j.1467-8519.2009.01744.x. [Internet]. Available from: http://grants.nih.gov/grants/policy/antiretroviral/ [accessed 27 May 2011] [DOI] [PubMed] [Google Scholar]
- Shaffer D N, Yebei V N, Ballidawa J B, Sidle J E, Greene J Y, Meslin E M, Kimaiyo S J, Tierney W M. Equitable Treatment for HIV/AIDS Clinical Trial Participants: A focus Group Study of Patients, Clinician Researchers, and Administrators in Western Kenya. Journal of Medical Ethics. 2006;32:55–60. doi: 10.1136/jme.2004.011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Elmer S, Grady C. Planning for Posttrial Access to Antiretroviral Treatment for Research Participants in Developing Countries. American Journal of Public Health. 2009;99:1556–1562. doi: 10.2105/AJPH.2008.157982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A W. Kantian Ethics, Exploitation, and Multinational Clinical Trials. In: Hawkins J S, Emanuel E J, editors. Exploitation and Developing Countries: The Ethics of Clinical Research. Princeton; Oxford: Princeton University Press; 2008. pp. 286–313. [Google Scholar]
- Slack C, Stobie M, Milford C, Lindegger G, Wassenaar D, Strode A, Ijsselmuiden C. Provision of HIV Treatment in HIV Preventive Vaccine Trials: A Developing Country Perspective. Social Science and Medicine. 2005;60:1197–1208. doi: 10.1016/j.socscimed.2004.06.049. [DOI] [PubMed] [Google Scholar]
- Sofaer N, Strech D. The Need for Systematic Reviews of Reasons. Bioethics. 2011 doi: 10.1111/j.1467-8519.2011.01858.x. doi:10.1111/j.1467-8519.2011.01858.x (Epub ahead of print 27 April 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofaer N, Thiessen C, Campbell E G, Ballou J, Goold S D, Getz K A, Koski G, Krueger R A, Weissman J S. Subjects’ Views of Obligations to Ensure Post-Trial Access to Drugs, Care, and Information: Qualitative Results from the Experiences of Participants in Clinical Trials (epic) Study. Journal of Medical Ethics. 2009;35:183–188. doi: 10.1136/jme.2008.024711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strech D, Synofzik M, Marckmann G. Systematic Reviews of Empirical Bioethics. Journal of Medical Ethics. 2008;34:472–477. doi: 10.1136/jme.2007.021709. [DOI] [PubMed] [Google Scholar]
- The European Group on Ethics in Science and New Technologies to the European Commission. Ethical Aspects of Clinical Research in Developing Countries. 2003. [Internet]. Available from: http;//europa.eu.int/comm/european_group_ethics/docs/avis17_en.pdf [accessed] [Google Scholar]
- UNAIDS Group. 2000. Ethical Considerations in HIV Preventive Vaccine Research: UNAIDS Guidance Document. [Google Scholar]
- Wellcome Trust Group. Research Involving People Living in Developing Countries: Position Statement and Guidance Notes for Applicants. 2004. [Internet]. Available from: http://www.wellcome.ac.uk/About-us/Policy/Policy-and-position-statements/WTD015295.htm [accessed 27 May 2011] [Google Scholar]
- Wertheimer A. Exploitation. Princeton, NJ: Princeton University Press; 1999. [Google Scholar]
- Wertheimer A. Exploitation in Clinical Research. In: Hawkins J S, Emanuel E J, editors. Exploitation and Developing Countries: The Ethics of Clinical Research. Princeton and Oxford: Princeton University Press; 2008. pp. 63–104. [Google Scholar]
- Wolinsky H. The Battle of Helsinki: Two Troublesome Paragraphs in the Declaration of Helsinki are Causing a Furore Over Medical Research Ethics. EMBO Report. 2006;7:670–672. doi: 10.1038/sj.embor.7400743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Ferney-Voltaire: World Medical Association. 2004. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. 2008. [Internet]. Ferney-Voltaire: World Medical Association, available from: http://www.wma.net/en/30publications/10policies/b3/17c.pdf [accessed 27 May 2011] [DOI] [PubMed] [Google Scholar]
- Yearby R. Good Enough to Use for Research, but Not Good Enough to Benefit from the Results of that Research: Are the Clinical HIV Vaccine Trials in Africa Unjust? De Paul Law Review. 2004;53:1127–1154. [PubMed] [Google Scholar]
- Zong Z. Should Post-Trial Provision of Beneficial Experimental Interventions be Mandatory in Developing Countries? Journal of Medical Ethics. 2008;34:188–192. doi: 10.1136/jme.2006.018754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.