Abstract
Resveratrol (RESV; 3,5,4′-tri-hydroxy stilbene), a naturally occurring phytoalexin, is found at a high concentration in the skin of red grapes and red wine. RESV mediates a wide-range of biological activities, which comprise an increased life span, anti-ischemic, anti-cancer, antiviral, anti-aging and anti-inflammatory properties. Studies in several animal prototypes of brain injury suggest that RESV is an effective neuroprotective compound. Ability to enter the brain after a peripheral administration and no adverse effects on the brain or body are other features that are appealing for using this compound as a therapy for brain injury or neurodegenerative diseases. The goal of this review is to discuss the promise of RESV for treating acute seizures, preventing the acute seizure or status epilepticus induced development of chronic epilepsy, and easing the chronic epilepsy typified by spontaneous recurrent seizures and cognitive dysfunction. First, the various beneficial effects of RESV on the normal brain are discussed to provide a rationale for considering RESV treatment in the management of acute seizures and epilepsy. Next, the detrimental effects of acute seizures or status epilepticus on the hippocampus and the implications of post-status epilepticus changes in the hippocampus towards the occurrence of chronic epilepsy and cognitive dysfunction are summarized. The final segment evaluates studies that have used RESV as a neuroprotective compound against seizures, and proposes studies that are critically needed prior to the clinical application of RESV as a prophylaxis against the development of chronic epilepsy and cognitive dysfunction after an episode of status epilepticus or head injury.
Keywords: Acute seizures, Cognitive dysfunction, Dentate gyrus, Epilepsy, Epileptogenesis, GABA-ergic interneurons, Hippocampal neurogenesis, Inflammation, Learning and memory, Neurodegeneration, Neuroinflammation, Neuroprotection, Oxidative stress, Polyphenol, Resveratrol, Red wine, Seizures, SIRT1, Status epilepticus, Temporal lobe epilepsy
1. Introduction
Resveratrol (RESV; 3,5,4′-tri-hydroxy stilbene) is a type of polyphenol and an antimicrobial substance synthesized de novo by plants (a phytoalexin). RESV is found in the skin of red grapes and is a component of red wine (Fremont, 2000; Orallo, 2008). The other sources of RESV include raspberries, mulberries, plums, peanuts, bilberries, blueberries, cranberries, Scots pine, and Japanese knotweed. RESV is synthesized instinctively by the above plants as a protection to counter the bacterial and fungal infections, stress and injury (Balestrazzi et al., 2009; Maddox et al., 2010). RESV received substantial notice with the emergence of “French paradox”, which is portrayed by the reduced prevalence of cardiovascular diseases in the red wine drinking southern France population notwithstanding eating foods that are rich in saturated fats (Vidavalur et al., 2006). Although RESV subsists as both cis- and transisomeric forms, the trans-isomer is the steady form of RESV, which is also the isomer that plays a role in nearly all biological actions of RESV (Fremont, 2000). RESV mediates a variety of biological activities which comprise extension of the life span even when fed a high caloric diet and cancer prevention (Howitz et al., 2003; Wood et al., 2004; Baur et al., 2006; Baur & Sinclair, 2006; Orallo, 2008). Studies in animal models also imply a number of other beneficial health effects of RESV, which comprise anti-ischemic, antiviral, anti-oxidant and anti-inflammatory properties (Belguendouz et al., 1997; Jang et al., 1999; Manna et al., 2000; Sato et al., 2000; Kraft et al., 2009; Campagna & Rivas, 2010; Robich et al., 2010; Sun et al., 2010). Furthermore, RESV shows promise for delaying the onset of a variety of age-related diseases (Orallo, 2008; Rossi et al., 2008; Karuppagounder et al., 2009).
Pertaining to the central nervous system, multiple cell culture investigations and in vivo studies in animal models of neurodegenerative diseases/brain injury point out that RESV is a potent neuroprotective compound. First, in cell culture studies, RESV treatment reduced: (i) ethanol induced neuronal cell death (Sun et al., 1997); (ii) sodium nitroprusside induced hippocampal cell death and intracellular reactive oxygen species (ROS) accumulation (Bastianetto et al., 2000); (iii) neuronal cell death in the presence of amyloid beta-peptide, a neurotoxic peptide believed to have a role in the pathogenesis of Alzheimer's disease (Jang & Surh, 2003; Han et al., 2004); and (iv) the loss of dopaminergic neurons in rat primary midbrain neuron–glial cultures treated with lipopolysaccharide (LPS) via anti-inflammatory activity (Zhang et al., 2010). Second, in animal models of stroke, RESV pre-treatment provided neuroprotection via its antioxidant actions and induction of heme oxygenase 1 (Sinha et al., 2002; Wang et al., 2002; Baur & Sinclair, 2006; Sakata et al., 2010). Third, RESV treatment provided neuroprotection and functional recovery in a rat model of spinal cord injury via its anti-oxidant, anti-apoptotic and anti-inflammatory actions (Liu et al., 2010). Fourth, oral administration of RESV attenuated neuronal damage and neurological dysfunction in a rat model of multiple sclerosis likely via anti-inflammatory activity (Shindler et al., 2010). Recent studies imply that p38 mitogen-activated protein kinase (p38MAPK) cascade is a key signal transduction pathway for eliciting the anti-inflammatory action of RESV through transcriptional induction of macrophage inhibitory cytokine-1 (Golkar et al., 2007; Paul et al., 2009). Fifth, RESV treatment provided neuroprotection in animal models of Huntington's disease via activation of Ras-extracellular signal-regulated kinase (ERK; Maher et al., 2010). Sixth, RESV attenuates behavioral impairments and reduces cortical and hippocampal neuron loss in a rat controlled cortical impact model of traumatic brain injury (Singleton et al., 2010). Thus, the capability of RESV for vigorous neuroprotection has been detected in animal models of multiple neurodegenerative diseases. Besides, its ability to traverse the blood–brain-barrier after peripheral administration (Mokni et al., 2007)and a lack of undesirable consequences on the brain are other characteristics that present this compound as attractive for therapeutic use in neurodegenerative disorders (Baur & Sinclair, 2006). Additionally, the activity of RESV in the brain after peripheral administration (including after an oral administration) can last up to 4 h (Wang et al., 2002; Abd El-Mohsen et al., 2006). Taken together, it is clear that RESV is a potent neuroprotective compound. Yet, the relevance of the above findings to clinical situations needs to be validated.
The purpose of this review is to discuss the promise of RESV administration for treating acute seizures, preventing acute seizure or status epilepticus (SE) induced chronic epilepsy, and for easing chronic epilepsy characterized by spontaneous recurrent motor seizures. The first section will discuss the known effects of RESV on the normal brain, which support the use of RESV for treating acute seizures and epilepsy. The second section summarizes the detrimental effects of acute seizures or status epilepticus (SE) on the hippocampus and the implications of post-SE changes in the hippocampus towards the occurrence of chronic epilepsy and cognitive dysfunction. The final segment evaluates studies that have used RESV as a neuroprotective compound against seizures and epilepsy. Furthermore, critical studies that are needed before considering RESV as a prophylaxis treatment against chronic epilepsy development and cognitive dysfunction after an initial precipitating event such as SE or head injury are discussed.
2. Resveratrol treatment and normal brain function
Because of a higher degree of oxygen consumption and availability of relatively lower levels of antioxidant defense enzymes, the adult brain is very vulnerable to free radical mediated damage (Halliwell, 2006). Studies on the effects of RESV treatment on normal brain function suggested many beneficial effects, which include maintenance of the mitochondrial function, neuroprotective properties, preservation of cognitive function and anti-excitatory properties. These issues are briefly discussed in the following sections to support the use of RESV for treating acute seizures and epilepsy.
2.1. Resveratrol and mitochondrial function
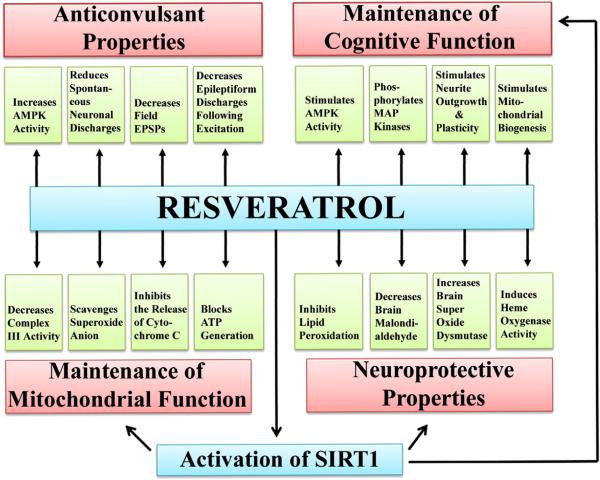
Zini et al. (1999) showed that RESV decreases complex III activity in the mitochondria through competition with coenzyme Q. This mode of action imparts beneficial effects as complex III is the site where ROS are generated. RESV was also able to scavenge the superoxide anion generated from the rat forebrain mitochondria in a concentration dependent manner (Zini et al., 1999). Thus, by reducing complex III activity, RESV could both oppose the production of ROS and scavenge the ROS that were produced. Furthermore, a follow up study by the same group (Zini et al., 2002) demonstrated that RESV inhibits the release of cytochrome c (an initial step of mitochondrial apoptosis) and decreases the superoxide anion production, which in turn protected the mitochondrial membranes in an anoxia–reoxygenation model. Another study using a prototype of anoxia–reoxygenation applied to suspensions of mitochondria isolated from the rat cortex, confirmed the effects of RESV on cytochrome c release and showed that RESV blocks ATP generation (Tillement, 2001). Thus, RESV intake appears to be useful for preserving mitochondrial function through antioxidant properties, actions on complex III, and a membrane stabilizing effect (Fig. 1).
Fig. 1.
The various beneficial effects of resveratrol (RESV) on the normal brain function that provide a strong rationale for administering RESV in conditions such as status epilepticus (SE) or brain injury as a neuroprotective compound. The neuroprotective properties of RESV are supported by its ability to inhibit the lipid peroxidation, reduce brain malondialdehyde levels, enhance the concentration of brain superoxide dismutase, and induce heme oxygenase activity (see the lower right region of the illustration). The role of RESV in the maintenance of mitochondrial function is indicated by its ability to decrease complex III activity, scavenge superoxide anions, inhibit the release of cytochrome C, and block adenosine triphosphate (ATP) generation (see the lower left region). The anticonvulsant properties of RESV are typified by its ability to increase 5′ AMP-activated protein kinase (AMPK) activity, reduce spontaneous neuronal discharges, decrease the field excitatory post-synaptic potentials, and decrease the epileptiform discharges following excitation (see the upper left region). The contribution of RESV towards the maintenance of cognitive function is supported by its ability to stimulate AMPK activity, phosphorylate mitogen-activated protein (MAP) kinases, stimulate the neurite outgrowth, and promote neural plasticity and mitochondrial biogenesis (see the upper right region).
2.2. Resveratrol and lipid peroxidation
Inhibition of the lipid peroxidation by RESV has been demonstrated in several studies (Lu et al., 2002; Zhuang et al., 2003; Mokni et al., 2007). In one of the studies, intraperitoneal administration of RESV in a healthy normal rat decreased brain malondialdehyde (MDA) levels and increased brain superoxide dismutase, catalase and peroxidase activities (Mokni et al., 2007). Optimal effects were seen with the RESV dose of 12.5 mg/kg body weight (bw). Another study using neuronal cell cultures demonstrated that RESV treatment induces heme oxygenase 1 activity with no detectable toxic effects (Zhuang et al., 2003). Because heme levels increase inside cells after stroke and heme (iron-protoporphyrin IX) is considered a pro-oxidant, its rapid degradation by heme oxygenase is believed to be neuroprotective. From this perspective, increased heme oxygenase activity is likely one of the mechanisms by which RESV functions as a neuroprotective compound. Thus, RESV exerts neuroprotective properties by regulating several detoxifying enzymes (Fig. 1).
2.3. Resveratrol and phosphorylation of MAP kinases and AMP kinase activity
Using human neuroblastoma SH-SY5Y cells in vitro, Tredici et al. (1999) showed that RESV induces phosphorylation of several MAP kinases which include extracellular signal-regulated kinase 1 (ERK1) and ERK2. As MAP kinases are critical for different aspects of signal transduction in cells, and phosphorylation of ERK2 is vital for synaptic changes in response to memory and learning processes, effects of RESV on MAP kinases are likely useful for preventing dementia. Indeed, epidemiological studies suggest that moderate wine intake decreases the incidence of dementia (Mehlig et al., 2008). Moreover, proteomics analyses of the rat brain proteins following RESV treatment suggests that RESV mediated protection against dementia involves prevention of the loss of proteins that are implicated in cognitive disorders (Kim et al., 2006). Furthermore, RESV stimulates AMP kinase activity in Neuro2a cells and primary neurons in vitro, and neurons in the adult brain (Dasgupta & Milbrandt, 2007). RESV mediated AMPK activation promoted robust neurite outgrowth in Neuro2a cells, which could be blocked by genetic and pharmacologic inhibition of AMPK. Further analyses also provided evidence that RESV induces mitochondrial biogenesis via AMPK activation. Thus, phosphorylation of MAPKs and activation of AMPK by RESV in neurons likely helps in the maintenance of cognitive functions and provides neuroprotection (Fig. 1).
2.4. Resveratrol and electrical activity of hippocampal neurons
Using a prototype of rat hippocampal neuronal cultures, Gao and Hu (2005) demonstrated that superfusion of RESV could reversibly inhibit the delayed rectifier (I(K)) and fast transient K(+) current (I[A]). As voltage-gated K(+) channels have been implicated in neuronal apoptosis, it is possible that inhibition of voltage-activated K(+) currents by RESV contributes to its neuroprotective effects. Furthermore, Li et al. (2005) using extracellular recording techniques in hippocampal slices demonstrate that RESV inhibits neuronal discharges in rat hippocampal CA1 area. Specifically, application of RESV reduced the spontaneous discharge rate in majority of neurons in a dose dependent manner. Furthermore, RESV suppressed epileptiform discharges in slices mediated by several compounds, which include L-glutamate, L-type calcium channel agonist, and nitric oxide synthase inhibitor L-NAME. Additionally, a study by Gao et al. (2006) showed that perfusion with RESV causes a concentration-dependent reversible inhibition of field excitatory postsynaptic potentials via suppression of glutamate-induced currents in postsynaptic CA1 pyramidal neurons, suggesting inhibition of postsynaptic glutamate receptors by RESV. Thus, RESV has the ability to inhibit the electrical activity of hippocampal pyramidal neurons through several mechanisms. This anticonvulsant property makes RESV ideal as a neuroprotective agent against acute seizures (Fig. 1).
2.5. Resveratrol and oxidative stress
Okawara et al. (2007) examined the neuroprotective effect of RESV on dopaminergic neurons in organotypic slice cultures prepared from the midbrain. They demonstrated that RESV prevents the loss of dopaminergic neurons when slice cultures were treated with a dopaminergic neurotoxin, 1-methyl-4-phenyl pyridinium. They also found that RESV provides concentration-dependent neuroprotective effects against a mitochondrial complex IV inhibitor sodium azide, and a microglia-activating agent thrombin. These beneficial effects appeared to be due to a direct antioxidant property of RESV, as inhibitors of silent mating type information regulation 2 homolog 1 (SIRT1, a class III histone deacetylase) did not attenuate the protective effect of RESV, and RESV treatment reduced the accumulation of ROS, depletion of cellular glutathione, and cellular oxidative damage. In another study, RESV pre-treatment effectively protected against cadmium-induced lipid peroxidation and ameliorated the adverse effect of cadmium (Eybl et al., 2006). Thus, RESV is an effective antioxidant, which makes it suitable as a neuroprotective compound against acute seizures or SE.
2.6. Resveratrol and inflammation
A study examined the effects of RESV administration on nitric oxide and tumor necrosis factor-alpha (TNF-alpha) production in cultured microglia that are activated through lipopolysaccharide (LPS) treatment (Bi et al., 2005). While the microglial cultures exposed to LPS alone exhibited increased levels of TNF-alpha and NO, microglial cultures exposed to LPS and RESV displayed no significant increases in TNF-alpha and NO. Additional analyses revealed that RESV administration suppressed LPS-induced expression of iNOS and phosphorylation of p38 MAPKs in microglial cells. Thus, RESV can potently suppress proinflammatory responses of microglia. Considering these, it appears that RESV administration would be beneficial for curtailing the inflammatory reaction in neurodegenerative diseases. This is particularly applicable to conditions where significant microglial activation is one of the pathological changes such as after acute seizure or SE induced brain injury.
2.7. Resveratrol and activation of SIRT1
SIRT1, a nuclear protein and the mammalian equivalent of the silent information regulator 2 (SIR2) that promotes longevity in yeast, flies and nematodes, has a role in the regulation of key metabolic and physiological processes (Chung et al., 2010). SIRT1 is a class III histone deacetylase (HDAC) and believed to underlie the health benefits of caloric restriction, a diet that defers aging and neurodegeneration in mammals (Kim et al., 2007). Studies demonstrate that activation of SIRT1 by polyphenols such as RESV has multiple favorable outcomes (Chung et al., 2010). These include regulation of the oxidative stress, inflammation, cellular senescence, autophagy, apoptosis, differentiation, stem cell pluripotency, metabolism, and mitochondrial biogenesis (Chung et al., 2010). SIRT1 knockout or knockdown results in an increased activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and proinflammatory cytokine release. NF-κB, a protein complex that controls the transcription of DNA, is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, ultraviolet irradiation and bacterial or viral antigens. Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases (Brasier, 2006; Perkins, 2007). Interestingly, stimulation of SIRT1 by activators such as RESV inhibits NF-κB-mediated inflammatory mediators release (Chung et al., 2010). Studies have also shown that SIRT1 can interact, deacetylate and activate FOXO3, a transcription factor that regulates a numberof cellular responses, such as the cell cycle arrest, cellular senescence, proliferation, resistance to oxidative stress and apoptosis (Brunet et al., 2004; Chung et al., 2010). SIRT1 plays an important role in regulating autophagy, which is a process important for the turnover of cellular organelles and proteins to maintain the cell homeostasis. Indeed, SIRT1-deficient mice have accumulation of damaged organelles and disruption of homeostasis (Lee et al., 2008). Thus, activation of SIRT1 can mediate multiple beneficial health outcomes and enhanced physiological functions. RESV is the first polyphenolic compound that has been shown to activate SIRT1 (Cohen et al., 2004; Kaeberlein et al., 2005; Baur et al., 2006). Recent in vivo studies have revealed that RESV supplementation leads to increased activity of mitochondria and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α; a transcriptional coactivator that regulates the genes involved in energy metabolism), with a concomitant decrease in their acetylation (Baur & Sinclair, 2006; Lagouge et al., 2006). SIRT1-mediated deacetylation of PGC-1α by RESV acts as a regulator of mitochondrial energy balance and biogenesis. Hence, activation of SIRT1 via RESV appears to be beneficial for treating various chronic inflammatory diseases (Yeung et al., 2004; Milne et al., 2007; Rajendrasozhan et al., 2008; Chung et al., 2010; Singh et al., 2010).
Pertaining to the brain, SIRT1 affects different processes that are potentially involved in the maintenance of brain integrity. These include chromatin remodeling, DNA repair, cell survival, and neurogenesis (Michan et al., 2010). Absence of SIRT1 impairs cognitive abilities, including immediate memory, classical conditioning and spatial learning, and synaptic plasticity (Michan et al., 2010). Another recent study has also shown that SIRT1 modulates synaptic plasticity and memory formation (Gao et al., 2010). Activation of SIRT1 enhanced whereas loss of SIRT1 function impaired synaptic plasticity in this study. Further analyses revealed that these effects were mediated through post-transcriptional regulation of cAMP response-element binding protein (CREB) expression by a brain-specific microRNA, miR-134. Under normal conditions, SIRT1 limits the expression of miR-134 through a repressor complex containing the transcription factor YY1 (Gao et al., 2010). Additional characterization unraveled that SIRT1 deficiency leads to an increased expression of miR-134, which induces downregulation in the expression of CREB as well as the neurotrophic factor BDNF and impairs synaptic plasticity (Gao et al., 2010). Thus, SIRT1 has a direct role in regulating cognitive function in the brain. Indeed, a study has demonstrated that administration of RESV in the inducible p25 transgenic mice (a model of Alzheimer's disease and amyotrophic lateral sclerosis) reduced hippocampal neurodegeneration, prevented learning impairment and decreased the acetylation of the known SIRT1 substrates, PGC-1α and p53 (Kim et al., 2007). Overall, it appears that activation of SIRT1 through compounds such as RESV has great importance for providing neuroprotection and alleviating cognitive dysfunction observed in various neurodegenerative disorders including the temporal lobe epilepsy (TLE) (Fig. 1).
3. Acute seizures or status epilepticus and development of chronic temporal lobe epilepsy
Status epilepticus (SE) is an emergency condition typified by prolonged seizure activity, which affects > 150,000 Americans every year with ~20% mortality (Sirven & Waterhouse, 2003; Boggs, 2004). In addition, a significant percentage of SE-survivors exhibit morbidity typified by cognitive impairments and/or an increased risk for chronic epilepsy. The first line antiepileptic drugs (AEDs) such as benzodiazepines and phenytoin used for the treatment of SE are ineffective in ~40% of patients (Shaner et al., 1988; Sirven & Waterhouse, 2003; Knake et al., 2009). The AEDs also have adverse side effects and do not seem to alter the long-term detrimental effects of SE, such as cognitive impairments and chronic epilepsy. Hence, efficient alternative therapies are necessary for preventing SE-induced mortality and morbidity.
3.1. Long-term implications of SE-induced hippocampal injury on learning and memory
The hippocampus is highly vulnerable to SE-induced injury (Sankar et al., 1998; Sloviter et al., 2003). This is exemplified by substantial damage to the hippocampus observed after SE elicited by chemoconvulsants such as kainic acid (KA) or pilocarpine (Dube et al., 2001; Bengzon et al., 2002; Rao et al., 2006). The pattern and extent of hippocampal neurodegeneration in a rat model of SE induced through i.p. KA administration are illustrated in Fig. 2. Acute seizures or SE induced by KA in rat leads to degeneration of fractions of dentate hilar neurons (including the excitatory mossy cells) and CA1 and CA3 pyramidal neurons (Hellier et al., 1998; Hattiangady et al., 2004; Rao et al., 2006; Rao et al., 2007), and a significant inflammation (Hattiangady et al., 2004; Hattiangady & Shetty, 2008). An example of inflammation characterized by the presence of a large number of activated microglial cells after KA-induced SE in a rat model is shown in Fig. 3. Hippocampal injury inflicted by SE can lead to impairments in learning and memory function (Liu et al., 2003; Alessio et al., 2004a; Strine et al., 2005; Groticke et al., 2007; Jones et al., 2008; Lenck-Santini & Holmes, 2008). Studies in animal models show that SE impairs spatial learning and memory function. This was evidenced by: (i) a longer latency to the criterion and more reference errors in a radial arm-maze test; and (ii) longer escape latencies and deficient memory for finding the platform location in a Morris water maze test (Letty et al., 1995; Rutten et al., 2002; Mikati et al., 2004; Sayin et al., 2004; Detour et al., 2005). Fig. 4 illustrates spatial learning and memory impairments in a rat model after KA-induced SE. The observations are consistent with the clinical reports that memory difficulties are a frequent cognitive complaint in patients after SE and in patients with chronic epilepsy (Neville et al., 2007; Vannest et al., 2008). Thus, SE induces persistent cognitive dysfunction. As these impairments are associated with hippocampal neurodegeneration, apt interventions that provide maximal neuroprotection during and shortly after SE might prevent or greatly diminish these adverse effects on learning and memory function.
Fig. 2.
The structure of the hippocampus at 4 days after status epilepticus (SE) when visualized with the neuron-specific nuclear antigen (NeuN) immunostaining. A1 and A3 show anterior and posterior regions of the hippocampus from a vehicle-treated control rat. B1 and B3 show anterior and posterior regions of the hippocampus from a KA-treated rat showing moderate hippocampal injury. C1 and C3 show hippocampal regions from a KA treated rat showing massive hippocampal injury. A2, B2 and C2 are magnified views of dentate hilar (DH) regions from A1, B1 and C1. Note that, in the moderate injury group (B1–B3), the loss of neurons is considerable in the dentate hilus (DH) and the CA1 subfield but modest in the CA3 region. In contrast, in the massive injury group (C1–C3), the loss of CA1 and CA3 pyramidal neurons is dramatic throughout the hippocampus. DG, dentate gyrus. Scale bar, A1, B1, C1 and A3, B3 and C3=500 μm; A2, B2 and C2=100 μm. The bar chart (D) depicts the absolute number of surviving neurons in different regions of the hippocampus of vehicle-treated rats, and KA-treated rats with moderate or massive hippocampal injury at 4 days post-administration. The loss of neurons in KA treated rats with moderate hippocampal injury is significant in the dentate hilus, and CA1 and CA3 pyramidal cell layers. However, there is no loss of neurons in the granule cell layer. The massive injury group exhibits greater loss of CA1 and CA3 pyramidal neurons than the moderate injury group. Moreover, the dentate gyrus of the massive injury group exhibits significant loss of both dentate granule cells and dentate hilar neurons. DH, dentate hilus; GCL, granule cell layer.
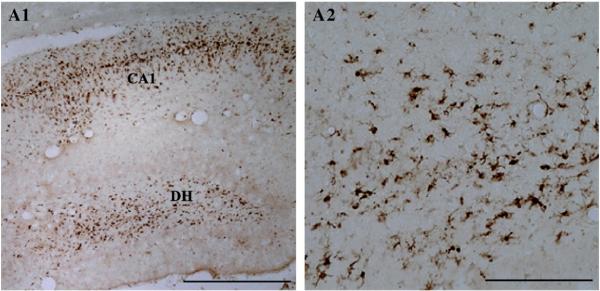
Fig. 3.
The extent of inflammation in the hippocampus after an episode of status epilepticus (SE) when visualized with the immunostaining for ED-1 antigen (a trans-membrane protein that identifies activated microglial cells). Figure A1 illustrates a higher density of activated microglia in regions of the neurodegeneration such as the dentate hilus (DH) and the CA1 subfield. A2 is magnified view of activated microglia from a region of the DH. For ED-1 immunostaining methods, see Hattiangady et al. (2011). Scale bar, A1=500 μm; A2=100 μm.
Fig. 4.
Status epilepticus impairs the ability for spatial learning and spatial memory retrieval even at extended time-points after an episode of SE. The figure A on the left side compares the spatial learning ability of rats that underwent SE and developed chronic epilepsy at ~4-months after SE (indicated by a red line) with the age-matched naive rats (indicated by a green line) in a water maze test (WMT). Note that, in comparison to intact rats, the average swim path lengths to reach the platform were much greater in rats exhibiting chronic epilepsy in all of the eleven training sessions (A). Naïve rats learn quickly to locate the hidden platform using spatial cues and hence their swim path lengths are much shorter after 3–4 sessions of learning. The epileptic rats are clearly slow learners and exhibit overnight forgetting. The figures in B and C show the results of a probe test (i.e. a 30 second memory retrieval test for each rat without the hidden platform) conducted one-day after the eleven learning sessions performed over 6 days. The circular diagram on the left side of figure B depicts the various water maze tank quadrants in different colors and the swim path of a naïve rat. Note that this naïve rat exhibits robust memory retention, as it swam straight to the quadrant where the platform was originally placed (gray-colored area) and spent most of its probe test time searching for the platform in this quadrant. The circular diagram on the right side of figure B illustrates the swim path of a chronically epileptic rat in various quadrants of the water maze tank. Note that this epileptic rat explored all quadrants of the maze without any specific affinity for the quadrant where the platform was originally placed. The bar chart in C compares the dwell time of rats from the naïve group and the epileptic group in different quadrants of the water maze tank. Note that control rats spend most of their probe test time in the platform quadrant (gray colored bar) whereas epileptic rats spend more or less equal amount of time in all four quadrants, clearly implying that chronically epileptic rats exhibit memory dysfunction. For methods pertaining to water maze tests, see Hattiangady et al. (2011).
3.2. Effects of SE on the behavior of neural stem/progenitor cells
In the hippocampus, new neurons are added to the granule cell layer of the dentate gyrus (DG) all through life. This occurs because of continued proliferation of neural stem/progenitor cells (NSCs) residing in the subgranular zone (SGZ) of the DG (Eriksson et al., 1998; Gould & Gross, 2002; Song et al., 2002; Abrous et al., 2005). Many studies advocate a linkage between the amount of DG neurogenesis and the hippocampal-dependent cognitive functions (van Praag et al., 2002; Aimone et al., 2006; Kee et al., 2007; Imayoshi et al., 2008). For example, there is an association between a decreased DG neurogenesis and impairments in some of the hippocampal-dependent learning and memory functions (Drapeau et al., 2003; Rola et al., 2004; Siwak-Tapp et al., 2007; Dupret et al., 2008; Jessberger et al., 2009). The various conditions that inflict hippocampal injury such as after an episode of acute seizures, ischemia, stroke or hypoxia greatly enhance DG neurogenesis for a certain period of time (Parent et al., 1997; Choi et al., 2003; Felling & Levison, 2003). Pertaining to seizures, multiple studies now corroborate that the reaction of DG neurogenesis swerves noticeably between the initial and delayed stages after acute seizures. Acute seizures or SE greatly enhance NSC proliferation and result in a greatly increased DG neurogenesis (Bengzon et al., 1997; Parent et al., 1997; Gray & Sundstrom, 1998; Nakagawa et al., 2000; Ekdahl et al., 2001; Hattiangady et al., 2004) (Fig. 5). The discharge of NSC mitogenic factors from dying neurons, deafferented granule cells and reactive glia is believed to be one of the underlying factors promoting this surge in neurogenesis, as the concentration of several neurotrophic factors is elevated in the hippocampus after SE/brain injury (Lowenstein et al., 1993; Shetty et al., 2003, 2004). Acute hyperexcitability and increased GABA levels following SE could be another factor promoting increased neurogenesis, as GABA is mitogenic to NSCs (Ge et al., 2007). It takes about 2–3 weeks for normalization in the rate of NSC proliferation and return to the baseline-level of neurogenesis after SE (Parent et al., 1997; Nakagawa et al., 2000). Addition of a large number of new neurons to the granule cell layer of the DG after SE appears to be beneficial. This is because, newly born neurons that are added to the granule cell layer after an episode of SE or acute seizures tend to exhibit reduced excitability, which may contribute towards decreasing the overall hyperexcitability of the DG after SE (Jakubs et al., 2006).
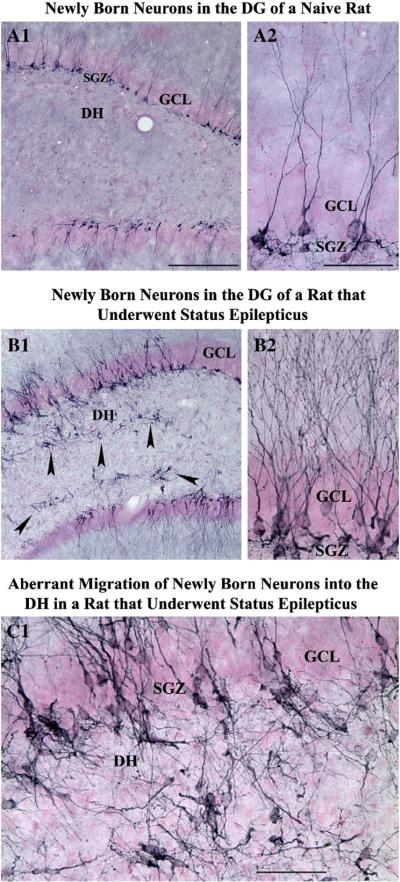
Fig. 5.
Changes in the dentate neurogenesis after an episode of status epilepticus (SE) induced by kainic acid. Newly born neurons in the dentate gyrus (DG) of a naïve adult rat (A1) and an adult rat that underwent status epilepticus 12 days prior to euthanasia (B1) were visualized with immunostaining for doublecortin (a marker of newly born neurons). A2 and B2 show magnified views of regions of dentate gyrus from A1 and B1 respectively. Note that, in comparison to the dentate gyrus of a control rat (A1, A2), a rat that underwent SE (B1, B2) exhibits considerably increased density of doublecortin + new neurons and abnormal migration of newly born neurons into the dentate hilus (indicated by arrowheads in B1). C1 is magnified view of a region from B1 showing aberrantly migrated newly born neurons in the dentate hilus. DH, dentate hilus; GCL, granule cell layer; SGZ, subgranular zone. Scale bar, A1 and B1=200 μm; A2, B2 and C1=50 μm.
However, the presence of anomalous migration of a fraction of newly born granule cells into the dentate hilus and/or the molecular layer after SE (Fig. 5 [C1]) is considered to be detrimental (Houser, 1990; Scharfman et al., 2000, 2002a,b; McCloskey et al., 2006; Parent et al., 2006; Parent, 2007; Shetty & Hattiangady, 2007). Loss of reelin (a secreted migration guidance cue) expression after SE has been suggested to be the reason for this aberrant integration of newly born dentate granule cells into ectopic locations (Gong et al., 2007). It has been shown that newly born granule cells that drift off uncharacteristically into the dentate hilus get incorporated abnormally with the CA3 network (Scharfman et al., 2000), get activated when spontaneous seizures occur (Scharfman et al., 2002b), and respond to stimulation of the hippocampal perforant path afferents from the entorhinal cortex with a longer latency to onset of evoked responses (Scharfman et al., 2003). Moreover, these ectopically placed granule cells establish afferent connectivity with mossy fiber terminals (i.e. with axons of granule cells in the granule cell layer of the DG; Pierce et al., 2005), exhibit natural eruptions of action potentials (Scharfman et al., 2000) and seem to be a factor in spontaneous recurrent seizures that occur in chronic epilepsy (Jung et al., 2004; McCloskey et al., 2006). From the above, it is clear that SE-induced abnormal DG neurogenesis promotes development of an aberrant circuitry in the DG. This aberrant circuitry is believed to contribute towards the evolution of SE into chronic epilepsy. Hence, it will be critical to examine in future studies whether the most efficacious neuroprotective treatments can also thwart seizure-induced abnormal neurogenesis.
The overall DG neurogenesis declines substantially at delayed time-points after SE (i.e. when spontaneous seizures occur) (Hattiangady et al., 2004; Kuruba & Shetty, 2007; Hattiangady & Shetty, 2008; Kuruba et al., 2009)(Fig. 6). A recent study demonstrated that declined neurogenesis in chronic epilepsy is not due to decreased production of new cells or poor survival of newly born cells. Rather, it is due to a dramatically decreased neuronal differentiation of newly born cells. It turns out that, in chronic epileptic conditions, most of the newly born cells differentiate into glia rather than into neurons (Hattiangady & Shetty, 2010; Fig. 7). Considering that chronic epilepsy is also associated with substantially decreased levels of several neurotrophic factors including BDNF and FGF-2(Hattiangady et al., 2004; Shetty et al., 2004), it is plausible that changes in DG milieu contribute to the declined neuronal differentiation of newly born cells. Furthermore, both decrease in DG neurogenesis and ectopic migration of newly born granule cells were more pronounced in rats exhibiting greater frequency of spontaneous recurrent seizures. Additionally, a vast majority of newly born neurons in chronically epileptic hippocampi displayed basal dendrites (Fig. 6), another feature that is believed to contribute towards the establishment of recurrent excitatory circuitry (Ribak et al., 2000; Shapiro & Ribak, 2006; Hattiangady & Shetty, 2008). In view of the importance of DG neurogenesis in hippocampal-dependent learning and memory functions, it is likely that the various hippocampal-dependent learning and memory deficits observed in chronic epilepsy are linked at least partially to the declined DG neurogenesis (Brown-Croyts et al., 2000; Oddo et al., 2003; Alessio et al., 2004a,b). Thus, it will be important to investigate in future studies whether the ideal neuroprotective therapies have the ability to maintain a normal level of hippocampal neurogenesis and cognitive function in the chronic phase after injury or SE.
Fig. 6.
Status of dentate neurogenesis in chronic epilepsy as revealed by doublecortin (DCX) immunostaining. A1–C1 illustrate the distribution of DCX positive newly born neurons in the dentate gyrus of an age-matched intact rat (A1), a rat exhibiting chronic epilepsy at 5 months after an intracerebroventricular (ICV) kainic acid (KA) administration (B1), and a rat displaying robust chronic epilepsy at 5-months after intraperitoneal (IP) KA-induced status epilepticus (SE) (C1). Note that, in comparison to the age-matched intact hippocampus, hippocampi from chronically epileptic animals exhibit dramatically reduced density of DCX positive newly generated neurons. Arrowheads point to regions in the subgranular zone (SGZ) where neurogenesis is active. The arrow in C1 denotes a neuron that has migrated into the dentate hilus. A2, B2, and C2 are magnified views of regions from A1, B1, and C1 demonstrating the morphology newly generated neurons in the three groups. In the dentate gyrus of the age-matched intact rat (A2), DCX positive new neurons exhibit long apical dendrites that extend into the molecular layer (ML) through the granule cell layer (GCL). Contrastingly, in hippocampi from epileptic animals (B2, C2), a vast majority of DCX positive neurons display basal dendrites (indicated by short arrows). The bar chart compares the absolute numbers of DCX positive new neurons in different groups. Note that, in comparison to the age-matched control rats, the overall dentate neurogenesis in hippocampi of chronically epileptic rats is drastically reduced. Furthermore, the decline is more pronounced in the hippocampus of rats exhibiting robust chronic epilepsy (i.e. the hippocampus at 5-months post-SE) than the hippocampus of rats exhibiting fewer spontaneous seizures (i.e. the hippocampus at 5-months post-ICV KA administration). DH, Dentate hilus; GCL, Scale bar, A1, B1, C1=200 μm; A2, B2, C2=50 μm.
Fig. 7.
Differentiation of newly born cells into neurons, astrocytes, and oligodendrocyte progenitors at 24 h after twelve daily injections of BrdU in the chronically epileptic hippocampus. Examples of newly born cells that differentiate into doublecortin + (DCX+) immature neuron (A1–A3), TuJ-1+ neuron (B1; indicated by an arrow), S-100β+ astrocyte (C1; denoted by an arrow), and NG2+ oligodendrocyte progenitor (D1; showed by an arrow) in the subgranular zone-granule cell layer (SGZ-GCL) are illustrated. Scale bar, A1–A3=5 μm; B1, C1, D1=10 μm. The bar chart in E1 compares percentages of newly born cells (i.e. BrdU + cells) that express DCX, TuJ-1, S-100β or NG2 in the subgranular zone-granule cell layer (SGZ-GCL) between the age-matched intact hippocampus and the chronically epileptic hippocampus. Note that the neuronal differentiation of newly born cells is dramatically decreased but differentiation of newly added cells into S-100β+ or NG2+ glia is considerably enhanced in the chronically epileptic hippocampus.
3.3. Effects of SE on hippocampal gamma-amino butyric acid positive (GABA-ergic) interneuron population
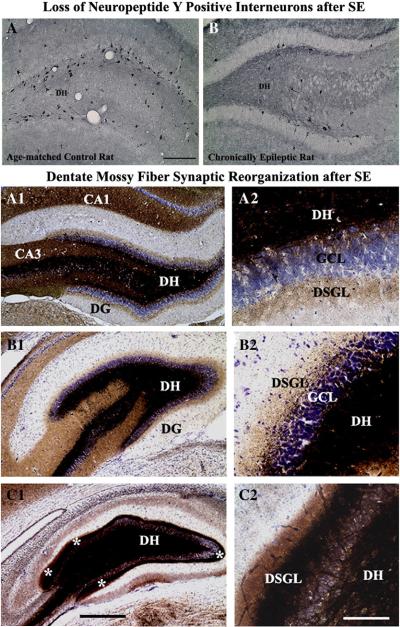
Loss of GABA-ergic interneurons after SE/brain injury is believed to have a significant role in epileptogenesis, as it can lead to a reduction of GABA-mediated inhibition and promote seizures. Multiple studies have analyzed the potential loss of GABA-ergic interneurons in various models of SE and brain injury. Studies that focused on early time-time points mostly suggested the resistance of GABA-ergic interneurons to SE/brain injury (Franck et al., 1988; Davenport et al., 1990a,b; Morin et al., 1998; Williamson et al., 1999). However, studies that analyzed the subclasses of interneurons expressing different calcium binding proteins and neuropeptides at delayed time-points after SE/brain injury showed a substantial loss of these interneurons (Sperk et al., 1986; Shetty & Turner, 1995a,b; Kobayashi et al., 2003). Studies quantifying the numbers of interneurons expressing the GABA synthesizing enzyme glutamate decarboxylase-67 at 1–6 months after KA-induced seizures/injury showed substantial reductions in their number throughout the hippocampus (Shetty & Turner, 2000, 2001; Shetty et al., 2009). The upper panel of Fig. 8 illustrates the loss of a subclass of GABA-ergic interneurons expressing the neuropeptide Y in the hippocampus of a rat that underwent SE. The above findings imply that reduced number of GABA-ergic interneurons likely contributes to the decreased functional inhibition observed during the chronic phase after SE. It is also possible that decreased inhibition is one of the factors contributing to the occurrence of spontaneous seizures during the chronic phase after SE (Sloviter, 1987; Cornish & Wheal, 1989; Sloviter, 1991; Obenaus et al., 1993; Houser & Esclapez, 1996; Dudek & Spitz, 1997; Bernard et al., 1998). This premise is supported by anticonvulsant actions observed after the administration of drugs that increase synaptic GABA (Treiman, 2001), or grafting of cells that synthesize and release GABA in epilepsy models (Loscher et al., 1998; Gernert et al., 2002; Thompson, 2005; Castillo et al., 2006; Hattiangady et al., 2008; Waldau et al., 2010). Thus, preservation of the GABA-ergic interneuron population after an episode of SE via neuroprotective interventions appears useful for preventing chronic epilepsy after SE (Coulter, 1999; Ben-Ari, 2006; Richardson et al., 2008).
Fig. 8.
The upper panel shows that, status epilepticus (SE) depletes the population of GABA-ergic interneurons expressing the neuropeptide, the neuropeptide Y (NPY). Figure A illustrates the distribution and density of NPY immunopositive interneurons in the dentate hilus of a naïve rat. Figure B shows the distribution and density of NPY + interneurons in the dentate hilus of an age-matched rat that underwent kainic acid induced SE and acquired chronic epilepsy. Note that the overall density of NPY + interneurons is greatly reduced after SE. For NPY immunostaining methods see Rao et al. (2006). Scale bar, A1 and A2=200 μm. The lower panel illustrates the extent of the aberrant mossy fiber sprouting in rats with moderate hippocampal injury (B1–B2) and rats with severe hippocampal injury (C1–C2), in comparison to age-matched intact rats (A1–A2), visualized by Timm's histochemical staining. Note that, in comparison to rats exhibiting moderate hippocampal injury (B1–B2), the rats showing severe hippocampal injury (C1–C2) have much robust aberrant sprouting of mossy fibers into the dentate supragranular layer (DSGL). Asterisks in C1 denote mossy fiber sprouting in the DSGL. DH, dentate hilus; GCL, granule cell layer. Scale bar, A1, B1 & C1=500 μm; A2, B2 and C2=200 μm.
3.4. Consequences of SE on synaptic reorganization in the dentate gyrus
Degeneration of a fraction of dentate hilar cells and CA3 pyramidal neurons, the target cells of granule cell axons (mossy fibers) is distinctively seen after an episode of SE/hippocampal injury induced by chemoconvulsants such as KA. This results in the evolution of an abnormal hippocampal circuitry typified by the sprouting of mossy fibers into the dentate supragranular layer (DSGL; Fig. 8 [lower panel]), analogous to the alteration observed in the human TLE (Houser et al., 1990; Babb et al., 1991; Mathern et al., 1996; Shetty & Turner, 1999). The aberrant mossy fiber sprouting (MFS) has been considered as one of the hallmarks after an episode of SE or in TLE (Houser et al., 1990; Franck et al., 1995). Studies on this anomalous sprouting in various animal prototypes of TLE hold up the hypothesis that the abnormal dentate MFS causes granule cells to stimulate one another and is the center of seizure activity, which are revealed by the following observations. To begin with, the sprouted mossy fibers form new asymmetric (excitatory) synapses on dendritic spines of granule cells that are not their parent neurons, which implies a strong influence of MFS on the circuit formation (Buckmaster et al., 2002; Koyama & Ikegaya, 2004). Furthermore, exogenous glutamate treatment to the dentate molecular layer results in excitatory post-synaptic currents or excitatory post-synaptic potentials in granule cells that are situated away from the treated site in epileptic rats (Molnar & Nadler, 1999; Lynch & Sutula, 2000). Moreover, antidromic stimulation of the granule cells in acute hippocampal slices from epileptic rats evokes prolonged seizure-like bursts of population spikes in the granule cell layer, when inhibition is depressed and/or the concentration of extracellular potassium is increased (Wuarin & Dudek, 1996; Okazaki et al., 1999). Besides, in animals with abnormal MFS, a single stimulation of the entorhinal-dentate (perforant) pathway generates 3–12 successive population spikes in contrast to the single spike/stimulus observed in the saline treated controls (Patrylo et al., 1999). This implied that the synaptic input from the entorhinal cortex is converted to epileptiform bursts through mossy fiber recurrent circuits (Koyama & Ikegaya, 2004). Additionally, in epileptic animals, an association has been identified between the progression of aberrant MFS and the frequency and intensity of spontaneous recurrent seizures (Sutula et al., 1988).
From the above, it appears that the aberrant MFS is closely linked to spontaneous recurrent seizures in TLE. However, some studies contradict this notion based on the observations that: (i) no correlation exists between the aberrant MFS and the first spontaneous seizure after SE (Nissinen et al., 2001); (ii) the presence of aberrant MFS is not a pre-requisite for epileptogenesis in postnatal models of SE (Bender et al., 2003); (iii) the MFS after SE also promotes an increased inhibition of the DG (Sloviter, 1992); and (iv) new excitatory circuits after SE develop in the CA1 region as well (Shao & Dudek, 2005). Thus, it remains to be determined whether the MFS is a cause or result of epilepsy. Nevertheless, because the sprouted mossy fibers make excitatory connections with the dendrites of granule cells, it is plausible that the aberrant MFS contributes to the chronic state of TLE (Zhang et al., 2002; Koyama & Ikegaya, 2004). Indeed, several studies suggest that the DG with aberrant MFS has increased seizure susceptibility (Mathern et al., 1993, 1996; Sutula, 2002; Nadler, 2003; Santhakumar et al., 2005). Thus, deterrence of aberrant MFS is likely to be vital for either blocking or reducing the intensity of DG hyperexcitability and spontaneous recurrent seizures after SE. Such prevention would however require considerable neuroprotection to dentate hilar neurons as well as CA3 pyramidal neurons during SE (Nadler, 2003; Shetty et al., 2003; Cavazos et al., 2004; Shetty et al., 2005; Dudek & Sutula, 2007; Nadler et al., 2007).
3.5. Evolution of SE into chronic epilepsy characterized by spontaneous seizures
In most cases, SE leads to the development of chronic epilepsy after a delay (Dube et al., 2001; Bengzon et al., 2002). In a F344 model of SE, virtually all rats that exhibit continuous stages III–V seizures for over 3 h after the KA administration acquire chronic epilepsy characterized by spontaneous recurrent motor seizures (Rao et al., 2006). In this study, each KA-treated rat displayed over 2.0 spontaneous seizures per hour at 3–5 months post-SE. The duration of individual seizures was ~61 s at 4 and 5 months post-SE. Electroencephalographic (EEG) recordings from the cortex (via a pre-implanted epidural metal electrode) and the hippocampus (via a pre-implanted intrahippocampal stainless steel electrode) confirmed robust chronic epilepsy in these animals. Fig. 9 illustrates an EEG trace during a stage IV/V spontaneous seizure (along with electrographic activities both before and after a spontaneous seizure). Thus, the various epileptogenic changes that ensue after SE (including an abnormal pattern of neurogenesis in the dentate gyrus, loss of GABA-ergic interneurons, and an aberrant mossy fiber sprouting) eventually lead to the development of chronic epilepsy characterized by spontaneous recurrent seizures.
Fig. 9.
Electrical activity in the hippocampus during a spontaneous seizure in a chronically epileptic rat, as recorded through electroencephalography (EEG). The activity was recorded through an electrode implanted into the dentate gyrus. Note the presence of persistent large amplitude and high frequency polyspikes for over 50 s. Polyspikes represent a complex paroxysmal EEG pattern with close association of two or more diphasic spikes occurring more or less rhythmically in bursts of variable duration, generally with large amplitudes. For methods of electrode implantation into the dentate gyrus, see Rao et al. (2006).
3.6. Significance of hippocampal neuroprotection against SE
Neuroprotection has been examined in animal models of SE. It is believed that seizure-induced hippocampal neuron loss, and the ensuing changes in NSC behavior, neurogenesis and circuitry contribute to impairments in new learning, formation of new memories and chronic epilepsy development. Therefore, application of neuroprotective strategies early after the onset of SE is considered beneficial (Ebert et al., 2002). In animal models of SE, the major neuronal damage occurs in structures belonging to the circuit of initiation and maintenance of seizures (i.e. the dentate gyrus and the hippocampus), though some damage also occurs in the propagation areas such as the entorhinal, perirhinal and piriform cortices, and thalamic and amygdalar nuclei. Certain antiepileptic drugs and NMDA receptor blockers protect fractions of hippocampal pyramidal neurons from dying following acute seizures but fail to prevent the loss of dentate hilar neurons and GABA-ergic interneurons (Andre et al., 2001; Brandt et al., 2003; Kapur, 2003; Rigoulot et al., 2004). Ideal neuroprotection strategies after the onset of SE should be capable of: (i) considerably rescuing all types of hippocampal neurons that are vulnerable to seizures; (ii) suppressing the abnormal behavior of NSCs and their progeny in the DG; (iii) preventing or diminishing the aberrant synaptic reorganization in the DG and other regions. If these requirements are met, it may be possible to avoid both hippocampal dysfunction and chronic epilepsy after SE.
4. Efficacy of RESV administration against acute seizures and epilepsy
4.1. Neuroprotective effects of RESV against excitotoxic brain injury and acute seizures
A study by Virgili and Contestabile (2000) was the first to suggest a neuroprotective property for RESV against excitotoxic brain injury. They compared the effect of systemic administration of an excitotoxin kainic acid (KA) between young adult rats that were chronically treated with RESV and control young adult rats. They found that chronic RESV treatment prior to the KA administration considerably reduced the damage caused by KA in the olfactory cortex and the hippocampus. Following this, a study demonstrated the protective effect of RESV pretreatment on KA induced seizures and oxidative stress (Gupta et al., 2002). They found that a single dose of RESV (at 40 mg/kg i.p.) five-minutes prior to KA treatment (10 mg/kg i.p.) increased the latency to convulsions but was unable to completely inhibit the convulsions. However, with multiple doses of RESV treatment (i.e. at 5 min prior to KA injection and at 30 and 90 min post-KA injection), the incidence of convulsions was significantly reduced. The above RESV treatment regimen also inhibited the KA-injury related increases in the level of MDA, suggesting that antioxidant function is one of the mechanisms by which RESV mediates neuroprotection against excitotoxic injury and acute seizures.
Furthermore, a study has shown neuroprotection when RESV was administered prior to intracortical placement of FeCl3 (Gupta et al., 2001). In the absence of RESV treatment, FeCl3 treated animals exhibited significant epileptiform EEG discharges and increased levels of the oxidative stress marker MDA in the brain tissue. However, in animals that received RESV (20 or 40 mg/kg i.p.) 30 min prior to FeCl3 treatment, the onset of the epileptiform EEG discharges was delayed and MDA levels were reduced. However, it was unclear whether the above protective effects are adequate for preventing neurodegeneration in the hippocampus, a learning and memory center of the brain that plays an important role in the development of chronic TLE following injury. A subsequent study investigated this issue and showed that prior RESV treatment protects against neurotoxicity induced by KA (Wang et al., 2004). In this study, a group of rats was concurrently treated with KA (8 mg/kg; once daily for 5 days) and RESV (30 mg/kg; once daily for 5 days) and another group of rats was treated with KA alone (i.e. 8 mg/kg; once daily for 5 days). Animals treated with KA alone displayed significant neurodegeneration, reactive astrocytes and activated microglial cells in the hippocampal CA1 and CA3 subfields and the dentate hilus. In contrast, animals treated with KA and RESV exhibited milder hippocampal neurodegeneration with reduced density of reactive astrocytes and activated microglial cells. Collectively, the above studies suggest the promise of RESV treatment (commencing either prior to or at the time of the excitotoxic injury) for minimizing the excitotoxin-induced seizures, oxidative stress and hippocampal neurodegeneration.
4.2. Potential mechanisms of RESV-mediated neuroprotection against acute seizures
Neuroprotective effects of RESV are likely mediated through multiple mechanisms, which might include the inhibition of the: (i) voltage-gated potassium currents (Gao & Hu, 2005); (ii) electrical activity of CA1 neurons (Li et al., 2005); and (iii) excitatory synaptic transmission in the hippocampus via inhibition of the post-synaptic glutamate receptors (Gao & Hu, 2005). Another study shows that RESV-mediated attenuation of neuronal cell death against SE was associated with suppression of activated astrocytes and microglia (Wang et al., 2004). As increased oxidative stress is a key factor in the mechanisms of KA-induced neurotoxicity, the neuroprotective effect afforded by RESV supports the purported ability of the RESV to act as free radical scavenger to protect against neuronal damage caused by excitotoxic insults. RESV is also capable of both scavenging the superoxide anion generated from rat forebrain mitochondria and decreasing complex III activity, suggesting that RESV can both scavenge and oppose the production of reactive oxygen species (Zini et al., 1999; See Fig. 1). Several cell culture studies, in addition, demonstrate robust anti-apoptotic properties of RESV (Sun et al., 1997; Nicolini et al., 2001). The anti-apoptotic properties of RESV could involve the regulation of apoptosis-related genes such as Bax, Bcl-2 and caspase-3. Overall, it is likely that RESV-mediated neuroprotection against acute seizures involves anti-convulsant, anti-oxidant, and anti-apoptotic mechanisms. However, additional rigorous studies are required in future to confirm the above possibilities.
4.3. Effects of RESV treatment on SE-induced epileptogenesis and chronic epilepsy
A recent study analyzed the effects of intragastric administration of RESV (15 mg/kg) for 10 days following an intrahippocampal injection of 1.0 μg of KA into anesthetized rats (Wu et al., 2009). Analyses of behavioral spontaneous seizures at an early time-point (8 weeks) after KA injection suggested that only a smaller percentage of animals exhibit spontaneous seizures among rats receiving both KA and RESV, in comparison to rats receiving KA alone. Furthermore, EEG recordings for a brief period of 2 h suggested that epileptiform-like waves were reduced in rats receiving both KA and RESV, in comparison to rats receiving KA alone. Histological analyses revealed considerable neuroprotection in the CA1 cell layer and CA3a sub region in the KA plus RESV treated rats, in comparison to a widespread neurodegeneration observed in rats receiving KA alone. However, the above regimen of RESV treatment was unable to protect neurons in the CA3b and CA3c sub regions. The synaptic reorganization of dentate mossy fibers (in the form of aberrant mossy fiber sprouting into the inner molecular layer of the dentate gyrus) was also reduced when examined at ~8 weeks after KA injection. The KA plus RESV treated animals also exhibited reduced expression of kainate receptors than rats treated with KA alone.
The above observations support the promise of RESV treatment commencing after the hippocampal injury or acute seizures for reducing the incidence/intensity of injury or acute seizure induced chronic TLE. However, there are quite a few limitations in the above study. First, as KA was administered directly into the hippocampus under anesthesia, acute seizures were minimal in this model and RESV administration did not prevent the direct KA-induced neurodegeneration in the dentate hilus and CA3b and CA3c sub regions. For promotion of RESV as a neuroprotective agent against acute seizures, it will be essential to demonstrate that the administration of RESV commencing after the onset of acute seizures (such as in SE models) offers significant neuroprotection. Second, analyses of various parameters of epileptogenesis were performed at an early time-point after KA injection. This is a major limitation because spontaneous seizures develop progressively and typically require ~4–6 months of time after KA administration to exhibit the full range of seizures (Rao et al., 2006, 2007; Waldau et al., 2010) and seizures sometimes occur in clusters.
4.4. Studies needed to authenticate the efficacy of RESV for treating SE/epilepsy
To authenticate the efficacy of RESV for neuroprotection against acute seizures or SE, rigorous analyses of spontaneous recurrent seizures using chronic EEG recordings are needed at extended time-points (e.g. 4–6 months) after an initial precipitating injury (such as SE) and RESV treatment. Such studies need to validate the potential efficacy of this compound for preventing or greatly diminishing the SE-induced multiple changes (as depicted in Fig. 10). Particularly, its ability for long-term suppression of the SE-induced changes that contribute to epileptogenesis will need to be unraveled. These include the: (i) loss of both principal and GABA-ergic neurons (neurodegeneration) in the hippocampus and the extrahippocampal regions such as the piriform cortex, entorhinal cortex, thalamus and amygdala; (ii) abnormal behavior of newly generated neurons in the DG (aberrant neurogenesis); (iii) abnormal synaptic reorganization (such as the aberrant sprouting of dentate mossy fibers and neosynaptogenesis); (iv) chronic epilepsy development typified by spontaneous recurrent seizures; and (v) cognitive dysfunction (Fig. 10). The other important questions to address include the following. (1) Does preservation of hippocampal principal neurons and GABA-ergic interneurons through RESV administration after SE prevent abnormal neurogenesis (such as the aberrant migration of newly born neurons)? (2) Does neuroprotection of dentate hilar neurons and CA3 pyramidal neurons through RESV administration after SE prevent the aberrant sprouting and the enhanced excitatory connectivity of dentate mossy fibers? (3) Does the preservation of normal hippocampal cytoarchitecture by RESV after SE (as described above) maintain normal level of neurogenesis at both early and late phases after SE? Furthermore, the much bigger question is, does the overall neuroprotection afforded by RESV against SE prevent the chronic epilepsy development characterized by spontaneous recurrent seizures and cognitive dysfunction? (Fig. 10).
Fig. 10.
Potential outcome of resveratrol (RESV) therapy for status epilepticus (SE) and epilepsy. The overall outcome of RESV treatment would likely depend on the time-point of its administration after an initial precipitating injury (IPI) such as SE or head injury. An IPI such as SE leads first to changes that comprise increased excitation of neurons, enhanced oxidative stress, considerable neurodegeneration, inflammatory reaction and abnormal neurogenesis, which are broadly classified as “early changes”. While most of these changes can occur in several brain regions, the hippocampus has been recognized as the most vulnerable region to SE for exhibiting all of these changes. This initial phase is typically followed by an intermediate “epileptogenesis” phase during which the various epileptogenic changes occur. These include the loss of GABA-ergic interneurons, decreases in the functional inhibition, aberrant sprouting of axons, and abnormal integration of newly born neurons. All of these changes are believed to contribute towards the development of an epileptogenic circuitry, which gradually leads to a state of “chronic epilepsy” typified by spontaneous recurrent seizures, decreased hippocampal neurogenesis and cognitive dysfunction. Considering the above, the commencement of RESV treatment immediately after the induction of SE may either prevent or greatly minimize the early changes, which may block “epileptogenesis” as well as the evolution of SE into “chronic epilepsy”. This hypothesis is based on the anticonvulsant, anti-oxidant, anti-inflammatory, and anti-apoptotic properties of RESV. On the other hand, the commencement of RESV treatment after “early changes” may reduce the extent of “epileptogenesis” through preservation of greater numbers of GABA-ergic interneurons, dampening of hippocampal hyperexcitability and maintenance of normal neurogenesis. These actions of RESV may considerably restrain the development/intensity of chronic epilepsy and cognitive dysfunction. This premise is based on the neuroprotective, anti-inflammatory and anticonvulsant properties of RESV. Furthermore, the commencement of RESV treatment after most of the epileptogenic changes occurs or after the establishment of a chronic epileptic state may still restrain the intensity of chronic epilepsy and improve the cognitive function. This proposition is based on the anti-inflammatory and anticonvulsant properties of RESV and the ability of RESV to activate SIRT1.
Furthermore, it is critical to identify the window of time after the onset of SE at which RESV provides maximal neuroprotection and prevents the progression of acute seizures into chronic epilepsy and cognitive dysfunction. Moreover, it needs to be determined whether RESV administration during and shortly after SE is sufficient or RESV treatment for a longer period after SE is required for a durable neuroprotection of the hippocampal and extrahippocampal regions. Additionally, the efficacy of RESV treatment for restraining epilepsy and cognitive dysfunction needs to be examined at different phases after SE, such as after: (i) the initial neurodegeneration and inflammatory reaction; (ii) the onset of various epileptogenic changes; and (iii) the commencement of spontaneous seizures (Fig. 11). Because of the anticonvulsant properties of RESV (Fig. 1), RESV administration in chronic epileptic conditions may suppress spontaneous recurrent seizures. Besides, RESV treatment in chronic epileptic conditions may also decrease inflammation and oxidative stress, improve neurogenesis, and ease cognitive dysfunction. These beneficial effects might also involve the activation of SIRT1, AMPK or MAPKs (Fig. 1).
5. Bioavailability and toxicity issues pertaining to RESV
5.1. Bioavailability of RESV
For short-term therapeutic interventions such as after SE, administration of RESV via intraperitoneal, intramuscular or subcutaneous injections appears to be ideal. However, if RESV treatment for SE or brain injury needs to be continued for several weeks or months after SE, then, oral administration will be the most feasible route. Therefore, it is important to know the bioavailability of RESV after an oral administration (Cottart et al., 2010). Pharmacokinetic studies measuring the plasma concentration after an oral intake of RESV in humans suggest the following. When RESV was administered orally at a dose of ~25 mg, the plasma concentration of the free form of RESV ranged from 1 to 5 ng/ml. When a higher dose (5 g, ~equivalent to a dose of 71 mg/kg bw) of RESV was administered, the plasma concentration of the free form of RESV reached 530 ng/ml (Boocock et al., 2007a). The maximum peak plasma concentration was attained in the first 30 min after a low dose intake, whereas intake of a higher dose of RESV delayed the peak plasma concentration to 1.5–2 h after the intake (Cottart et al., 2010). In another study, by administering a single dose of RESV ranging from 0.5 to 5 g, Boocock et al. (2007a) confirmed that the free RESV is absorbed rapidly (peak plasma concentration at 0.83–1.5 h after ingestion) at a relatively low mean plasma concentration (from 73 ng/ml to 539 ng/ml for a 0.5 and 5 g RESV intake respectively). A light rebound was observed after 5–6 h after the administration supporting the occurrence of the enterohepatic cycle (Boocock et al., 2007a; Cottart et al., 2010). Interestingly, the corresponding concentrations of the main metabolites of RESV (resveratrol-3-O-sulfate and monoglucuronides) surpassed those of free RESV by ~20-folds. Furthermore, the plasma half-lives of RESV and its main conjugates were similar (between 2.9 and 11.5 h). In the urine, within 24 h after the administration, excretion rates peaked during the initial 4-hour collection period. Traces of RESV metabolites were detected in the feces, which is consistent with the enterohepatic recirculation. This study showed that even with a high-dose trans-RESV administration, only a small amount of the free form is present in the plasma. However, it has been suggested that RESV concentrations are probably underestimated in this study as the efficiency of extraction in plasma and urine is ~60–70% (Boocock et al., 2007b).
Overall, from the available studies, it emerges that RESV is well tolerated even at higher doses. It is rapidly absorbed and metabolized mainly as sulfo- and glucuro-conjugates, which are excreted in the urine (Cottart et al., 2010). As the concentrations of free RESV detected in various studies are low in the plasma, one can argue that the overall bioavailability of free RESV is low after an oral administration. However, Cottart et al. (2010) point out several valid issues that need to be considered while evaluating the low bioavailability of RESV. First, when assessing the plasma RESV concentrations, the bound part is not taken into account. This is an important issue because RESV is highly lipophilic (is bound by LDL and albumin). Second, conjugated metabolites may also have RESV-like biological activity. Third, it is possible that a large part of the molecule is bound by cell membranes or lipophilic tissue. From this point of view, free RESV levels in serum are underestimated because of large amounts potentially contained in the cellular fraction. Considering these, the effects of RESV may not be due to the amount of visible plasma fraction of RESV but rather due to RESV cellular fractions that were not quantified (Cottart et al., 2010).
5.2. Potential toxicity of RESV
If RESV is to be used long-term for treating SE or epilepsy, it is important to assess the potential toxicity of RESV. A study by Crowell et al. (2004) examined the effects of administration of 0.3, 1 and 3 g/kg/day trans-RESV for 4 weeks in rats (which is equivalent to 21, 70, and 210 g/day respectively in a human weighing 70 kg; (Cottart et al., 2010)). Only two of the 40 rats receiving the highest dose (3 g/kg/day) died due to nephrotoxicity. However, no adverse event was observed in animals treated with 300 mg/kg/day for 4 weeks. In another study, Williams et al. (2009), using both low and high doses of RESV (up to 750 mg/kg/day for 3 months in rabbits and rats) reported that RESV is well tolerated and non-toxic, has no embryo/fetal toxicity, and has no effect on the reproductive capacity of both male and female rats. In humans, 5 g/70 kg (~71 mg/kg bw) did not result in any adverse event (Cottart et al., 2010). Thus, relatively higher doses of RESV intake appear to be safe based on toxicity assays in RESV-treated animals.
6. Conclusions
Analyses of the efficacy of compounds that have a promise for affording robust hippocampal neuroprotection when administered after the onset of SE, acute seizures or head injury have immense value. This is because robust hippocampal neuroprotection might prevent the acute seizure/injury induced learning and memory impairments and chronic epilepsy development. From a clinical standpoint, such interventions will be highly beneficial for patients who are susceptible to develop chronic TLE after an initial precipitating event such as severe head trauma, acute seizures or SE. From the above discussion, it appears that RESV has a promise as a therapeutic drug for easing injury or acute seizure-induced chronic epilepsy development and cognitive impairments. Nevertheless, to validate the efficacy of RESV, rigorous long-term studies are needed in acute seizure or SE models that evolve into chronic epilepsy with time. Clinical application of RESV can be easily promoted for SE, head trauma and stroke, if RESV treatment after SE or brain injury shows efficacy for greatly diminishing epileptogenesis, cognitive impairments and chronic epilepsy occurrence in animal models. The translational potential is very high for RESV because administration of RESV was shown to be safe in a pharmacokinetic phase 1 study (Boocock et al., 2007a). Furthermore, studies in humans using RESV to prevent cancer, to treat colon and colorectal cancer, metabolic disease and Alzheimer's disease are currently underway (Marques et al., 2009); http://clinicaltrials.gov/ct2/results?term=resveratrol). Additionally, RESV is considered as a food supplement, and a large number of people are already taking RESV as a dietary supplement and higher doses of RESV intake appear to be safe based on toxicity assays conducted so far.
Acknowledgment
Supported by grants from the Department of Veterans Affairs (VA Merit Review Award to A.K.S.), the National Institute of Neurological Disorders and Stroke (R01 NS054780 to A.K.S.) and the National Center for Complementary & Alternative Medicine (R21AT006256). The author thanks Drs. B. Hattiangady, M. S. Rao, R.Kuruba,B.Waldau, and B. Shuai for their excellent contributions to epilepsy research in Dr. Shetty's laboratory that are discussed in this review.
Abbreviations
- AED
Antiepileptic drug
- AMP
5′ AMP-activated protein
- AMPK
5′ AMP-activated protein kinase
- ATP
Adenosine triphosphate
- BDNF
Brain-derived neurotrophic factor
- cAMP
Cyclic adenosine monophosphate
- CA1
Cornu ammonis 1
- CA3
Cornu ammonis 3
- CREB
cAMP response element binding protein
- DG
Dentate gyrus
- DNA
Deoxyribonucleic acid
- DSGL
Dentate supragranular layer
- EEG
Electroencephalographic
- ERK1
Extracellular signal-regulated kinase 1
- ERK2
Extracellular signal-regulated kinase 2
- FeCl3
Ferric chloride
- FGF-2
Fibroblast growth factor-2
- FOXO3
Forkhead boxO3
- GABA
Gamma-amino butyric acid
- HDAC
Histone deacetylase
- KA
Kainic acid
- iNOS
Induced nitric oxide synthase
- LDL
Low-density lipoprotein
- L-NAME
L-NG-Nitroarginine methyl ester
- LPS
Lipopolysaccharide
- MAP
Mitogen activated protein
- MAPK
Mitogen activated protein kinase
- MDA
Malondialdehyde
- miR
Micro-RNA
- MFS
Mossy fiber sprouting
- NMDA
N-methyl d-aspartate
- NSC
Neural stem cell
- NF-kB
Nuclear factor kappa B
- NO
Nitric oxide
- p53
Protein 53
- PGC-1α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- RESV
Resveratrol
- RNA
Ribonucleic acid
- ROS
Reactive oxygen species
- SE
Status epilepticus
- SGZ
Subgranular zone
- SIR2
Silent Information Regulator 2
- SIRT1
Sirtuin 1 (Silent mating type information regulation 2 homolog 1)
- TLE
Temporal lobe epilepsy
- TNF-α
Tumor necrosis factor-alpha
References
- Abd El-Mohsen M, Bayele H, Kuhnle G, Gibson G, Debnam E, Kaila Srai S, et al. Distribution of [3 H]trans-resveratrol in rat tissues following oral administration. Br J Nutr. 2006;96:62–70. doi: 10.1079/bjn20061810. [DOI] [PubMed] [Google Scholar]
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: From precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Alessio A, Damasceno BP, Camargo CH, Kobayashi E, Guerreiro CA, Cendes F. Differences in memory performance and other clinical characteristics in patients with mesial temporal lobe epilepsy with and without hippocampal atrophy. Epilepsy Behav. 2004a;5:22–27. doi: 10.1016/j.yebeh.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Alessio A, Kobayashi E, Damasceno BP, Lopes-Cendes I, Cendes F. Evidence of memory impairment in asymptomatic individuals with hippocampal atrophy. Epilepsy Behav. 2004b;5:981–987. doi: 10.1016/j.yebeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Andre V, Ferrandon A, Marescaux C, Nehlig A. Vigabatrin protects against hippocampal damage but is not antiepileptogenic in the lithium-pilocarpine model of temporal lobe epilepsy. Epilepsy Res. 2001;47:99–117. doi: 10.1016/s0920-1211(01)00299-6. [DOI] [PubMed] [Google Scholar]
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- Balestrazzi A, Bonadei M, Calvio C, Mattivi F, Carbonera D. Leaf-associated bacteria from transgenic white poplar producing resveratrol-like compounds: Isolation, molecular characterization, and evaluation of oxidative stress tolerance. Can J Microbiol. 2009;55:829–840. doi: 10.1139/w09-038. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Zheng WH, Quirion R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br J Pharmacol Chemother. 2000;131:711–720. doi: 10.1038/sj.bjp.0703626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belguendouz L, Fremont L, Linard A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochem Pharmacol. 1997;53:1347–1355. doi: 10.1016/s0006-2952(96)00820-9. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Seizures beget seizures: The quest for GABA as a key player. Crit Rev Neurobiol. 2006;18:135–144. doi: 10.1615/critrevneurobiol.v18.i1-2.140. [DOI] [PubMed] [Google Scholar]
- Bender RA, Dube C, Gonzalez-Vega R, Mina EW, Baram TZ. Mossy fiber plasticity and enhanced hippocampal excitability, without hippocampal cell loss or altered neurogenesis, in an animal model of prolonged febrile seizures. Hippocampus. 2003;13:399–412. doi: 10.1002/hipo.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengzon J, Kokaia Z, Elmer E, Nanobashvili A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci U S A. 1997;94:10432–10437. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengzon J, Mohapel P, Ekdahl CT, Lindvall O. Neuronal apoptosis after brief and prolonged seizures. Prog Brain Res. 2002;135:111–119. doi: 10.1016/S0079-6123(02)35011-8. [DOI] [PubMed] [Google Scholar]
- Bernard C, Esclapez M, Hirsch JC, Ben-Ari Y. Interneurones are not so dormant in temporal lobe epilepsy: A critical reappraisal of the dormant basket cell hypothesis. Epilepsy Res. 1998;32:93–103. doi: 10.1016/s0920-1211(98)00043-6. [DOI] [PubMed] [Google Scholar]
- Bi XL, Yang JY, Dong YX, Wang JM, Cui YH, Ikeshima T, et al. Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. Int Immunopharmacol. 2005;5:185–193. doi: 10.1016/j.intimp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Boggs JG. Mortality associated with status epilepticus. Epilepsy Curr. 2004;4:25–27. doi: 10.1111/j.1535-7597.2004.04110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007a;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- Boocock DJ, Patel KR, Faust GE, Normolle DP, Marczylo TH, Crowell JA, et al. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2007b;848:182–187. doi: 10.1016/j.jchromb.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C, Potschka H, Loscher W, Ebert U. N-methyl-D-aspartate receptor blockade after status epilepticus protects against limbic brain damage but not against epilepsy in the kainate model of temporal lobe epilepsy. Neuroscience. 2003;118:727–740. doi: 10.1016/s0306-4522(03)00027-7. [DOI] [PubMed] [Google Scholar]
- Brasier AR. The NF-kappaB regulatory network. Cardiovasc Toxicol. 2006;6:111–130. doi: 10.1385/ct:6:2:111. [DOI] [PubMed] [Google Scholar]
- Brown-Croyts LM, Caton PW, Radecki DT, McPherson SL. Phenobarbital pre-treatment prevents kainic acid-induced impairments in acquisition learning. Life Sci. 2000;67:643–650. doi: 10.1016/s0024-3205(00)00658-5. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;03:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Zhang GF, Yamawaki R. Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. J Neurosci. 2002;22:6650–6658. doi: 10.1523/JNEUROSCI.22-15-06650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna M, Rivas C. Antiviral activity of resveratrol. Biochem Soc Trans. 2010;38:50–53. doi: 10.1042/BST0380050. [DOI] [PubMed] [Google Scholar]
- Castillo CG, Mendoza S, Freed WJ, Giordano M. Intranigral transplants of immortalized GABAergic cells decrease the expression of kainic acid-induced seizures in the rat. Behav Brain Res. 2006;171:109–115. doi: 10.1016/j.bbr.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Jones SM, Cross DJ. Sprouting and synaptic reorganization in the subiculum and CA1 region of the hippocampus in acute and chronic models of partial-onset epilepsy. Neuroscience. 2004;126:677–688. doi: 10.1016/j.neuroscience.2004.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Lee MY, Sung KW, Jeong SW, Choi JS, Park HJ, et al. Regional differences in enhanced neurogenesis in the dentate gyrus of adult rats after transient forebrain ischemia. Mol Cells. 2003;16:232–238. [PubMed] [Google Scholar]
- Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys. 2010;501:79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Cornish SM, Wheal HV. Long-term loss of paired pulse inhibition in the kainic acid-lesioned hippocampus of the rat. Neuroscience. 1989;28:563–571. doi: 10.1016/0306-4522(89)90005-5. [DOI] [PubMed] [Google Scholar]
- Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL. Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 2010;54:7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- Coulter DA. Chronic epileptogenic cellular alterations in the limbic system after status epilepticus. Epilepsia. 1999;40(Suppl 1):S23–S33. doi: 10.1111/j.1528-1157.1999.tb00875.x. [DOI] [PubMed] [Google Scholar]
- Crowell JA, Korytko PJ, Morrissey RL, Booth TD, Levine BS. Resveratrol-associated renal toxicity. Toxicol Sci. 2004;82:614–619. doi: 10.1093/toxsci/kfh263. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport CJ, Brown WJ, Babb TL. GABAergic neurons are spared after intrahippocampal kainate in the rat. Epilepsy Res. 1990a;5:28–42. doi: 10.1016/0920-1211(90)90063-2. [DOI] [PubMed] [Google Scholar]
- Davenport CJ, Brown WJ, Babb TL. Sprouting of GABAergic and mossy fiber axons in dentate gyrus following intrahippocampal kainate in the rat. Exp Neurol. 1990b;109:180–190. doi: 10.1016/0014-4886(90)90072-z. [DOI] [PubMed] [Google Scholar]
- Detour J, Schroeder H, Desor D, Nehlig A. A 5-month period of epilepsy impairs spatial memory, decreases anxiety, but spares object recognition in the lithium-pilocarpine model in adult rats. Epilepsia. 2005;46:499–508. doi: 10.1111/j.0013-9580.2005.38704.x. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube C, da Silva Fernandes MJ, Nehlig A. Age-dependent consequences of seizures and the development of temporal lobe epilepsy in the rat. Dev Neurosci. 2001;23:219–223. doi: 10.1159/000046147. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Spitz M. Hypothetical mechanisms for the cellular and neurophysiologic basis of secondary epileptogenesis: Proposed role of synaptic reorganization. J Clin Neurophysiol. 1997;14:90–101. doi: 10.1097/00004691-199703000-00002. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Sutula TP. Epileptogenesis in the dentate gyrus: A critical perspective. Prog Brain Res. 2007;163:755–773. doi: 10.1016/S0079-6123(07)63041-6. [DOI] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert U, Brandt C, Loscher W. Delayed sclerosis, neuroprotection, and limbic epileptogenesis after status epilepticus in the rat. Epilepsia. 2002;43(Suppl 5):86–95. doi: 10.1046/j.1528-1157.43.s.5.39.x. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Mohapel P, Elmer E, Lindvall O. Caspase inhibitors increase short-term survival of progenitor-cell progeny in the adult rat dentate gyrus following status epilepticus. Eur J Neurosci. 2001;14:937–945. doi: 10.1046/j.0953-816x.2001.01713.x. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Eybl V, Kotyzova D, Koutensky J. Comparative study of natural antioxidants – curcumin, resveratrol and melatonin – in cadmium-induced oxidative damage in mice. Toxicology. 2006;225:150–156. doi: 10.1016/j.tox.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Felling RJ, Levison SW. Enhanced neurogenesis following stroke. J Neurosci Res. 2003;73:277–283. doi: 10.1002/jnr.10670. [DOI] [PubMed] [Google Scholar]
- Franck JE, Kunkel DD, Baskin DG, Schwartzkroin PA. Inhibition in kainate-lesioned hyperexcitable hippocampi: physiologic, autoradiographic, and immunocytochemical observations. J Neurosci. 1988;8:1991–2002. doi: 10.1523/JNEUROSCI.08-06-01991.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck JE, Pokorny J, Kunkel DD, Schwartzkroin PA. Physiologic and morphologic characteristics of granule cell circuitry in human epileptic hippocampus. Epilepsia. 1995;36:543–558. doi: 10.1111/j.1528-1157.1995.tb02566.x. [DOI] [PubMed] [Google Scholar]
- Fremont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- Gao ZB, Hu GY. Trans-resveratrol, a red wine ingredient, inhibits voltage-activated potassium currents in rat hippocampal neurons. Brain Res. 2005;1056:68–75. doi: 10.1016/j.brainres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Gao ZB, Chen XQ, Hu GY. Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. Brain Res. 2006;1111:41–47. doi: 10.1016/j.brainres.2006.06.096. [DOI] [PubMed] [Google Scholar]
- Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABAsets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Gernert M, Thompson KW, Loscher W, Tobin AJ. Genetically engineered GABA-producing cells demonstrate anticonvulsant effects and long-term transgene expression when transplanted into the central piriform cortex of rats. Exp Neurol. 2002;176:183–192. doi: 10.1006/exnr.2002.7914. [DOI] [PubMed] [Google Scholar]
- Golkar L, Ding XZ, Ujiki MB, Salabat MR, Kelly DL, Scholtens D, et al. Resveratrol inhibits pancreatic cancer cell proliferation through transcriptional induction of macrophage inhibitory cytokine-1. J Surg Res. 2007;138:163–169. doi: 10.1016/j.jss.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Gong C, Wang TW, Huang HS, Parent JM. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J Neurosci. 2007;27:1803–1811. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Gross CG. Neurogenesis in adult mammals: Some progress and problems. J Neurosci. 2002;22:619–623. doi: 10.1523/JNEUROSCI.22-03-00619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WP, Sundstrom LE. Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res. 1998;790:52–59. doi: 10.1016/s0006-8993(98)00030-4. [DOI] [PubMed] [Google Scholar]
- Groticke I, Hoffmann K, Loscher W. Behavioral alterations in the pilocarpine model of temporal lobe epilepsy in mice. Exp Neurol. 2007;207:329–349. doi: 10.1016/j.expneurol.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Gupta YK, Chaudhary G, Sinha K, Srivastava AK. Protective effect of resveratrol against intracortical FeCl3-induced model of posttraumatic seizures in rats. Methods Find Exp Clin Pharmacol. 2001;23:241–244. doi: 10.1358/mf.2001.23.5.662120. [DOI] [PubMed] [Google Scholar]
- Gupta YK, Briyal S, Chaudhary G. Protective effect of trans-resveratrol against kainic acid-induced seizures and oxidative stress in rats. Pharmacol Biochem Behav. 2002;71:245–249. doi: 10.1016/s0091-3057(01)00663-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Han YS, Zheng WH, Bastianetto S, Chabot JG, Quirion R. Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: Involvement of protein kinase C. Br J Pharmacol. 2004;141:997–1005. doi: 10.1038/sj.bjp.0705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Implications of decreased hippocampal neurogenesis in chronic temporal lobe epilepsy. Epilepsia. 2008;49(Suppl 5):26–41. doi: 10.1111/j.1528-1167.2008.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Shetty AK. Decreased neuronal differentiation of newly generated cells underlies reduced hippocampal neurogenesis in chronic temporal lobe epilepsy. Hippocampus. 2010;20:97–112. doi: 10.1002/hipo.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty AK. Grafting of striatal precursor cells into hippocampus shortly after status epilepticus restrains chronic temporal lobe epilepsy. Exp Neurol. 2008;212:468–481. doi: 10.1016/j.expneurol.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Kuruba R, Shetty AK. Acute seizures in old age leads to a greater loss of CA1 pyramidal neurons, and increased propensity for developing chronic TLE and a severe cognitive dysfunction. Aging Dis. 2011;2:1–17. [PMC free article] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: Assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Houser CR. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res. 1990;535:195–204. doi: 10.1016/0006-8993(90)91601-c. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res. 1996;26:207–218. doi: 10.1016/s0920-1211(96)00054-x. [DOI] [PubMed] [Google Scholar]
- Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Daelgado-Escuet AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci. 1990;10:267–282. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Kokaia M, et al. Environment matters: Synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron. 2006;52:1047–1059. doi: 10.1016/j.neuron.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Jang JH, Surh YJ. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic Biol Med. 2003;34:1100–1110. doi: 10.1016/s0891-5849(03)00062-5. [DOI] [PubMed] [Google Scholar]
- Jang DS, Kang BS, Ryu SY, Chang IM, Min KR, et al. Inhibitory effects of resveratrol analogs on unopsonized zymosan-induced oxygen radical production. Biochem Pharmacol. 1999;57:705–712. doi: 10.1016/s0006-2952(98)00350-5. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr., Consiglio A, Lie DC, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Puccio AM, Harshman KJ, Falcione B, Benedict N, Jankowitz BT, et al. Levetiracetam versus phenytoin for seizure prophylaxis in severe traumatic brain injury. Neurosurg Focus. 2008;25:E3. doi: 10.3171/FOC.2008.25.10.E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Chu K, Kim M, Jeong SW, Song YM, Lee ST, et al. Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur J Neurosci. 2004;19:3219–3226. doi: 10.1111/j.0953-816X.2004.03412.x. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- Kapur J. Role of neuronal loss in the pathogenesis of recurrent spontaneous seizures. Epilepsy Curr. 2003;3:166–167. doi: 10.1046/j.1535-7597.2003.03506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochem Int. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kim H, Deshane J, Barnes S, Meleth S. Proteomics analysis of the actions of grape seed extract in rat brain: Technological and biological implications for the study of the actions of psychoactive compounds. Life Sci. 2006;78:2060–2065. doi: 10.1016/j.lfs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knake S, Hamer HM, Rosenow F. Status epilepticus: A critical review. Epilepsy Behav. 2009;15:10–14. doi: 10.1016/j.yebeh.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Wen X, Buckmaster PS. Reduced inhibition and increased output of layer II neurons in the medial entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci. 2003;23:8471–8479. doi: 10.1523/JNEUROSCI.23-24-08471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama R, Ikegaya Y. Mossy fiber sprouting as a potential therapeutic target for epilepsy. Curr Neurovasc Res. 2004;1:3–10. doi: 10.2174/1567202043480242. [DOI] [PubMed] [Google Scholar]
- Kraft TE, Parisotto D, Schempp C, Efferth T. Fighting cancer with red wine? Molecular mechanisms of resveratrol. Crit Rev Food Sci Nutr. 2009;49:782–799. doi: 10.1080/10408390802248627. [DOI] [PubMed] [Google Scholar]
- Kuruba R, Shetty AK. Could hippocampal neurogenesis be a future drug target for treating temporal lobe epilepsy? CNS Neurol Disord Drug Targets. 2007;6:342–357. doi: 10.2174/187152707783220884. [DOI] [PubMed] [Google Scholar]
- Kuruba R, Hattiangady B, Shetty AK. Hippocampal neurogenesis and neural stem cells in temporal lobe epilepsy. Epilepsy Behav. 2009;14(Suppl 1):65–73. doi: 10.1016/j.yebeh.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenck-Santini PP, Holmes GL. Altered phase precession and compression of temporal sequences by place cells in epileptic rats. J Neurosci. 2008;28:5053–5062. doi: 10.1523/JNEUROSCI.5024-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letty S, Lerner-Natoli M, Rondouin G. Differential impairments of spatial memory and social behavior in two models of limbic epilepsy. Epilepsia. 1995;36:973–982. doi: 10.1111/j.1528-1157.1995.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Li M, Wang QS, Chen Y, Wang ZM, Liu Z, Guo SM. Resveratrol inhibits neuronal discharges in rat hippocampal CA1 area. Sheng Li Xue Bao. 2005;57:355–360. [PubMed] [Google Scholar]
- Liu X, Muller RU, Huang LT, Kubie JL, Rotenberg A, Rivard B, et al. Seizure-induced changes in place cell physiology: Relationship to spatial memory. J Neurosci. 2003;23:11505–11515. doi: 10.1523/JNEUROSCI.23-37-11505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Shi Z, Fan L, Zhang C, Wang K, Wang B. Resveratrol improves neuron protection and functional recovery in rat model of spinal cord injury. Brain Res. 2010;1374:100–109. doi: 10.1016/j.brainres.2010.11.061. [DOI] [PubMed] [Google Scholar]
- Loscher W, Ebert U, Lehmann H, Rosenthal C, Nikkhah G. Seizure suppression in kindling epilepsy by grafts of fetal GABAergic neurons in rat substantia nigra. J Neurosci Res. 1998;51:196–209. doi: 10.1002/(SICI)1097-4547(19980115)51:2<196::AID-JNR8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Seren MS, Longo FM. Prolonged increases in neurotrophic activity associated with kainate-induced hippocampal synaptic reorganization. Neuroscience. 1993;56:597–604. doi: 10.1016/0306-4522(93)90359-n. [DOI] [PubMed] [Google Scholar]
- Lu M, Cai YJ, Fang JG, Zhou YL, Liu ZL, Wu LM. Efficiency and structure-activity relationship of the antioxidant action of resveratrol and its analogs. Pharmazie. 2002;57:474–478. [PubMed] [Google Scholar]
- Lynch M, Sutula T. Recurrent excitatory connectivity in the dentate gyrus of kindled and kainic acid-treated rats. J Neurophysiol. 2000;83:693–704. doi: 10.1152/jn.2000.83.2.693. [DOI] [PubMed] [Google Scholar]
- Maddox CE, Laur LM, Tian L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr Microbiol. 2010;60:53–58. doi: 10.1007/s00284-009-9501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P, Dargusch R, Bodai L, Gerard PE, Purcell JM, Marsh JL. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington's disease. Hum Mol Genet. 2010;20:261–270. doi: 10.1093/hmg/ddq460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- Marques FZ, Markus MA, Morris BJ. Resveratrol: Cellular actions of a potent natural chemical that confers a diversity of health benefits. Int J Biochem Cell Biol. 2009;41:2125–2128. doi: 10.1016/j.biocel.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Cifuentes F, Leite JP, Pretorius JK, Babb TL. Hippocampal EEG excitability and chronic spontaneous seizures are associated with aberrant synaptic reorganization in the rat intrahippocampal kainate model. Electroencephalogr Clin Neurophysiol. 1993;87:326–339. doi: 10.1016/0013-4694(93)90186-y. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Leite JP, Pretorius K, Yeoman KM, Kuhlman PA. The pathogenic and progressive features of chronic human hippocampal epilepsy. Epilepsy Res. 1996;26:151–161. doi: 10.1016/s0920-1211(96)00052-6. [DOI] [PubMed] [Google Scholar]
- McCloskey DP, Hintz TM, Pierce JP, Scharfman HE. Stereological methods reveal the robust size and stability of ectopic hilar granule cells after pilocarpine-induced status epilepticus in the adult rat. Eur J Neurosci. 2006;24:2203–2210. doi: 10.1111/j.1460-9568.2006.05101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlig K, Skoog I, Guo X, Schütze M, Gustafson D, Waern M, et al. Alcoholic beverages and incidence of dementia: 34-year follow-up of the prospective population study of women in Goteborg. Am J Epidemiol. 2008;167:684–691. doi: 10.1093/aje/kwm366. [DOI] [PubMed] [Google Scholar]
- Michan S, Li Y, Chou MM, Parrella E, Ge H, Long JM, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikati MA, Holmes GL, Werner S, Bakkar N, Carmant L, Liu Z, et al. Effects of nimodipine on the behavioral sequalae of experimental status epilepticus in prepubescent rats. Epilepsy Behav. 2004;5:168–174. doi: 10.1016/j.yebeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokni M, Elkahoui S, Limam F, Amri M, Aouani E. Effect of resveratrol on antioxidant enzyme activities in the brain of healthy rat. Neurochem Res. 2007;32:981–987. doi: 10.1007/s11064-006-9255-z. [DOI] [PubMed] [Google Scholar]
- Molnar P, Nadler JV. Mossy fiber-granule cell synapses in the normal and epileptic rat dentate gyrus studied with minimal laser photostimulation. J Neurophysiol. 1999;82:1883–1894. doi: 10.1152/jn.1999.82.4.1883. [DOI] [PubMed] [Google Scholar]
- Morin F, Beaulieu C, Lacaille JC. Selective loss of GABA neurons in area CA1 of the rat hippocampus after intraventricular kainate. Epilepsy Res. 1998;32:363–369. doi: 10.1016/s0920-1211(98)00033-3. [DOI] [PubMed] [Google Scholar]
- Nadler JV. The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res. 2003;28:1649–1658. doi: 10.1023/a:1026004904199. [DOI] [PubMed] [Google Scholar]
- Nadler JV, Tu B, Timofeeva O, Jiao Y, Herzog H. Neuropeptide Y in the recurrent mossy fiber pathway. Peptides. 2007;28:357–364. doi: 10.1016/j.peptides.2006.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa E, Aimi Y, Yasuhara O, Tooyama I, Shimada M, McGeer PL, et al. Enhancement of progenitor cell division in the dentate gyrus triggered by initial limbic seizures in rat models of epilepsy. Epilepsia. 2000;41:10–18. doi: 10.1111/j.1528-1157.2000.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Neville BG, Chin RF, Scott RC. Childhood convulsive status epilepticus: Epidemiology, management and outcome. Acta Neurol Scand. 2007;186:21–24. [PubMed] [Google Scholar]
- Nicolini G, Rigolio R, Miloso M, Bertelli AA, Tredici G. Anti-apoptotic effect of trans-resveratrol on paclitaxel-induced apoptosis in the human neuroblastoma SH-SY5Y cell line. Neurosci Lett. 2001;302:41–44. doi: 10.1016/s0304-3940(01)01654-8. [DOI] [PubMed] [Google Scholar]
- Nissinen J, Lukasiuk K, Pitkänen A. Is mossy fiber sprouting present at the time of the first spontaneous seizures in rat experimental temporal lobe epilepsy? Hippocampus. 2001;11:299–310. doi: 10.1002/hipo.1044. [DOI] [PubMed] [Google Scholar]
- Obenaus A, Esclapez M, Houser CR. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci. 1993;13:4470–4485. doi: 10.1523/JNEUROSCI.13-10-04470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Solis P, Consalvo D, Giagante B, Silva W, D'Alessio L, et al. Mesial temporal lobe epilepsy and hippocampal sclerosis: Cognitive function assessment in Hispanic patients. Epilepsy Behav. 2003;4:717–722. doi: 10.1016/j.yebeh.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Okawara M, Katsuki H, Kurimoto E, Shibata H, Kume T, Akaike A. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol. 2007;73:550–560. doi: 10.1016/j.bcp.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Okazaki MM, Molnar P, Nadler JV. Recurrent mossy fiber pathway in rat dentate gyrus: Synaptic currents evoked in presence and absence of seizure-induced growth. J Neurophysiol. 1999;81:1645–1660. doi: 10.1152/jn.1999.81.4.1645. [DOI] [PubMed] [Google Scholar]
- Orallo F. Trans-resveratrol: A magical elixir of eternal youth? Curr Med Chem. 2008;15:1887–1898. doi: 10.2174/092986708785132951. [DOI] [PubMed] [Google Scholar]
- Parent JM. Adult neurogenesis in the intact and epileptic dentate gyrus. Prog Brain Res. 2007;163:529–540. doi: 10.1016/S0079-6123(07)63028-3. [DOI] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006;59:81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- Patrylo PR, Schweitzer JS, Dudek FE. Abnormal responses to perforant path stimulation in the dentate gyrus of slices from rats with kainate-induced epilepsy and mossy fiber reorganization. Epilepsy Res. 1999;36:31–42. doi: 10.1016/s0920-1211(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Paul S, Rimando AM, Lee HJ, Ji Y, Reddy BS, Suh N. Anti-inflammatory action of pterostilbene is mediated through the p38 mitogen-activated protein kinase pathway in colon cancer cells. Cancer Prev Res. 2009;2:650–657. doi: 10.1158/1940-6207.CAPR-08-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Melton J, Punsoni M, McCloskey DP, Scharfman HE. Mossy fibers are the primary source of afferent input to ectopic granule cells that are born after pilocarpine-induced seizures. Exp Neurol. 2005;196:316–331. doi: 10.1016/j.expneurol.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:861–870. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Reddy DS, Shetty AK. Hippocampal neurodegeneration, spontaneous seizures, and mossy fiber sprouting in the F344 rat model of temporal lobe epilepsy. J Neurosci Res. 2006;83:1088–1105. doi: 10.1002/jnr.20802. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Rai KS, Shetty AK. Strategies for promoting anti-seizure effects of hippocampal fetal cells grafted into the hippocampus of rats exhibiting chronic temporal lobe epilepsy. Neurobiol Dis. 2007;27:117–132. doi: 10.1016/j.nbd.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Tran PH, Spigelman I, Okazaki MM, Nadler JV. Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J Comp Neurol. 2000;428:240–253. doi: 10.1002/1096-9861(20001211)428:2<240::aid-cne4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Richardson RM, Barbaro NM, Alvarez-Buylla A, Baraban SC. Developing cell transplantation for temporal lobe epilepsy. Neurosurg Focus. 2008;24:E17. doi: 10.3171/FOC/2008/24/3-4/E16. [DOI] [PubMed] [Google Scholar]
- Rigoulot MA, Koning E, Ferrandon A, Nehlig A. Neuroprotective properties of topiramate in the lithium-pilocarpine model of epilepsy. J Pharmacol Exp Ther. 2004;308:787–795. doi: 10.1124/jpet.103.057091. [DOI] [PubMed] [Google Scholar]
- Robich MP, Osipov RM, Nezafat R, Feng J, Clements RT, Bianchi C, et al. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation. 2010;122:S142–S149. doi: 10.1161/CIRCULATIONAHA.109.920132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Rossi L, Mazzitelli S, Arciello M, Capo CR, Rotilio G. Benefits from dietary polyphenols for brain aging and Alzheimer's disease. Neurochem Res. 2008;33:2390–2400. doi: 10.1007/s11064-008-9696-7. [DOI] [PubMed] [Google Scholar]
- Rutten A, van Albada M, Silveira DC, Cha BH, Liu X, Hu YN, et al. Memory impairment following status epilepticus in immature rats: Time-course and environmental effects. Eur J Neurosci. 2002;16:501–513. doi: 10.1046/j.1460-9568.2002.02103.x. [DOI] [PubMed] [Google Scholar]
- Sakata Y, Zhuang H, Kwansa H, Koehler RC, Doré S. Resveratrol protects against experimental stroke: Putative neuroprotective role of heme oxygenase 1. Exp Neurol. 2010;224:325–329. doi: 10.1016/j.expneurol.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar R, Shin DH, Liu H, Mazarati A, Pereira de Vasconcelos A, Wasterlain CG. Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J Neurosci. 1998;18:8382–8393. doi: 10.1523/JNEUROSCI.18-20-08382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Aradi I, Soltesz I. Role of mossy fiber sprouting and mossy cell loss in hyperexcitability: A network model of the dentate gyrus incorporating cell types and axonal topography. J Neurophysiol. 2005;93:437–453. doi: 10.1152/jn.00777.2004. [DOI] [PubMed] [Google Scholar]
- Sato M, Ray PS, Maulik G, Maulik N, Engelman RM, Bertelli AA, et al. Myocardial protection with red wine extract. J Cardiovasc Pharmacol. 2000;35:263–268. doi: 10.1097/00005344-200002000-00013. [DOI] [PubMed] [Google Scholar]
- Sayin U, Sutula TP, Stafstrom CE. Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia. 2004;45:1539–1548. doi: 10.1111/j.0013-9580.2004.54903.x. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: Functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL, Croll SD. Spontaneous limbic seizures after intrahippocampal infusion of brain-derived neurotrophic factor. Exp Neurol. 2002a;174:201–214. doi: 10.1006/exnr.2002.7869. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AL, Goodman JH. Spontaneous recurrent seizures after pilocarpine-induced status epilepticus activate calbindin-immunoreactive hilar cells of the rat dentate gyrus. Neuroscience. 2002b;111:71–81. doi: 10.1016/s0306-4522(01)00599-1. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AE, Berger RE, Goodman JH, Pierce JP. Perforant path activation of ectopic granule cells that are born after pilocarpine-induced seizures. Neuroscience. 2003;121:1017–1029. doi: 10.1016/s0306-4522(03)00481-0. [DOI] [PubMed] [Google Scholar]
- Shaner DM, McCurdy SA, Herring MO, Gabor AJ. Treatment of status epilepticus: A prospective comparison of diazepam and phenytoin versus phenobarbital and optional phenytoin. Neurology. 1988;38:202–207. doi: 10.1212/wnl.38.2.202. [DOI] [PubMed] [Google Scholar]
- Shao LR, Dudek FE. Detection of increased local excitatory circuits in the hippocampus during epileptogenesis using focal flash photolysis of caged glutamate. Epilepsia. 2005;46(S5):100–106. doi: 10.1111/j.1528-1167.2005.01018.x. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Ribak CE. Newly born dentate granule neurons after pilocarpine-induced epilepsy have hilar basal dendrites with immature synapses. Epilepsy Res. 2006;69:53–66. doi: 10.1016/j.eplepsyres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B. Concise review: Prospects of stem cell therapy for temporal lobe epilepsy. Stem Cells. 2007;25:2396–2407. doi: 10.1634/stemcells.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Enhanced cell survival in fetal hippocampal suspension transplants grafted to adult rat hippocampus following kainate lesions: a three-dimensional graft reconstruction study. Neuroscience. 1995a;67:561–582. doi: 10.1016/0306-4522(95)00025-e. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Intracerebroventricular kainic acid administration in adult rat alters hippocampal calbindin and non-phosphorylated neurofilament expression. J Comp Neurol. 1995b;363:581–599. doi: 10.1002/cne.903630406. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Aging impairs axonal sprouting response of dentate granule cells following target loss and partial deafferentation. J Comp Neurol. 1999;414:238–254. doi: 10.1002/(sici)1096-9861(19991115)414:2<238::aid-cne7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Fetal hippocampal grafts containing CA3 cells restore host hippocampal glutamate decarboxylase-positive interneuron numbers in a rat model of temporal lobe epilepsy. J Neurosci. 2000;20:8788–8801. doi: 10.1523/JNEUROSCI.20-23-08788.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Glutamicacid decarboxylase-67-positive hippocampal interneurons undergo a permanent reduction in number following kainic acid-induced degeneration of ca3 pyramidal neurons. Exp Neurol. 2001;169:276–297. doi: 10.1006/exnr.2001.7668. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Zaman V, Shetty GA. Hippocampal neurotrophin levels in a kainate model of temporal lobe epilepsy: a lack of correlation between brain-derived neurotrophic factor content and progression of aberrant dentate mossy fiber sprouting. J Neurochem. 2003;87:147–159. doi: 10.1046/j.1471-4159.2003.01979.x. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Rao MS, Hattiangady B, Zaman V, Shetty GA. Hippocampal neurotrophin levels after injury: Relationship to the age of the hippocampus at the time of injury. J Neurosci Res. 2004;78:520–532. doi: 10.1002/jnr.20302. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Zaman V, Hattiangady B. Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy. J Neurosci. 2005;25:8391–8401. doi: 10.1523/JNEUROSCI.1538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Rao MS. Vulnerability of hippocampal GABA-ergic interneurons to kainate-induced excitotoxic injury during old age. J Cell Mol Med. 2009;13:2408–2423. doi: 10.1111/j.1582-4934.2008.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler KS, Ventura E, Dutt M, Elliott P, Fitzgerald DC, Rostami A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J Neuroophthalmol. 2010;30:328–339. doi: 10.1097/WNO.0b013e3181f7f833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M. Resveratrol (trans-3,5,4′-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther. 2010;332:829–839. doi: 10.1124/jpet.109.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton RH, Yan HQ, Fellows-Mayle W, Dixon CE. Resveratrol attenuates behavioral impairments and reduces cortical and hippocampal loss in a rat controlled cortical impact model of traumatic brain injury. J Neurotrauma. 2010;27:1091–1099. doi: 10.1089/neu.2010.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha K, Chaudhary G, Gupta YK. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002;71:655–665. doi: 10.1016/s0024-3205(02)01691-0. [DOI] [PubMed] [Google Scholar]
- Sirven JI, Waterhouse E. Management of status epilepticus. Am Fam Physician. 2003;68:469–476. [PubMed] [Google Scholar]
- Siwak-Tapp CT, Head E, Muggenburg BA, Milgram NW, Cotman CW. Neurogenesis decreases with age in the canine hippocampus and correlates with cognitive function. Neurobiol Learn Mem. 2007;88:249–259. doi: 10.1016/j.nlm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: The “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Possible functional consequences of synaptic reorganization in the dentate gyrus of kainate-treated rats. Neurosci Lett. 1992;137:91–96. doi: 10.1016/0304-3940(92)90306-r. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Harvey BD, Bumanglag AV, Bender RA, Frotscher M. “Dormant basket cell” hypothesis revisited: Relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J Comp Neurol. 2003;459:44–76. doi: 10.1002/cne.10630. [DOI] [PubMed] [Google Scholar]
- Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- Sperk G, Wieser R, Widmann R, Singer EA. Kainic acid induced seizures: Changes in somatostatin, substance P and neurotensin. Neuroscience. 1986;17:1117–1126. doi: 10.1016/0306-4522(86)90081-3. [DOI] [PubMed] [Google Scholar]
- Strine TW, Kobau R, Chapman DP, Thurman DJ, Price P, Balluz LS. Psychological distress, comorbidities, and health behaviors among U.S. adults with seizures: Results from the 2002 National Health Interview Survey. Epilepsia. 2005;46:1133–1139. doi: 10.1111/j.1528-1167.2005.01605.x. [DOI] [PubMed] [Google Scholar]
- Sun AY, Chen YM, James-Kracke M, Wixom P, Cheng Y. Ethanol-induced cell death by lipid peroxidation in PC12 cells. Neurochem Res. 1997;22:1187–1192. doi: 10.1023/a:1021968526696. [DOI] [PubMed] [Google Scholar]
- Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula T. Seizure-induced axonal sprouting: Assessing connections between injury, local circuits, and epileptogenesis. Epilepsy Curr. 2002;2:86–91. doi: 10.1046/j.1535-7597.2002.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula T, Xiao-Xian H, Cavazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- Thompson KW. Genetically engineered cells with regulatable GABA production can affect afterdischarges and behavioral seizures after transplantation into the dentate gyrus. Neuroscience. 2005;133:1029–1037. doi: 10.1016/j.neuroscience.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Tillement JP. In vitro protection of cerebral mitochondrial function by E-resveratrol in anoxia followed by re-oxygenation. Bull Acad Natl Med. 2001;185:1429–1443. [PubMed] [Google Scholar]
- Tredici G, Miloso M, Nicolini G, Galbiati S, Cavaletti G, Bertelli A. Resveratrol, map kinases and neuronal cells: Might wine be a neuroprotectant? Drugs Exp Clin Res. 1999;25:99–103. [PubMed] [Google Scholar]
- Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42(Suppl 3):8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannest J, Szaflarski JP, Privitera MD, Schefft BK, Holland SK. Medial temporal fMRI activation reflects memory lateralization and memory performance in patients with epilepsy. Epilepsy Behav. 2008;12:410–418. doi: 10.1016/j.yebeh.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Vidavalur R, Otani H, Singal PK, Maulik N. Significance of wine and resveratrol in cardiovascular disease: French paradox revisited. Exp Clin Cardiol. 2006;11:217–225. [PMC free article] [PubMed] [Google Scholar]
- Virgili M, Contestabile A. Partial neuroprotection of in vivo excitotoxic brain damage by chronic administration of the red wine antioxidant agent, trans-resveratrol in rats. Neurosci Lett. 2000;281:123–126. doi: 10.1016/s0304-3940(00)00820-x. [DOI] [PubMed] [Google Scholar]
- Waldau B, Hattiangady B, Kuruba R, Shetty AK. Medial ganglionic eminence-derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy. Stem Cells. 2010;28:1153–1164. doi: 10.1002/stem.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, et al. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- Wang Q, Yu S, Simonyi A, Rottinghaus G, Sun GY, Sun AY. Resveratrol protects against neurotoxicity induced by kainic acid. Neurochem Res. 2004;29:2105–2112. doi: 10.1007/s11064-004-6883-z. [DOI] [PubMed] [Google Scholar]
- Williams LD, Burdock GA, Edwards JA, Beck M, Bausch J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem Toxicol. 2009;47:2170–2182. doi: 10.1016/j.fct.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Williamson A, Patrylo PR, Spencer DD. Decrease in inhibition in dentate granule cells from patients with medial temporal lobe epilepsy. Ann Neurol. 1999;45:92–99. doi: 10.1002/1531-8249(199901)45:1<92::aid-art15>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Wu Z, Xu Q, Zhang L, Kong D, Ma R, Wang L. Protective effect of resveratrol against kainate-induced temporal lobe epilepsy in rats. Neurochem Res. 2009;34:1393–1400. doi: 10.1007/s11064-009-9920-0. [DOI] [PubMed] [Google Scholar]
- Wuarin J-P, Dudek FE. Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slice from kainate treated epileptic rats. J Neurosci. 1996;16:4438–4448. doi: 10.1523/JNEUROSCI.16-14-04438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cui SS, Wallace AE, Hannesson DK, Schmued LC, Saucier DM, et al. Relations between brain pathology and temporal lobe epilepsy. J Neurosci. 2002;22:6052–6061. doi: 10.1523/JNEUROSCI.22-14-06052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Shi JS, Zhou H, Wilson B, Hong JS, Gao HM. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol Pharmacol. 2010;78:466–477. doi: 10.1124/mol.110.064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang H, Kim YS, Koehler RC, Dore S. Potential mechanism by which resveratrol, a red wine constituent, protects neurons. Ann NY Acad Sci. 2003;993:276–286. doi: 10.1111/j.1749-6632.2003.tb07534.x. [DOI] [PubMed] [Google Scholar]
- Zini R, Morin C, Bertelli A, Bertelli AA, Tillement JP. Effects of resveratrol on the rat brain respiratory chain. Drugs Exp Clin Res. 1999;25:87–97. [PubMed] [Google Scholar]
- Zini R, Morin C, Bertelli A, Bertelli AA, Tillement JP. Resveratrol-induced limitation of dysfunction of mitochondria isolated from rat brain in an anoxia-reoxygenation model. Life Sci. 2002;71:3091–3108. doi: 10.1016/s0024-3205(02)02161-6. [DOI] [PubMed] [Google Scholar]