Abstract
Inflammation when unchecked is associated with many prevalent disorders such as the classic inflammatory diseases arthritis and periodontal disease, as well as the more recent additions that include diabetes and cardiovascular maladies. Hence mechanisms to curtail the inflammatory response and promote catabasis are of immense interest. In recent years, evidence has prompted a paradigm shift whereby the resolution of acute inflammation is a biochemically active process regulated in part by endogenous PUFA (polyunsaturated fatty acid)-derived autacoids. Among these are a novel genus of SPMs (specialized proresolving mediators) that comprise novel families of mediators including lipoxins, resolvins, protectins and maresins. SPMs have distinct structures and act via specific G-protein seven transmembrane receptors that signal intracellular events on selective cellular targets activating proresolving programmes while countering pro-inflammatory signals. An appreciation of these endogenous pathways and mediators that control timely resolution opened a new terrain for therapeutic approaches targeted at stimulating resolution of local inflammation. In the present review, we provide an overview of the biosynthesis and actions of resolvin E1, underscoring its protective role in vascular systems and regulating platelet responses. We also give an overview of newly described resolution circuitry whereby resolvins govern miRNAs (microRNAs), and transcription factors that counter-regulate pro-inflammatory chemokines, cytokines and lipid mediators.
Keywords: lipid mediator, microRNA, omega-3 fatty acid, platelet, resolution
Abbreviations: AA, arachidonic acid; ALX/FPR2, G-protein-coupled receptor for lipoxin A4; apoE, apolipoprotein E; CD, cluster of differentiation; ChemR23, G-protein-coupled receptor for RvE1; COX, cyclo-oxygenase; CRP, C-reactive protein; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; ERK, extracellular-signal-regulated; GPR32, G-protein-coupled receptor for RvD1; HETE, hydroxyeicosatetraenoic acid; IFN, interferon; IκB, inhibitory κB; IL, interleukin; LC-MS/MS, liquid chromatography-tandem MS; LDL, low-density lipoprotein; LOX, lipoxygenase; LTB4, leukotriene B4; LX, lipoxin; MAPK, mitogen-activated protein kinase; miRNA, microRNA; NF-κB, nuclear factor κB; p70S6K, ribosomal protein S6 kinase; PDGF, platelet-derived growth factor; PDGFR, PDGF receptor; PI3K, phosphoinositide 3-kinase; PGI2, prostacyclin; PGI3, Δ17-prostacyclin; PGLYRP, peptidoglycan recognition protein; PMN, polymorphonuclear cell/neutrophil; PUFA, polyunsaturated fatty acid; rS6, ribosomal protein S6; RvD1, resolvin D1; RvE1, resolvin E1; SPM, specialized proresolving mediator; TF, transcription factor; 7-TM, G-protein-coupled seven-transmembrane receptor; TLR, Toll-like receptor; TNF, tumour necrosis factor; TX, thromboxane; VMSC, vascular smooth muscle cell
HOMOEOSTASIS: THE PUSH AND PULL OF PRO-INFLAMMATORY AND PRORESOLVING MEDIATORS
Oedema formation and leucocyte emigration are essential components of the acute inflammatory response [1] (Figure 1A). Initially protective, acute inflammation is a physiological programme that protects the host against invading pathogens and local injury [1]. If uncontrolled, inflammation can become chronic leading to fibrosis and tissue damage. A prominent cause of tissue damage is excessive leucocyte accumulation as in the cases of arthritis or periodontal disease [2]. Hence, mechanisms for removal of leucocytes from inflammatory sites and the clearance of remnants of the host's combat between leucocytes, invading microbes, and/or other initiators of inflammation are of considerable interest.
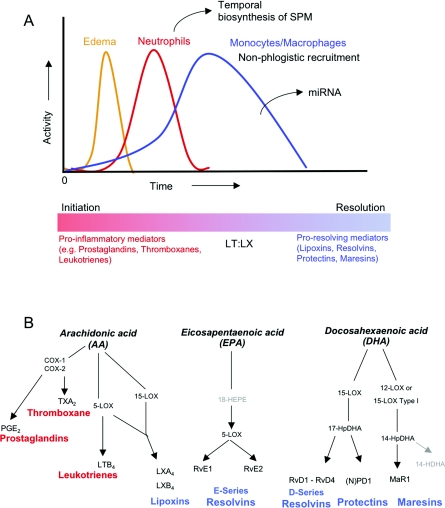
Figure 1. Acute inflammation cellular and chemical mediators.
(A) At the onset of acute inflammation, vascular leakage (oedema) occurs. Neutrophils are among the first responders during an acute inflammatory response, followed by monocytes and macrophages. The first cellular hallmark of tissue resolution is a decrease in neutrophil infiltration. Updated from [1]. Classically, eicosanoids such as PGE2, TXA2 and LTB4 (B, red) are known to exert pro-inflammatory actions such as vasodilatation, platelet activation and chemotaxis respectively. Proresolving molecules are generated during inflammation that block vasodilatation/oedema formation and limit further chemotaxis thus allowing for the return to homoeostasis. (B, blue) (B) Scheme of eicosanoid and SPM generation. AA-derived eicosanoids are in red. EPA-derived E-series resolvins, DHA-derived D-series resolvins, protectins and maresins are in blue.
It is becoming increasingly apparent that the resolution of inflammation and the return from disease state (catabasis) requires active counter-regulation of pro-inflammatory signals [3]. Counter-regulation of the inflammatory response is achieved by several physiological mechanisms acting at the systemic level; biosynthesis of local bioactive lipid autacoids, an increase in circulating levels of glucocorticoids, or activation of the acute-phase response, provide systemically active and protective responses to stress and inflammation. Within the vasculature as an example, AA (arachidonic acid)-derived PGI2 (prostacyclin) and TX (thromboxane) A2 are important counter-regulators whereby both chemical mediators are necessary for vascular homoeostasis [4].
Resolution of inflammation is operative at the local tissue level to limit inflammatory injury and restore homoeostasis [5]. In addition to AA-derived lipoxins, a novel genus of enzymatically oxygenated lipid mediators derived from ω–3 PUFAs (poly-unsaturated fatty acids), such as EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid), were elucidated in this laboratory that function as SPMs (specialized proresolution mediators) that actively ‘turn off’ the inflammatory response [6]. These SPM families include lipoxins, resolvins, protectins and maresins (Figure 1B) and comprise novel families of autacoids with potent anti-inflammatory, tissue-protective and resolution-stimulating functions [6]. Of note, each SPM has a unique structural feature to evoke biological functions. The biosynthesis and general actions were recently reviewed in detail in [7,8].
The formation of specific lipid mediator autacoids during resolution was monitored by LC-MS/MS (liquid chromatography-tandem MS)-based lipidomic analysis of resolving, self-limited inflammatory exudates. The biosynthesis with human cells was assembled employing individual cell types and human recombinant enzymes [9] using a systems approach with exudates from experimental animals (reviewed in detail in [6]).
SPMs regulate homoeostasis
The first anti-inflammatory and proresolving SPMs recognized were LX (lipoxin) A4 and LXB4 [10]. Lipoxins are lipoxygenase-derived eicosanoids, derived enzymatically from AA, an ω–6 fatty acid that is released and mobilized during inflammation [11]. In human systems, they are synthesized via transcellular biosynthesis steps engaged during leucocyte interactions with mucosal cells, that is, epithelia of the gastrointestinal tract or bronchial tissue, and within the vasculature during platelet–leucocyte interactions [6] (For further details regarding protective action in vivo, see Table 1). Aspirin has an unexpected impact within resolution. In humans, aspirin ‘jump-starts’ this process by its ability to trigger the endogenous biosynthesis of specific lipid mediators [10]. The LXs were first appreciated for their anti-inflammatory actions in vivo, but took nearly two decades to learn that they are agonists of resolution [12].
Table 1. In vivo actions of lipoxins and aspirin-triggered lipoxins with a LXA4/ATL mediator.
| Species/disease model | Action(s) |
|---|---|
| Mouse/dermal inflammation | Inhibits neutrophil recruitment and vascular leakage [103] |
| Mouse/dorsal air pouch | Inhibits neutrophil recruitment [104] |
| Rabbit/periodontitis | Reduces PMN infiltration and prevents connective tissue and bone loss [105] |
| Mouse/peritonitis | Inhibits neutrophil recruitment and lymphatic removal of phagocytes [3,12] |
| Mouse/colitis | Attenuates pro-inflammatory gene expression and reduces severity of colitis, inhibits weight loss, inflammation and immune dysfunction [106] |
| Mouse/asthma | Inhibits airway hyper-responsiveness and pulmonary inflammation [107] |
| Mouse/cystic fibrosis | Decreases neutrophilic inflammation, pulmonary bacterial burden and disease severity [108] |
| Mouse/ischaemia/reperfusion (I/R) | Attenuates hind-limb I/R-induced lung injury [109]. Detachment of adherent leucocytes in mesenteric I/R [110]. Reduces myocardial infarct size and area at risk in myocardial I/R (infarction) |
| Mouse/cornea | Accelerates cornea re-epithelialization, limits sequelae of thermal injury (i.e. neovascularization, opacity) and promotes host defence [86] |
| Mouse/angiogenesis | Reduces angiogenic phenotype: endothelial cell proliferation and migration [111] |
| Mouse/bone marrow transplant (BMT) | Protects against BMT-induced graft-versus-host diseases (GvHD) [112] |
| Rat/glomerulonephritis | Reduces leucocyte rolling and adherence, decreases neutrophil recruitment [113] |
| Rat/hyperalgesia | Prolongs paw withdraw latency, reducing hyperalgesic index and reduces paw oedema [114] |
| Rat/pleuritis | Shortens the duration of pleural exudation [115] |
| Mouse/tumour growth | Suppresses the growth of transplanted H22 tumour in mice through inhibiting tumour-related angiogenesis [116] |
| Mouse/allograft rejections | Prevents acute rejection of vascularized cardiac and renal allografts [117] |
| Mouse/arthritis | Inhibits oedema formation and PMN influx, reduces TNFα and LTB4 levels [118] |
| Rat/acute pancreatitis | Reduces intercellular adhesion molecule 1 (ICAM-1) and NF-κB p65 expression in the pancreas, and expression of ICAM-1 in the lungs in animals with pancreatitis [119] |
Resolvins are a family of new local mediators enzymatically generated within resolving inflammatory exudates. They were initially identified using a systems approach with LC-MS/MS-based lipidomics and informatics, and subsequently complete structural elucidation of these bioactive mediators and related compounds was achieved [13–17]. The term resolvins or resolution-phase interaction products refers to endogenous mediators that are biosynthesized from the major ω–3 fatty acids EPA and DHA, denoted as E series (RvE) and D series (RvD) resolvins respectively [6]. Similar to lipoxins, resolvins are also generated by the COX (cyclo-oxygenase)-2 pathway in the presence of aspirin yielding ‘AT’ (aspirin-triggered) forms. Increasing evidence indicates that resolvins possess potent anti-inflammatory and immunoregulatory actions that include blocking the production of pro-inflammatory mediators (i.e. chemokines and cytokines) and the organized leucocyte traffic to inflammatory sites (reviewed in [18]), as well as clearance of neutrophils from mucosal surfaces [19]. Specifically, resolvins are multi-focal-acting mediators that act via limiting further PMN (polymorphonuclear cell/neutrophil) transendothelial migration in vitro and infiltration in vivo [13,14], but also enhance pro-inflammatory chemokine scavenging [20], non-phlogistic recruitment on monocytes and phagocytosis, as well as phagocyte clearance via the lymphatics [12].
The actions of these endogenous SPMs are mediated via specific 7-TMs (G-protein-coupled seven-transmembrane receptors). Hence, SPMs are resolution agonists that stimulate proresolving pathways, rather than obstruct anti-inflammatory signals. RvE1 (resolvin E1) as an example, acts as an agonist on ChemR23 (G-protein-coupled receptor for RvE1) and as a partial agonist on the LTB4 (leukotriene B4) receptor (BLT1) thus competing with LTB4 for binding [17,21]. Recent research has revealed that RvE1 stimulates phosphorylation of Akt and p70S6K (ribosomal protein S6 kinase) in a time- and dose-dependent manner via direct activation of ChemR23 [22]. RvE1 therefore displays a distinct mechanism of action compared with LXA4 that inhibits downstream tyrosine phosphorylation in eosinophils [23]. Recently two separate 7-TMs that RvD1 specifically binds on human leucocytes, namely the ALX/FPR2 (LXA4 receptor) and GPR32 (G-protein-coupled receptor for RvD1) an orphan receptor were reported [24]. Identification of receptors for the other ω–3-derived SPMs are yet to be uncovered, but are likely to also be high-affinity 7-TMs on the basis of the potency and stereoselective actions of each SPM member. Hence, two GPCRs for each RvE1 and RvD1 have been identified. The notion that one ligand can act on a repertoire of receptors is not surprising in light of recent evidence on neuronal responses to formyl receptor-like peptides that possess ALX/FPR2 and are activated by LXA4 [25]. Also, anti-inflammatory peptides annexin 1 and chemerin specifically activate these receptors as well [26].
SPMs are made available to evolving exudates
Early phases of the acute inflammatory response involve rapid changes in local blood vessel perfusion and permeability not only to permit extravasation of circulating leucocytes and plasma proteins, but also provide a means to deliver nutrient from the circulation, namely substrates for exudate SPM biosynthesis. In zymosan-stimulated peritonitis exudate the levels of free or unesterified ω−3 PUFA, AA, EPA and DHA increase rapidly, reaching maximal levels approximately 2–4 h post-stimulation [3]. Systemically administered deuterium-labelled ω−3 PUFAs rapidly appear in the developing infiltrate within the inflammatory exudates and a second wave of circulating substrates are delivered during resolution [27]. A close inspection indicates that the SPM precursors EPA and DHA appear in the inflammatory milieu with similar kinetics as the extravasation of serum proteins (identified using proteomics [3]), paralleling oedema yet before the infiltration of neutrophils. These findings point to the importance of oedema with exudating serum proteins, to effectively deliver ω−3 PUFAs to an inflamed tissue and promote resolution.
Without control of the inflammatory response, tissues would be overwhelmed by oedema, persistent inflammatory cell infiltrates and subsequent tissue damage incurred by activated inflammatory leucocytes [28–30]. Thus active counter-regulation and resolution of inflammation are essential for the maintenance of homoeostasis and health [31]. This present review focuses on the protective actions of resolvins, specifically the first elucidated resolvin denoted RvE1, on selective cellular targets such as platelets, smooth muscle cells and macrophages, as well as novel concepts underlying resolution circuitry.
CHANGING THE PARADIGM FOR ω−3 FATTY ACID PROTECTION IN HUMAN PATHOLOGIES: FOCUS ON CARDIOVASCULAR DISEASE
Essential fatty acids, such as EPA and DHA, are well known for their protective actions in many diseases including, cancer [32], Alzheimer's disease [33] and cardiovascular diseases [34–38]. Their cardioprotective actions were first observed in the 1970s [39–43].
Dyerberg et al. [44] demonstrated that in the presence of EPA, the formation of TXA3 catalysed by platelet endoperoxides [45], which is mainly driven by COX-1. Unlike AA-derived TXA2 which is a potent platelet agonist [46], TXA3 does not have platelet-aggregating properties. AA and EPA can both be utilized by COX-1 to generate the intermediate endoperoxide and subsequent TXA2 or TXA3 respectively, creating a competition between these substrates for the enzyme [47,48]. In a milieu enriched in EPA, the COX-1 appears to favour EPA over utilizing AA as a substrate generating the biologically inactive TXA3 [44]. Moreover, EPA can be utilized by the endothelium to make an anti-aggregating substance, PGI3 (Δ17-prostacyclin) [44,48]. This finding suggests that, in vivo, high levels of EPA and low levels of AA could lead to an anti-thrombotic state that is mediated by PGI3 and a non-active TXA3 [44]. Thus utilization of EPA rather than AA in vivo would presumably shift the balance between the pro- and anti-aggregating (i.e. TXA2/PGI2) agents toward the anti-aggregatory (TXA3/PGI3) [44,49,50].
The GISSI (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico) study demonstrated reduced mortality from cardiovascular complications (e.g. cardiac death and coronary death) in >11000 cardiovascular patients taking ω−3 PUFAs [51,52]. It is noteworthy that, given the cardiovascular status of these patients, all were taking aspirin [51,52]. Aspirin is one of the most widely used anti-inflammatory drugs, and low-dose aspirin (81 mg) is currently recommended by the American Heart Association (http://www.americanheart.org) for both primary and secondary prevention of myocardial infarction, stroke and unstable angina. The beneficial actions of aspirin in the cardiovascular system have been widely attributed to the well-documented ability of aspirin to block prostaglandins and prothrombotic TXA2 generation via acetylation of COX-1 [53,54]. Notably, aspirin has additional anti-inflammatory actions, such as blocking leucocyte trafficking to inflamed tissues, which cannot only be attributed to aspirin's ability to inhibit prostanoid biosynthesis [18,55]. As noted above, aspirin acetylation of COX-2 not only inhibits prostanoid formation, but alters the active site of COX-2 and thereby permits conversion of AA into 15R-HETE (hydroxyeicosatetraenoic acid) or EPA to 18-HEPE in vascular endothelial cells as examples. These intermediates can be further transformed to epimeric lipoxins, RvE1, or aspirin-triggered resolvins by leucocytes [6]. The formation of 15-epi-lipoxins, as an example, is documented in healthy individuals taking low-dose aspirin and is shown to be both age and gender dependent [56,57]. Recently, the anti-inflammatory actions of aspirin were documented during acute inflammation in humans [58,59]. Oral administration of low-dose aspirin reduced leucocyte accumulation in cantharidin-induced skin blisters and stimulated both 15-epi-lipoxin biosynthesis and an increase in ALX expression [58].
Hence, recent evidence now points to bioactive lipid mediators that are structurally distinct and act via specific 7-TMs to actively counter-regulate pro-inflammatory signals. The next sections highlight the biosynthesis, structural elucidation and the targeted cellular actions of EPA-derived RvE1.
RvE1 BIOSYNTHETIC ROUTES AND SELECTIVE CELLULAR ACTIONS
RvE1 biosynthesis and structural elucidation
RvE1 is generated by human cell types during cell–cell interactions from EPA typified by endothelial cell and leucocyte interaction [13]. In vascular endothelial cells, aspirin-acetylated COX-2 converts EPA into 18R-hydro(peroxy)-EPE, which is quickly reduced to 18R-HEPE. 18R-HEPE is then released from endothelium and rapidly transformed by activated human PMNs in the proximity to a bioactive trihydroxy-containing product, termed RvE1 that reduced PMN transmigration and was anti-inflammatory in vivo more than 100× more potent than aspirin or dexamethasone [17]. Total organic synthesis confirmed the original structural assignment and bioactions as well as established the complete stereochemistry of natural RvE1,5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-EPA [17]. RvE1 is also produced in vivo through aspirinindependent routes via mechanisms involving P450 [60] and was also found to be produced by Candida albicans [61]. Thus there are multiple biosynthetic pathways for RvE1 production in biological systems, those being aspirin triggered, P450 dependent and/or de novo from microbial sources. RvE1 has protective actions in preclinical animal models of disease including experimental periodontitis [62,63], colitis [64] and dry eye [65] to name a few (see Table 2 for details). RvE1 also has actions on several cell types including leucocytes and platelets [66].
Table 2. In vivo actions of resolvins, protectins and maresins.
| Mediator | Species/disease model | Action(s) |
|---|---|---|
| RvE1 | Mouse/dorsal air pouch | Inhibits neutrophil recruitment [13] |
| Mouse/peritonitis | Inhibits neutrophil recruitment, regulates chemokine/cytokine production [3,17] and promotes lymphatic removal of phagocytes [12] | |
| Rabbit/periodontitis | Reduces PMN infiltration, prevents connective tissue and bone loss, promotes healing of diseased tissues and promotes regeneration of lost soft tissue and bone [62,63] | |
| Mouse/retinopathy | Protects against neovascularization [120] | |
| Mouse/colitis | Decreases PMN recruitment and pro-inflammatory gene expression, improves survival and reduces weight loss [64,121] | |
| Mouse/asthma | Reduces IL-23 and IL-6, and increases IFNγ and LXA4 in lungs to dampen airway inflammation [122]. RvE1 decreases eosinophil and lympocyte recruitment [123,124] | |
| Mouse/obesity | Regulates adipokines and protects against liver steatosis [125] | |
| Mouse/inflammatory pain | Inhibits spontaneous pain, and heat and mechanical hypersensitivity [126] | |
| Rat/cardiac ischaemia/reperfusion injury | Reduces infarct size [127] | |
| Mouse/allograft rejections | Prevents acute rejection of vascularized cardiac and renal allografts [117] | |
| Mouse/dry eye | Promotes tear production, corneal epithelial integrity, and decreases in inflammatory inducible COX-2. RvE1 inhibits keratocyte transformation to myofibroblasts and lowers the number of monocytes/macrophages [65] | |
| Mouse/herpes simplex virus | Reduces severity of herpes simplex virus-induced ocular lesions, reduces angiogenesis and stromal keratitis [128] | |
| RvD1 | Mouse/peritonitis | Inhibits neutrophil recruitment [15,100] |
| Mouse/dorsal air pouch | Inhibits neutrophil recruitment [14,15] | |
| Mouse/kidney ischemia-reperfusion | Protects from ischaemia/reperfusion-induced kidney damage and loss of function. Regulates macrophages [129] | |
| Mouse/retinopathy | Protects against neovascularization [120] | |
| Mouse/inflammatory pain | Inhibits spontaneous pain, heat and mechanical hypersensitivity [126] | |
| Rats/post-operative pain | Reduces post-operative pain, tactile allodynia and hyperalgesia [130] | |
| PD1/NPD1 | Mouse/peritonitis | Inhibits neutrophil recruitment and regulates chemokine/cytokine production [3,16] |
| Promotes lymphatic removal of phagocytes [12] and regulates T-cell migration [131] | ||
| Mouse/asthma | Protects from lung damage, airway inflammation and hyperresponsiveness [132] | |
| Human/asthma | PD1 is generated in human asthma [132] | |
| Mouse/kidney ischaemia/reperfusion | Protects from ischaemia/reperfusion-induced kidney damage and loss of function; regulates macrophages [129] | |
| Mouse/retinopathy | Protects against neovascularization [120] | |
| Rat/ischaemic stroke | Inhibits leucocyte infiltration, NF-κB and COX-2 induction [133] | |
| Human/Alzheimer's disease | Diminished PD1 production in human Alzheimer's disease [33] | |
| Mouse/liver injury | Protects necroinflammatory liver injury [125] | |
| Mouse/Alzheimer's disease | Potently down-regulates inflammatory signalling, amyloidogenic amyloid precursor protein cleavage and apoptosis [134] | |
| RvD2 | Mouse/peritonitis | Potently blocks PMN infiltration into the peritoneum [135] |
| Mouse/sepsis | Prevents hypothermia, decreases bacterial load in the blood and peritoneum, promotes survival [135] | |
| MaR1 | Mouse/peritonitis | Potently blocks PMN infiltration into the peritoneum [136] |
RvE1 actions in human whole blood, platelets and smooth muscle cells
EPA-derived RvE1 is endogenously generated in whole blood [17] and selectively acts on PMNs and monocytes to reduce L-selectin and CD (cluster of differentiation)18 surface expression [66]. Corroborating results via intravital microscopy in mice also demonstrated that RvE1 reduced leucocyte rolling along the endothelium. Additionally, RvE1 stimulation of whole blood regulated various cytokines and chemokines. Notably, RvE1 reduced IL (interleukin)-8 and increased IL-10 levels in human whole blood [66].
Platelets are critical in coagulation, atherogenesis, wound healing and inflammation. Platelet–platelet interactions are essential in thrombosis, which has further consequences on platelet–leucocyte and platelet–endothelium interactions [67]. These active cell–cell communications are responsible for and provide the links between thrombosis, inflammation and atherogenesis [68,69]. In view of the beneficial impact of EPA and aspirin in the cardiovascular arena, it was of particular interest to investigate whether RvE1 directly acts on human platelets. Indeed, EPA-derived RvE1 reduced ADP-stimulated platelet aggregation and TX generation [66]. In addition to ADP, RvE1 also reduced platelet aggregation stimulated by U46619, a TX receptor agonist, with similar kinetics [66]. In contrast, RvE1 did not affect collagen-stimulated platelet aggregation, indicating an agonist-selective action of RvE1 on human platelet aggregation. PD1 (protectin D1) reduces ADP-stimulated platelet aggregation in the micromolar range [66]. Of interest, newly named DHA-derived products coined poxytrins block collagen-stimulated platelet aggregation, but require micromolar concentrations for their actions [70,71]. The vital platelet homoeostatic responses (e.g. collagen- and thrombin-induced aggregation for wound healing) of poxytrins in the micromolar range raise the question regarding their physiological role in humans.
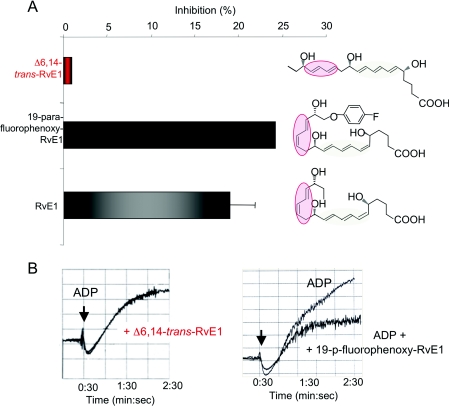
ChemR23, one of the receptors for RvE1, is present on human platelets [66,72] and to address whether RvE1's actions on platelets were stereoselective, RvE1 was directly compared with its biologically inactive isomer, the Δ6, 14-trans RvE1 isomer [17] and it biologically stable isomer 19-para-fluorophenoxy RvE1 (Figure 2). RvE1 and 19-para-fluorophenoxy RvE1 markedly reduced ADP-stimulated platelet aggregation, whereas RvE1's biologically isomer Δ6,14-trans RvE1 did not in this concentration range (Figure 2), this family demonstrated a selective action for RvE1 in attenuating human platelet aggregation in PRP (platelet-rich plasma).
Figure 2. RvE1 has potent and stereoselective action on human platelets.
(A) RvE1 (grey) and its stable isomer 19-para-fluorophenoxy-RvE1 (black) both reduced ADP-stimulated platelet aggregation. The biologically inactive isomer of RvE1, Δ6,14-trans isomer (red) did not block ADP-stimulated platelet aggregation. (B) Representative real-time aggregation tracings for Δ6,14-trans isomer (left-hand panel) and 19-para-fluorophenoxy-RvE1 (right-hand panel).
The actions of ADP on platelets are of particular interest because ADP is a well appreciated local mediator of haemostasis and thrombosis, and when aberrantly regulated can contribute to the pathogenesis of cardiovascular diseases [4,73]. On platelets, ADP specifically binds and activates two G-protein-coupled receptors P2Y1 and P2Y12. Transduction of the ADP signal involves inhibition of adenyl cyclase (via P2Y12) and a concomitant transient increase of intracellular Ca2+ (via P2Y1) [73]. Further downstream signalling results in robust shape changes, inside-out activation of platelet receptors, such as GPIIbIIIa, and granule secretion [73]. Current anti-platelet therapies targeted at blocking purinergic receptor signalling, specifically P2Y12 antagonists, are the focal point of platelet therapeutics [74] and are among the most widely used pharmacotherapy in cardiovascular diseases [75,76].
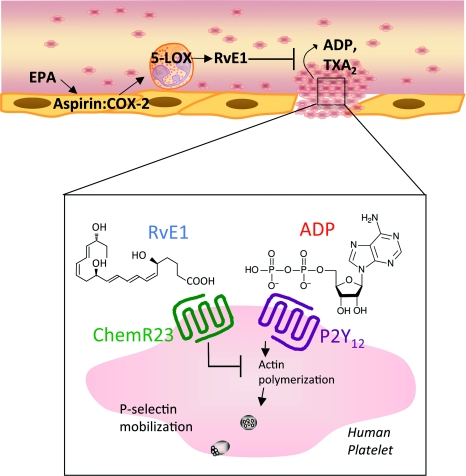
RvE1 and its 19-para-fluorophenoxy-RvE1 analogue are more potent than the native EPA, and limited ADP-stimulated P-selectin surface mobilization [72]. Additionally RvE1 dampened the potentiation of ADP-stimulated morphology changes [72] (Figure 3). RvE1's counter-regulatory actions on human platelets were calcium independent since RvE1 alone did not stimulate intracellular calcium nor did it block ADP-stimulated calcium mobilization [72], a direct consequence of P2Y1 signalling. Using a recombinant overexpressing β-arrestin/P2Y12 system, transient transfection of ChemR23 or mock indicated that RvE1 counter-regulatory actions of P2Y12 signals were ChemR23 dependent [72].
Figure 3. RvE1 has protective actions on human platelets.
Aspirin acetylated COX-2 in vascular endothelial cells contributes to the formation of RvE1 that stereoselectively generates 18R-hydroperoxy-EPE [18R-H(p)EPE]. 18R-HEPE is further converted via sequential actions of leucocyte 5-LOX, leading to formation of RvE1. RvE1 acts directly on human platelets to reduce ADP-stimulated platelet aggregation, TX generation, P-selectin mobilization and actin polymerization in a calcium-independent manner. RvE1 counter-regulation of ADP activation is ChemR23 dependent.
In addition to platelets, VMSCs (vascular smooth muscle cells) also regulate the maintenance of vascular homoeostasis and when aberrantly activated via inflammatory mediators such as hsCRP (high-sensitivity C-reactive protein) can lead to cardiovascular complications such as PAD (peripheral arterial disease) [77]. Exogenous application of human CRP to VSMCs derived from human saphenous vein increased PDGF (platelet-derived growth factor)-stimulated chemotaxis in a PDGFRβ (PDGF receptor β)-dependent fashion [78]. The migration of VSMCs is a characteristic feature of atherosclerotic lesion formation and neointima formation following arterial injury and vein bypass grafting. In a recent investigation, RvE1 decreased PDGF-stimulated VSMC chemotaxis as well as PDGFR activation [79]. Of note, ChemR23 is expressed on the VSMCs which may account for its actions. Hence RvE1's selective actions on leucocytes in whole blood, platelets and VSMCs all point to its potential protective role in the cardiovascular arena.
Failed resolution programmes? Implications for cardiovascular diseases
Cardiovascular diseases are the number one cause of death in the Western world [80]. Atherosclerosis, as an example, is widely viewed as a chronic inflammatory disease characterized by the excessive recruitment and activation of peripheral blood mononuclear cells, such as monocytes and T-cells [81]. Monocytes differentiate into macrophages within the plaque milieu and attempt to clear excess oxidized lipoproteins and cholesterol from the tissue. This precipitates the generation of lipid-laden foam cells that fail to clear from the plaque, continue to secrete pro-inflammatory mediators and eventually undergo post-apoptotic secondary necrosis [82]. Emerging evidence highlights that atherosclerosis could be viewed as failed resolution of inflammation [82].
Given the central role of macrophage efferocytosis in resolution, new evidence suggests that defective clearance of plaque macrophages may underlie the progression of advanced atherosclerotic lesions, characterized by macrophage necrosis [83]. Peritoneal macrophages isolated from obese-diabetic mice crossed with [LDLR LDL (low-density lipoprotein)-receptor] deficient mice (LDLR−/−) display defects in their ability to phagocytose apoptotic cells [83]. Moreover, defective phagocytosis and clearance of apoptotic cells was observed in advanced atherosclerotic lesions from these mice. Interestingly, saturated fatty acids (e.g. palmitic and stearic) are increased in obese mice relative to endogenous ω−3 fatty acids and were implicated in defective macrophage efferocytosis. Accordingly, supplementation of ω−3 fatty acids EPA and DHA reversed deficits in macrophage efferocytosis in obese/LDLR−/− mice [83]. These findings underscore that progression of chronic inflammatory diseases could result in part because of impaired resolution and implicates a protective role for endogenous lipid mediator pathways.
Interestingly, mice lacking both 12/15-LOX (lipoxygenase) and apoE (alipoprotein E) display exacerbated atherosclerotic lesion formation compared with apoE-null mice [84]. Targeted macrophage-specific overexpression of 12/15-LOX protected from lesion development. Importantly, 12/15-LOX gene dosage correlated with LXA4 formation in isolated macrophages, as well as the production of 17-hydroxyDHA, a marker of the D-series resolvin biosynthetic pathway. Both lipoxins and resolvins display potent actions on isolated macrophages and endothelial cells, regulating production of pro-inflammatory cytokines/chemokines and adhesion receptors [VCAM (vascular cell adhesion molecule)-1 and P-selectin] [84]. Notably, LXA4 and RvD1 each enhance macrophage phagocytosis of apoptotic cells. These results corroborate earlier findings in rabbits demonstrating the atheroprotective effect of macrophage-specific transgenic overexpression of 15-LOX [85]. The anti-inflammatory proresolving role of this pathway was independently confirmed in murine models of arthritis and ocular injury [86–88]. Hence the enzymes involved in the active biosynthesis of SPMs are critical for homoeostasis. Further investigation of direct SPM actions in cardiovascular diseases, such as atherosclerosis where macrophages play a crucial role [82] would be of interest.
RESOLVIN E1 SIGNALLING IN MACROPHAGES
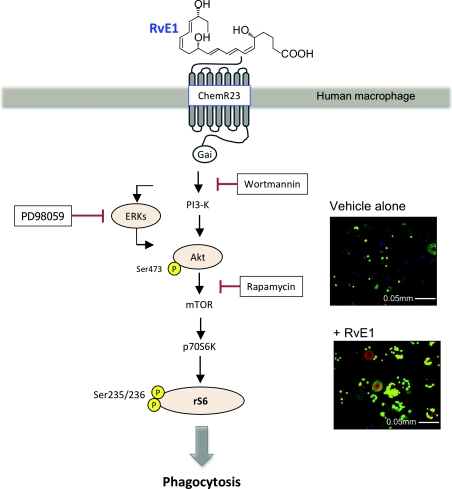
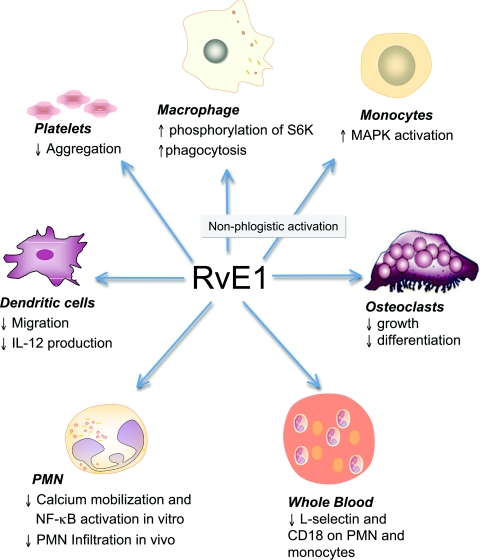
Macrophages have several phenotypes and their actions can either orchestrate inflammation or mediate resolution. Non-phlogistic (i.e. non-fever-causing) recruitment of monocytes [89] and phagocytosis by macrophages is the hallmark of tissue resolution. Hence it was of interest to further examine RvE1/ChemR23 signalling in macrophages. Figure 4 illustrates that RvE1-exposed human macrophages activate a pathway recently identified which exhibits intense rS6 (ribosomal S6 protein) phosphorylation [22]. RvE1-enhanced rS6 phosphorylation was reduced with wortmannin [an inhibitor of PI3K (phosphoinositide 3-kinase)], rapamycin [a mTOR (mammalian target of rapamycin) inhibitor] and PD98059 [an ERK (extracellular-signal-regulated kinase) inhibitor]. In contrast, SB203580 [a p38 MAPK (mitogen-activated protein kinase) inhibitor] and Bim [a PKC (protein kinase C) inhibitor] did not dramatically reduce the phosphorylation of rS6 [22].
Figure 4. Hypothetical scheme for RvE1/ChemR23-dependent signalling in human macrophages.
The scheme outlines the key phosphorylation-signalling components with RvE1 and the points of inhibitor action in this system. Inset, representative immunofluorescence of human macrophages phagocytosis of opsonized FITC-zymosan (see [22] for details).
rS6, a part of the small ribosomal subunit involved in translation, is known as a phospho-protein stimulated with growth factor or hormones [90]. It is also known that rS6 is a major substrate of p70S6K, a kinase downstream of PI3K (phosphoinositide 3-kinase)/Akt as well as Raf/ERK signal transductions. When rS6 is phosphorylated by p70S6K, the cells are undergoing growth [90]. There is the same motif, in which phospho-Akt (S) antibody recognized phosphorylation, from rS6 phosphorylated sites (Ser235 and Ser236) [91].
To investigate RvE1/ChemR23-dependent rS6 phosphorylation, an anti-ChemR23 antibody (a competitor for receptor binding), PD98059 and rapamycin were investigated and each significantly reduced rS6 phosphorylation to the baseline value. In contrast, isotype control IgG3κ, used as a negative control for the anti-ChemR23 antibody, did not modulate the phosphorylation. In addition to enhanced phagocytosis by murine macrophages in vivo [12] and in vitro [92], RvE1 also enhanced phagocytosis in human macrophages (Figure 4). Again, the anti-ChemR23 antibody, the inhibitors PD98059 and rapamycin each significantly reduced phagocytizing cells to baseline. This reduction was not observed with the IgG3κ antibody, which was used as a control for the anti-ChemR23 antibody. These phosphorylation-signalling pathways identified for RvE1 receptor–ligand interactions underscore the importance of endogenous proresolving agonists in resolving acute inflammation. Together, RvE1 acts on selective cellular targets for the maintenance of homoeostasis (Figure 5).
Figure 5. RvE1's selective cellular targets and actions.
Scheme of RvE1's actions on specific cells.
SPM RECEPTOR-MEDIATED CIRCUITS
Mapping temporal changes of miRNAs (microRNAs) during inflammation and resolution in vivo
The resolution of acute inflammation is a highly regulated process controlled by soluble (e.g. cytokines, chemokines and lipid autacoids) as well as cell-associated receptors and growth factors [14,93,94]. An emerging line of investigation indicates that many processes, at cellular, organ and systems level, are finely tuned by miRNAs [95]. miRNAs are small (22–24 nt) non-coding RNA sequences that act primarily as translational repressors of gene transcripts by interacting with their 3′ UTR (untranslated regions) [96], reviewed in [97]. miRNAs are involved in many physiological and pathological processes including several cancers [95] and in the immune response [98].
Previously, we sought evidence for miRNA involved in the resolution of zymosan-stimulated inflammation. RvD1 and other SPMs regulate cytokine production [84], NF-κB (nuclear factor κB) and pro-inflammatory eicosanoids (e.g. certain prostaglandins and leukotrienes) [24], and miRNAs are involved in regulating these molecules [98]. In order to investigate miRNAs involved in self-limited inflammation and resolution, zymosan A-stimulated self-limited peritonitis was carried out. Resolution indices [3] were calculated in order to obtain the peak of inflammation (defined by the peak in PMN numbers) compared with the time point in which the mice were in the resolving phase (when PMN numbers reach half maximum). Characterizing the resolution phase allows for the investigation of temporal changes in miRNA expression in exudates collected 4, 12 and 24 h post zymosan challenge, which correspond with the onset, maximum and resolution phases respectively, was monitored.
Among the few miRNAs highly expressed in the exudates at 12 h, miR-21, miR-146b, miR-208 and miR-203 displayed >2.5-fold change when compared with 4 h [99]. Conversely, a large group of miRNAs showed lower expression between 12 and 4 h time points such as miR-142-5p and miR-3p, miR-219 and miR-302d, indicating temporal changes in miRNA regulation during inflammation and resolution. Hence miRNAs are differentially regulated during inflammation and resolution. The next question is whether the SPM RvD1 further modulates these miRNAs during self-limited inflammation and resolution.
RvD1-regulated miRNAs: a temporal regulation in vivo
RvD1 is biosynthesized during resolution [14], regulates further PMN infiltration and stimulates non-phlogistic macrophage phagocytic activity [24,27,84,100]. Resolution indices were used here to define and pinpoint RvD1 actions in resolution. Administration of RvD1 resulted in a significant decrease in leucocyte infiltration at 12 h, consistent with RvD1-defined actions [14,100]. miRNA array analysis showed RvD1 increased levels of miR-146b, miR-142-3p, miR-5p and miR-219 in exudate cells at the peak of PMN infiltration. RvD1 slightly down-regulated miR-21, miR-203, miR-208 and miR-302d at this time point. Interestingly RvD1-treated mice had a significant reduction in expression levels of all miRNAs analysed at 48 h, with the exception of miR-302d that was not regulated in RvD1-treated mice at this time point. These findings suggest that RvD1 temporally controls specific sets of miRNAs in exudates in vivo.
RvD1 regulation of miRNAs, genes and transcription factors in human macrophages and monocytes
Human macrophages were investigated because they are key players in innate immunity and resolution. Regulatory actions of RvD1 using human macrophages overexpressing the specific RvD1 receptors ALX/FPR2 and GPR32 [24] demonstrated that RvD1 down-regulated miR-21, miR-146b and miR-219, whereas it increased levels of miR-208 and miR-302d. These results indicate that RvD1 regulates specific miRNA molecules via its interactions with specific RvD1 receptors.
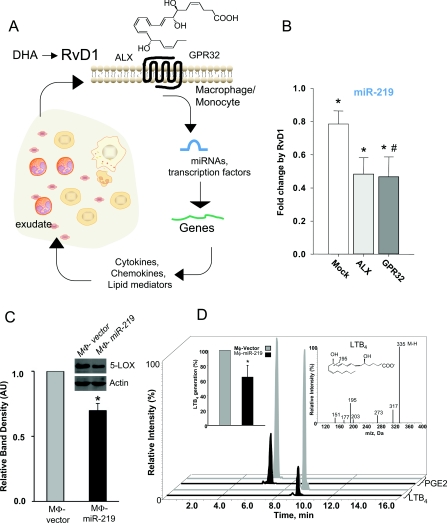
The target genes regulated by miR-146b, miR-208 and miR-219 were assessed. Overexpression of miR-146b in human macrophages resulted in significant down-regulation of transcripts of several cytokines, chemokines and their receptors, such as IL-8, IL-10, IL-12 receptor β2, CCR3 (chemokine CC motif receptor 3), IFN (interferon) α1 and β1, and members of the IL-1 family to name a few. Also, genes involved in the innate immune response and pathogen recognition, i.e. S100A12, LBP (lipopolysaccharide binding protein), CRP, C8a (complement component 8 α polypeptide), TLR (Toll-like receptor) 9, TLR10, PGLYRP (peptidoglycan recognition protein) 1 and PGLYRP2, were significantly reduced in macrophage miR-146b. Additionally, there were reductions in the mRNAs for CHUK (conserved helix–loop–helix ubiquitous kinase) also known as IKKα [IκB (inhibitory κB) kinase α], TRAF6 [TNF (tumour necrosis factor)-receptor-associated factor 6], nitric oxide synthase 2, PTAFR (platelet-activating factor receptor) and CD40. In miR-208a-overexpressing macrophages, reductions were obtained for mRNAs encoding for CD14, CD40L (CD40 ligand), PTGIR (prostaglandin I2 receptor), TBXA2R (TXA2 receptor) and PDCD4 (programmed cell death 4), a tumour suppressor molecule that regulates NF-κB activation and decreases IL-10 production [101]. Of note, miR-219 overexpression gave significant reduction in CD14, TNF receptor II, phospholipase Cγ2 and AA 5-LOX. Further investigation of miR-219 overexpression yielded decreased 5-LOX protein and leukotriene production (Figure 6).
Figure 6. Resolution circuitry.
(A) Scheme of RvD1 miRNA circuit. RvD1 is generated within inflammatory exudates and acts directly on selective cell types such as monocytes/macrophages. RvD1 actions are via its receptors, ALX or GPR32 to regulate miRNAs, transcription factors, gene expression and cellular function. (B) RvD1 down-regulates miR-219 via its receptors ALX and GPR32, which leads to less 5-LOX protein (C) and reduced LTB4 generation (D). Adapted from [99]. *P<0.05 compared against vehicle; #P<0.05 compared against mock.
Since many of the target genes of RvD1-regulated miRNA networks were involved in TFs (transcription factor) regulation, a panel of TFs with human monocytes to gain insight into potential translation was screened. Of interest, RvD1 significantly reduced nuclear translocation of NF-κB and Smad compared with vehicle. RvD1 reduced TNF-α induced phosphorylation of IκB, a critical step in NF-κB activation and nuclear translocation. Therefore these results suggest that RvD1 regulates the NF-κB pathway in human monocytes.
A miRNA signature of resolution within resolving self-limited inflammatory exudates was mapped. The proresolving mediator RvD1 regulated resolution indices and controlled specific miRNA expression in exudates in vivo that were also activated via recombinant receptors ALX/FPR2 and GPR32 regulating miR-146b, miR-208a and miR-219 (ex vivo). These results establish a novel RvD1-G-protein-coupled receptor-dependent miRNA axis in resolution circuits (Figure 6).
CONCLUDING REMARKS AND FUTURE DIRECTIONS
The formation of endogenous autacoids derived from PUFAs and specifically dietary ω−3 fatty acids may explain in part the well-known essential roles of the ω−3 fish oils in human health and disease [102]. ω−3-derived SPMs temporally regulate the resolution of inflammation via systematic changes in specific cell types, their activity and counter-regulation of pro-inflammatory signals upon perturbation or challenge of the organ and/or tissues. SPMs are endogenous lipid mediators that are enzymatically biosynthesized and act on selective cellular targets via specific 7-TMs for bioaction. Uncovering these novel SPMs emboldened the notion that agonists can quiesce the propagation of inflammation while stimulating resolution. SPMs were the first mediators demonstrated to actively promote resolution, but since their discovery many laboratories have found that other mediators have proresolving actions including peptides [26]. As reviewed herein, SPMs have specific cellular targets of shorter duration in the peripheral blood such as PMNs and platelets, where the SPMs evoke their actions within seconds of their formation, as well as receptor–ligand interactions that stimulate changes in specific miRNA of longer duration (i.e. hours) to return tissues to homoeostasis. Proresolving agonists open a new terrain for identifying endogenous biochemical pathways that exploit natural mechanisms operating in vivo to terminate the inflammatory response and tissue injury.
ACKNOWLEDGEMENT
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental and Craniofacial Research, the National Institute of General Medical Sciences, or the National Institutes of Health.
FUNDING
The work of the authors is supported by the National Institutes of Health [grant numbers R01DE019938 and R01GM038765].
References
- 1.Kumar V., Abbas A. K., Fausto N., Robbins S. L., Cotran R. S. Robbins and Cotran Pathologic Basis of Disease. Philadelphia: Elsevier/Saunders; 2005. [Google Scholar]
- 2.de Pablo P., Chapple I. L., Buckley C. D., Dietrich T. Periodontitis in systemic rheumatic diseases. Nat. Rev. Rheumatol. 2009;5:218–224. doi: 10.1038/nrrheum.2009.28. [DOI] [PubMed] [Google Scholar]
- 3.Bannenberg G. L., Chiang N., Ariel A., Arita M., Tjonahen E., Gotlinger K. H., Hong S., Serhan C. N. Molecular circuits of resolution: formation and actions of resolvins and protectins. J. Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 4.Marcus A. J. Platelets: their role in hemostasis, thrombosis, and inflammation. In: Gallin J. I., Snyderman R., editors. Inflammation: Basic Principles and Clinical Correlates. Philadelphia: Lippincott, Williams & Wilkins; 1999. pp. 77–95. [Google Scholar]
- 5.Willoughby D. A., Moore A. R., Colville-Nash P. R., Gilroy D. Resolution of inflammation. Int. J. Immunopharmacol. 2000;22:1131–1135. doi: 10.1016/s0192-0561(00)00064-3. [DOI] [PubMed] [Google Scholar]
- 6.Serhan C. N. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 7.Bannenberg G., Serhan C. N. Specialized pro-resolving lipid mediators in the inflammatory response: an update. Biochim. Biophys. Acta. 2010;1801:1260–1273. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spite M., Serhan C. N. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ. Res. 2010;107:1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh S. F., Pillai P. S., Recchiuti A., Yang R., Serhan C. N. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Invest. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan C. N. Special issue on lipoxins and aspirin-triggered lipoxins. Prostaglandins Leukotrienes Essent. Fatty Acids. 2005;73:139–321. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Samuelsson B., Dahlen S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 12.Schwab J. M., Chiang N., Arita M., Serhan C. N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan C. N., Clish C. B., Brannon J., Colgan S. P., Chiang N., Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serhan C. N., Hong S., Gronert K., Colgan S. P., Devchand P. R., Mirick G., Moussignac R. L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong S., Gronert K., Devchand P. R., Moussignac R. L., Serhan C. N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 16.Serhan C. N., Gotlinger K., Hong S., Lu Y., Siegelman J., Baer T., Yang R., Colgan S. P., Petasis N. A. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J. Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 17.Arita M., Bianchini F., Aliberti J., Sher A., Chiang N., Hong S., Yang R., Petasis N. A., Serhan C. N. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serhan C. N., Chiang N., Van Dyke T. E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell E. L., Louis N. A., Tomassetti S. E., Canny G. O., Arita M., Serhan C. N., Colgan S. P. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 20.Ariel A., Fredman G., Sun Y. P., Kantarci A., Van Dyke T. E., Luster A. D., Serhan C. N. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat. Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arita M., Ohira T., Sun Y. P., Elangovan S., Chiang N., Serhan C. N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 22.Ohira T., Arita M., Omori K., Recchiuti A., Van Dyke T. E., Serhan C. N. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J. Biol. Chem. 2009;285:3451–3461. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starosta V., Pazdrak K., Boldogh I., Svider T., Kurosky A. Lipoxin A4 counterregulates GM-CSF signaling in eosinophilic granulocytes. J. Immunol. 2008;181:8688–8699. doi: 10.4049/jimmunol.181.12.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnamoorthy S., Recchiuti A., Chiang N., Yacoubian S., Lee C.-H., Yang R., Petasis N. A., Serhan C. N. Resolvin D1 binds human phagocytes with evidence for pro-resolving receptors. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riviere S., Challet L., Fluegge D., Spehr M., Rodriguez I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature. 2009;459:574–577. doi: 10.1038/nature08029. [DOI] [PubMed] [Google Scholar]
- 26.Perretti M., D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 27.Kasuga K., Yang R., Porter T. F., Agrawal N., Petasis N. A., Irimia D., Toner M., Serhan C. N. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J. Immunol. 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varani J., Ward P. A. Mechanisms of endothelial cell injury in acute inflammation. Shock. 1994;2:311–319. doi: 10.1097/00024382-199411000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Dallegri F., Ottonello L. Tissue injury in neutrophilic inflammation. Inflammation Res. 1997;46:382–391. doi: 10.1007/s000110050208. [DOI] [PubMed] [Google Scholar]
- 30.Weiss S. J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 31.Han J., Ulevitch R. J. Limiting inflammatory responses during activation of innate immunity. Nat. Immunol. 2005;6:1198–1205. doi: 10.1038/ni1274. [DOI] [PubMed] [Google Scholar]
- 32.Larsson S. C., Kumlin M., Ingelman-Sundberg M., Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am. J. Clin. Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 33.Lukiw W. J., Bazan N. G. Docosahexaenoic acid and the aging brain. J. Nutr. 2008;138:2510–2514. doi: 10.3945/jn.108.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 35.Albert C. M., Hennekens C. H., O'Donnell C. J., Ajani U. A., Carey V. J., Willett W. C., Ruskin J. N., Manson J. E. Fish consumption and risk of sudden cardiac death. JAMA, J. Am. Med. Assoc. 1998;279:23–28. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- 36.Albert C. M., Campos H., Stampfer M. J., Ridker P. M., Manson J. E., Willett W. C., Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N. Engl. J. Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 37.Harris W. S., Von Schacky C. The omega-3 index: a new risk factor for death from coronary heart disease? Prev. Med. 2004;39:212–220. doi: 10.1016/j.ypmed.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 38.Leon H., Shibata M. C., Sivakumaran S., Dorgan M., Chatterley T., Tsuyuki R. T. Effect of fish oil on arrhythmias and mortality: systematic review. BMJ. 2008;337:a2931. doi: 10.1136/bmj.a2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bang H. O., Dyerberg J., Nielsen A. B. Plasma lipid and lipoprotein pattern in Greenlandic West-coast Eskimos. Lancet. 1971;1:1143–1145. doi: 10.1016/s0140-6736(71)91658-8. [DOI] [PubMed] [Google Scholar]
- 40.Bang H. O., Dyerberg J., Hjoorne N. The composition of food consumed by Greenland Eskimos. Acta Med. Scand. 1976;200:69–73. doi: 10.1111/j.0954-6820.1976.tb08198.x. [DOI] [PubMed] [Google Scholar]
- 41.Dyerberg J., Bang H. O. Lipid metabolism, atherogenesis, and haemostasis in Eskimos: the role of the prostaglandin-3 family. Haemostasis. 1979;8:227–233. doi: 10.1159/000214314. [DOI] [PubMed] [Google Scholar]
- 42.Bang H. O., Dyerberg J., Sinclair H. M. The composition of the Eskimo food in north western Greenland. Am. J. Clin. Nutr. 1980;33:2657–2661. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 43.Dyerberg J., Bang H. O. Haemostatic function and platelet polyunsaturated fatty acids in Eskimos. Lancet. 1979;2:433–435. doi: 10.1016/s0140-6736(79)91490-9. [DOI] [PubMed] [Google Scholar]
- 44.Dyerberg J., Bang H. O., Stoffersen E., Moncada S., Vane J. R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978;2:117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- 45.Needleman P., Moncada S., Bunting S., Vane J. R., Hamberg M., Samuelsson B. Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature. 1976;261:558–560. doi: 10.1038/261558a0. [DOI] [PubMed] [Google Scholar]
- 46.Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc. Natl. Acad. Sci. U.S.A. 1975;72:2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simopoulos A. P. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- 48.Needleman P., Raz A., Minkes M. S., Ferrendelli J. A., Sprecher H. Triene prostaglandins: prostacyclin and thromboxane biosynthesis and unique biological properties. Proc. Natl. Acad. Sci. U.S.A. 1979;76:944–948. doi: 10.1073/pnas.76.2.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Needleman P., Wyche A., LeDuc L., Sankarappe S. K., Jakschik B. A., Sprecher H. Fatty acids as sources of potential ‘magic bullets’ for the modification of platelet and vascular function. Prog. Lipid Res. 1981;20:415–422. doi: 10.1016/0163-7827(81)90073-4. [DOI] [PubMed] [Google Scholar]
- 50.Needleman P., Minkes M., Raz A. Thromboxanes: selective biosynthesis and distinct biological properties. Science. 1976;193:163–165. doi: 10.1126/science.945611. [DOI] [PubMed] [Google Scholar]
- 51.Tavazzi L., Maggioni A. P., Marchioli R., Barlera S., Franzosi M. G., Latini R., Lucci D., Nicolosi G. L., Porcu M., Tognoni G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomized, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 52.Marchioli R., Barzi F., Bomba E., Chieffo C., Di Gregorio D., Di Mascio R., Franzosi M. G., Geraci E., Levantesi G., Maggioni A. P., et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 53.Vane J. R. The mode of action of aspirin and similar compounds. J. Allergy Clin. Immunol. 1976;58:691–712. doi: 10.1016/0091-6749(76)90181-0. [DOI] [PubMed] [Google Scholar]
- 54.Vane J. R., Botting R. M. The mechanism of action of aspirin. Thromb. Res. 2003;110:255–258. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 55.Gilroy D. W., Colville-Nash P. R., Willis D., Chivers J., Paul-Clark M. J., Willoughby D. A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 56.Chiang N., Bermudez E. A., Ridker P. M., Hurwitz S., Serhan C. N. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiang N., Hurwitz S., Ridker P. M., Serhan C. N. Aspirin has a gender-dependent impact on antiinflammatory 15-epi-lipoxin A4 formation: a randomized human trial. Arterioscler. Thromb. Vasc. Biol. 2006;26:e14–e17. doi: 10.1161/01.ATV.0000196729.98651.bf. [DOI] [PubMed] [Google Scholar]
- 58.Morris T., Stables M., Hobbs A., de Souza P., Colville-Nash P., Warner T., Newson J., Bellingan G., Gilroy D. W. Effects of low-dose aspirin on acute inflammatory responses in humans. J. Immunol. 2009;183:2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 59.Morris T., Stables M., Colville-Nash P., Newson J., Bellingan G., de Souza P. M., Gilroy D. W. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc. Natl. Acad. Sci. U.S.A. 2010;107:8842–8847. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serhan C. N., Clish C. B., Brannon J., Colgan S. P., Gronert K., Chiang N. Anti-microinflammatory lipid signals generated from dietary N-3 fatty acids via cyclooxygenase-2 and transcellular processing: a novel mechanism for NSAID and N-3 PUFA therapeutic actions. J. Physiol. Pharmacol. 2000;51:643–654. [PubMed] [Google Scholar]
- 61.Haas-Stapleton E. H., Lu Y., Hong S., Arita M., Favoreto S., Nigam S., Serhan C. N., Agabian N. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator Resolvin E1. PLoS ONE. 2007;2:e1316. doi: 10.1371/journal.pone.0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hasturk H., Kantarci A., Ohira T., Arita M., Ebrahimi N., Chiang N., Petasis N. A., Levy B. D., Serhan C. N., Van Dyke T. E. RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 63.Hasturk H., Kantarci A., Goguet-Surmenian E., Blackwood A., Andry C., Serhan C. N., Van Dyke T. E. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J. Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 64.Arita M., Yoshida M., Hong S., Tjonahen E., Glickman J. N., Petasis N. A., Blumberg R. S., Serhan C. N. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li N., He J., Schwartz C. E., Gjorstrup P., Bazan H. E. Resolvin e1 improves tear production and decreases inflammation in a dry eye mouse model. J. Ocul. Pharmacol. Ther. 2010;26:431–439. doi: 10.1089/jop.2010.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dona M., Fredman G., Schwab J. M., Chiang N., Arita M., Goodarzi A., Cheng G., von Andrian U. H., Serhan C. N. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marcus A. J. Stratton lecture 1989. Thrombosis and inflammation as multicellular processes: pathophysiologic significance of transcellular metabolism. Blood. 1990;76:1903–1907. [PubMed] [Google Scholar]
- 68.Gawaz M., Langer H., May A. E. Platelets in inflammation and atherogenesis. J. Clin. Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knapp H. R., Reilly I. A. G., Alessandrini P., FitzGerald G. A. In vivo indexes of platelet and vascular function during fish-oil administration in patients with atherosclerosis. N. Engl. J. Med. 1986;314:937–942. doi: 10.1056/NEJM198604103141501. [DOI] [PubMed] [Google Scholar]
- 70.Chen P., Fenet B., Michaud S., Tomczyk N., Vericel E., Lagarde M., Guichardant M. Full characterization of PDX, a neuroprotectin/protectin D1 isomer, which inhibits blood platelet aggregation. FEBS Lett. 2009;583:3478–3484. doi: 10.1016/j.febslet.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Chen P., Vericel E., Lagarde M., Guichardant M. Poxytrins, a class of oxygenated products from polyunsaturated fatty acids, potently inhibit blood platelet aggregation. FASEB J. 2011;25:382–388. doi: 10.1096/fj.10-161836. [DOI] [PubMed] [Google Scholar]
- 72.Fredman G., Van Dyke T. E., Serhan C. N. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler. Thromb. Vasc. Biol. 2010;30:2005–2013. doi: 10.1161/ATVBAHA.110.209908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michelson A. D. Platelets. Amsterdam: Academic Press/Elsevier; 2007. [Google Scholar]
- 74.Gachet C. P2 receptors, platelet function and pharmacological implications. Thromb. Haemost. 2008;99:466–472. doi: 10.1160/TH07-11-0673. [DOI] [PubMed] [Google Scholar]
- 75.Bhatt D. L. Platelets in cardiovascular disease. London: Imperial College Press; 2008. [Google Scholar]
- 76.Michelson A. D. Antiplatelet therapies for the treatment of cardiovascular disease. Nat. Rev. Drug Discovery. 2010;9:154–169. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- 77.Ridker P. M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 78.Ho K. J., Owens C. D., Longo T., Sui X. X., Ifantides C., Conte M. S. C-reactive protein and vein graft disease: evidence for a direct effect on smooth muscle cell phenotype via modulation of PDGF receptor-beta. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1132–H1140. doi: 10.1152/ajpheart.00079.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ho K. J., Spite M., Owens C. D., Lancero H., Kroemer A. H., Pande R., Creager M. A., Serhan C. N., Conte M. S. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am. J. Pathol. 2010;177:2116–2123. doi: 10.2353/ajpath.2010.091082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Braunwald E. Shattuck lecture: cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N. Engl. J. Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 81.Hansson G. K., Libby P. The immune response in atherosclerosis: a double-edged sword. Nat. Rev. Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 82.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li S., Sun Y., Liang C. P., Thorp E. B., Han S., Jehle A. W., Saraswathi V., Pridgen B., Kanter J. E., Li R., et al. Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ. Res. 2009;105:1072–1082. doi: 10.1161/CIRCRESAHA.109.199570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Merched A. J., Ko K., Gotlinger K. H., Serhan C. N., Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shen J., Herderick E., Cornhill J. F., Zsigmond E., Kim H. S., Kuhn H., Guevara N. V., Chan L. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J. Clin. Invest. 1996;98:2201–2208. doi: 10.1172/JCI119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gronert K., Maheshwari N., Khan N., Hassan I. R., Dunn M., Laniado Schwartzman M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J. Biol. Chem. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- 87.Kronke G., Katzenbeisser J., Uderhardt S., Zaiss M. M., Scholtysek C., Schabbauer G., Zarbock A., Koenders M. I., Axmann R., Zwerina J., et al. 12/15-lipoxygenase counteracts inflammation and tissue damage in arthritis. J. Immunol. 2009;183:3383–3389. doi: 10.4049/jimmunol.0900327. [DOI] [PubMed] [Google Scholar]
- 88.Leedom A. J., Sullivan A. B., Dong B., Lau D., Gronert K. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am. J. Pathol. 2010;176:74–84. doi: 10.2353/ajpath.2010.090678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maddox J. F., Serhan C. N. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J. Exp. Med. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alessi D. R., Caudwell F. B., Andjelkovic M., Hemmings B. A., Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 91.Ly C., Arechiga A. F., Melo J. V., Walsh C. M., Ong S. T. Bcr-Abl kinase modulates the translation regulators ribosomal protein S6 and 4E-BP1 in chronic myelogenous leukemia cells via the mammalian target of rapamycin. Cancer Res. 2003;63:5716–5722. [PubMed] [Google Scholar]
- 92.Hong S., Porter T. F., Lu Y., Oh S. F., Pillai P. S., Serhan C. N. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J. Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 93.Rossi A. G., Sawatzky D. A. The Resolution of Inflammation. Basel: Birkhäuser Verlag; 2008. [Google Scholar]
- 94.Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 95.Iorio M. V., Croce C. M. MicroRNAs in cancer: small molecules with a huge impact. J. Clin. Oncol. 2009;27:5848–5856. doi: 10.1200/JCO.2009.24.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee R. C., Feinbaum R. L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 97.Bartel D. P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sheedy F. J., O'Neill L. A. Adding fuel to fire: microRNAs as a new class of mediators of inflammation. Ann. Rheum. Dis. 2008;67:iii50–55. doi: 10.1136/ard.2008.100289. [DOI] [PubMed] [Google Scholar]
- 99.Recchiuti A., Krishnamoorthy S., Fredman G., Chiang N., Serhan C. N. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 2010;25:544–560. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sun Y. P., Oh S. F., Uddin J., Yang R., Gotlinger K., Campbell E., Colgan S. P., Petasis N. A., Serhan C. N. Resolvin D1 and its aspirin-triggered 17R epimerStereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J. Biol. Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 101.Sheedy F. J., Palsson-McDermott E., Hennessy E. J., Martin C., O'Leary J. J., Ruan Q., Johnson D. S., Chen Y., O'Neill L. A. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat. Immunol. 2009;11:141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 102.Burr G. O., Burr M. M. A new deficiency disease produced by the rigid exclusion of fat from the diet. J. Biol. Chem. 1929;82:345–367. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 103.Takano T., Clish C. B., Gronert K., Petasis N., Serhan C. N. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J. Clin. Invest. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clish C. B., O'Brien J. A., Gronert K., Stahl G. L., Petasis N. A., Serhan C. N. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8247–8252. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Serhan C. N., Jain A., Marleau S., Clish C., Kantarci A., Behbehani B., Colgan S. P., Stahl G. L., Merched A., Petasis N. A., et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J. Immunol. 2003;171:6856–6865. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 106.Gewirtz A. T., Neish A. S., Madara J. L. Mechanisms of active intestinal inflammation and potential down-regulation via lipoxins. Adv. Exp. Med. Biol. 2002;507:229–236. doi: 10.1007/978-1-4615-0193-0_35. [DOI] [PubMed] [Google Scholar]
- 107.Levy B. D., De Sanctis G. T., Devchand P. R., Kim E., Ackerman K., Schmidt B. A., Szczeklik W., Drazen J. M., Serhan C. N. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat. Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 108.Karp C. L., Flick L. M., Park K. W., Softic S., Greer T. M., Keledjian R., Yang R., Uddin J., Guggino W. B., Atabani S. F., et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat. Immunol. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 109.Chiang N., Gronert K., Clish C. B., O'Brien J. A., Freeman M. W., Serhan C. N. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J. Clin. Invest. 1999;104:309–316. doi: 10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scalia R., Gefen J., Petasis N. A., Serhan C. N., Lefer A. M. Lipoxin A4 stable analogs inhibit leukocyte rolling and adherence in the rat mesenteric microvasculature: role of P-selectin. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9967–9972. doi: 10.1073/pnas.94.18.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fierro I. M., Kutok J. L., Serhan C. N. Novel lipid mediator regulators of endothelial cell proliferation and migration: aspirin-triggered-15R-lipoxin A(4) and lipoxin A(4) J. Pharmacol. Exp. Ther. 2002;300:385–392. doi: 10.1124/jpet.300.2.385. [DOI] [PubMed] [Google Scholar]
- 112.Devchand P. R., Schmidt B. A., Primo V. C., Zhang Q. Y., Arnaout M. A., Serhan C. N., Nikolic B. A synthetic eicosanoid LX-mimetic unravels host–donor interactions in allogeneic BMT-induced GvHD to reveal an early protective role for host neutrophils. FASEB J. 2005;19:203–210. doi: 10.1096/fj.04-2565com. [DOI] [PubMed] [Google Scholar]
- 113.Papayianni A., Serhan C. N., Phillips M. L., Rennke H. G., Brady H. R. Transcellular biosynthesis of lipoxin A4 during adhesion of platelets and neutrophils in experimental immune complex glomerulonephritis. Kidney Int. 1995;47:1295–1302. doi: 10.1038/ki.1995.184. [DOI] [PubMed] [Google Scholar]
- 114.Svensson C. I., Zattoni M., Serhan C. N. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J. Exp. Med. 2007;204:245–252. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bandeira-Melo C., Serra M. F., Diaz B. L., Cordeiro R. S., Silva P. M., Lenzi H. L., Bakhle Y. S., Serhan C. N., Martins M. A. Cyclooxygenase-2-derived prostaglandin E2 and lipoxin A4 accelerate resolution of allergic edema in Angiostrongylus costaricensis-infected rats: relationship with concurrent eosinophilia. J. Immunol. 2000;164:1029–1036. doi: 10.4049/jimmunol.164.2.1029. [DOI] [PubMed] [Google Scholar]
- 116.Chen Y., Hao H., He S., Cai L., Li Y., Hu S., Ye D., Hoidal J., Wu P., Chen X. Lipoxin A4 and its analogue suppress the tumor growth of transplanted H22 in mice: the role of antiangiogenesis. Mol. Cancer Ther. 2010;9:2164–2174. doi: 10.1158/1535-7163.MCT-10-0173. [DOI] [PubMed] [Google Scholar]
- 117.Levy B. D., Zhang Q. Y., Bonnans C., Primo V., Reilly J. J., Perkins D. L., Liang Y., Amin Arnaout M., Nikolic B., Serhan C. N. The endogenous pro-resolving mediators lipoxin A4 and resolvin E1 preserve organ function in allograft rejection. Prostaglandins Leukotrienes Essent. Fatty Acids. 2011;84:43–50. doi: 10.1016/j.plefa.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Conte F. P., Menezes-de-Lima O., Jr, Verri W. A., Jr, Cunha F. Q., Penido C., Henriques M. G. Lipoxin A(4) attenuates zymosan-induced arthritis by modulating endothelin-1 and its effects. Br. J. Pharmacol. 2010;161:911–924. doi: 10.1111/j.1476-5381.2010.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou M., Chen B., Sun H., Deng Z., Andersson R., Zhang Q. The protective effects of Lipoxin A(4) during the early phase of severe acute pancreatitis in ratsScand. J. Gastroenterol. 2011;46:211–219. doi: 10.3109/00365521.2010.525715. [DOI] [PubMed] [Google Scholar]
- 120.Connor K. M., SanGiovanni J. P., Lofqvist C., Aderman C. M., Chen J., Higuchi A., Hong S., Pravda E. A., Majchrzak S., Carper D., et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ishida T., Yoshida M., Arita M., Nishitani Y., Nishiumi S., Masuda A., Mizuno S., Takagawa T., Morita Y., Kutsumi, et al. Resolvin E1, an endogenous lipid derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflammatory Bowel Dis. 2010;16:87–95. doi: 10.1002/ibd.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Haworth O., Cernadas M., Yang R., Serhan C. N., Levy B. D. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat. Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aoki H., Hisada T., Ishizuka T., Utsugi M., Ono A., Koga Y., Sunaga N., Nakakura T., Okajima F., Dobashi K., Mori M. Protective effect of resolvin E1 on the development of asthmatic airway inflammation. Biochem. Biophys. Res. Commun. 2010;400:128–133. doi: 10.1016/j.bbrc.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 124.Aoki H., Hisada T., Ishizuka T., Utsugi M., Kawata T., Shimizu Y., Okajima F., Dobashi K., Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem. Biophys. Res. Commun. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 125.González-Périz A., Horrillo R., Ferré N., Gronert K., Dong B., Morán-Salvador E., Titos E., Martínez-Clemente M., López-Parra M., Arroyo V., Clària J. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu Z.-Z., Zhang L., Liu T., Park J.-Y., Berta T., Yang R., Serhan C. N., Ji R.-R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Keyes K. T., Ye Y., Lin Y., Zhang C., Perez-Polo J. R., Gjorstrup P., Birnbaum Y. Resolvin E1 protects the rat heart against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H153–H164. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- 128.Rajasagi N. K., Reddy P. B., Suryawanshi A., Mulik S., Gjorstrup P., Rouse B. T. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin e1. J. Immunol. 2011;186:1735–1746. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Duffield J. S., Hong S., Vaidya V. S., Lu Y., Fredman G., Serhan C. N., Bonventre J. V. Resolvin D series and protectin D1 mitigate acute kidney injury. J. Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 130.Huang L., Wang C.-F., Serhan C. N., Strichartz G. Enduring prevention and transient reduction of post-operative pain by intrathecal resolvin D1. Pain. 2011;152:557–565. doi: 10.1016/j.pain.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ariel A., Li P. L., Wang W., Tang W. X., Fredman G., Hong S., Gotlinger K. H., Serhan C. N. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J. Biol. Chem. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 132.Levy B. D., Kohli P., Gotlinger K., Haworth O., Hong S., Kazani S., Israel E., Haley K. J., Serhan C. N. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J. Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Marcheselli V. L., Hong S., Lukiw W. J., Tian X. H., Gronert K., Musto A., Hardy M., Gimenez J. M., Chiang N., Serhan C. N., Bazan N. G. Novel docosanoids inhibit brain ischemia–reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 134.Zhao Y., Calon F., Julien C., Winkler J. W., Petasis N. A., Lukiw W. J., Bazan N. G. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase and PPARgamma-mediated mechanisms in Alzheimer's disease models. PLoS ONE. 2011;6:e15816. doi: 10.1371/journal.pone.0015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Spite M., Norling L. V., Summers L., Yang R., Cooper D., Petasis N. A., Flower R. J., Perretti M., Serhan C. N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Serhan C. N., Yang R., Martinod K., Kasuga K., Pillai P. S., Porter T. F., Oh S. F., Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]