Abstract
The heterogeneous expression of voltage-gated channels in dendrites suggests that neurons perform local microdomain computations at different regions. It has been shown that A-type K+ channels have a non-uniform distribution along the primary apical dendrite in CA1 pyramidal neurons, increasing with distance from the soma. Kv4.2 channels, which are responsible for the somatodendritic A-type K+ current in CA1 pyramidal neurons, shape local synaptic input and regulate the back-propagation of APs into dendrites. Experiments were performed to test the hypothesis that Kv4.2 channels are differentially trafficked at different regions along the apical dendrite during basal activity and upon stimulation in CA1 neurons. Proximal (50–150 µm from the soma, primary and oblique) and distal (>200 µm) apical dendrites were selected. The fluorescence recovery after photobleaching (FRAP) technique was used to measure basal cycling rates of EGFP-tagged Kv4.2 (Kv4.2g). We found that the cycling rate of Kv4.2 channels was one order of magnitude slower at both primary and oblique dendrites between 50–150 µm from the soma. Kv4.2 channel cycling increased significantly at 200–250 µm from the soma. Expression of a Kv4.2 mutant lacking a phosphorylation site for protein kinase-A (Kv4.2gS552A) abolished this distance-dependent change in channel cycling; demonstrating that phosphorylation by PKA underlies the increased mobility in distal dendrites. Neuronal stimulation by α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) treatment increased cycling of Kv4.2 channels significantly at distal sites only. This activity-dependent increase in Kv4.2 cycling at distal dendrites was blocked by expression of Kv4.2gS552A. These results indicate that distance-dependent Kv4.2 mobility is regulated by activity-dependent phosphorylation of Kv4.2 by PKA.
Keywords: Potassium channel, trafficking, PKA, FRAP, photobleach
Introduction
Rapidly inactivating, A-type K+ currents are responsible for the modulation of many physiological processes, including synaptic plasticity, synaptic integration and shaping dendritic excitability (Hoffman et al., 1997; Kim et al., 2007; Schoppa et al., 1999 ; Watanabe et al., 2002). These currents are activated at a subthreshold range of voltages and determine action potential (AP) onset time, threshold and interspike intervals, as well as determining the time of synaptic inputs, affecting input resistance, and AP repolarization (Chen et al., 2006; Kim et al., 2005; Lopez-Barneo et al., 1988; Malin et al., 2001; Schoppa et al.. 1999). The Kv4.2 channel, which underlies transient A-type K+ current is expressed in multiple brain regions including the frontal cortex, cerebellum and hippocampus (Serodio et al., 1998). Kv4.2 channels are comprised of α-subunits each containing six transmembrane α-helical domains, with both the N-and C-termini being intracellular domains. The insertion of Kv4.2 channels into the plasma membrane is enhanced by the presence of two families of accessory proteins, KChIPs (Ca2+-binding Kv channel interacting proteins) and DPPLs (dipeptidyl-peptidase-like-proteins) (Jerng et al., 2004; Jerng et al., 2005; Nadal et al., 2003; Shibata et al., 2003). KChIPs bind to the N-terminus and DPP6 binds extracellularly near the first transmembrane domain (Ren et al., 2005; Zhou et al., 2004).
In the hippocampus, Kv4.2 channels have an uneven distribution in the somatodendritic compartment of CA1 pyramidal neurons, with highest expression occurring at the distal dendrites (Hoffman et al., 1997; Chen et al., 2006). This specialized distribution of channels combined with activity-dependent control of their membrane expression (Jung et al., 2009; Kim et al., 2007) makes them excellent candidates for controlling synaptic integration and plasticity. Indeed, Kv4.2 channel down-regulation increases the probability of coincident EPSPs and dendritic, back-propagating action potentials (bAPs) modulating spike timing-dependent plasticity (Bi et al., 1998; Watanabe et al., 2002). Recently, it has been demonstrated that activity-dependent internalization of Kv4.2 channels occurs during synaptic plasticity in CA1 pyramidal neurons (Jung et al., 2009; Kim et al., 2007). Furthermore, this activity-dependent Kv4.2 internalization is NMDA receptor- and Ca2+ -dependent (Kim et al., 2007), two common requirements for the induction of synaptic plasticity in CA1 neurons.
Kv4.2 α-subunits contain two consensus sites for PKA phosphorylation, three C-terminal ERK sites, two C-terminal PKC sites and two C-terminal CaMKII sites (Adams et al., 2000; Anderson et al., 2000; Varga et al., 2004). Expression of constitutively active CAMKII in CA1 pyramidal neurons results in an increase in A-type K+ currents, suggesting that the basal surface expression of Kv4.2 channels can be regulated by phosphorylation (Varga et al., 2004). Neuronal stimulation with AMPA induced Kv4.2 internalization in primary neuronal cultures. This effect was blocked in cultures that expressed a Kv4.2 channel that contained a mutated PKA phosphorylation site (Hammond et al., 2008). This suggests that activity-dependent Kv4.2 channel cycling is dependent in part on PKA phosphorylation of the channel.
Because A-type K+ channels have a heterogeneous distribution along the primary apical dendrite in CA1 pyramidal neurons, we asked whether Kv4.2 channels are differentially cycled at different regions along the apical dendrite in neurons. Here we observed that the basal cycling rate of Kv4.2g increases with distance from the soma. Neuronal stimulation by AMPA application caused a significant increase in Kv4.2g cycling rate only at distal dendrites. Expression of a phospho-deficient Kv4.2-PKA mutant abolished the differences observed in basal cycling rates at proximal and distal dendrites and upon stimulation. Taken together these results suggest that distance-dependent Kv4.2 cycling is regulated by the phosphorylation of Kv4.2 by PKA.
Materials and Methods
Organotypic hippocampal slice cultures
Hippocampal organotypic slice cultures were prepared from postnatal day 6–8 Sprague-Dawley rats as described (Nestor et al., 2007; Stoppini et al., 1991). Briefly, hippocampal slices were cut using a McIllwain tissue chopper at a thickness of 350 µm and placed on Millipore cell insert membranes in serum-containing medium (Millipore, Billerica, MA). Cultures were allowed to mature for 4 DIV in a water-jacketed incubator at 35°C and 5% CO2 before infection with Sindbis virus. The culture medium consisted of: 25% horse serum, 25% EBSS, 50% modified eagle medium + Glutemax, and B12 supplement. All animal procedures were conducted with accordance of the National Institutes of Health Guide for the Care and Use of Laboratory Animals under a protocol approved by the National Institutes of Child Health and Human Development’s Animal Care and Use Committee.
Sindbis viral infection
After 4 DIV, hippocampal CA1 neurons were infected on microinjection at two positions within the CA1 region 400 mm apart (Nanoliter 2000, World Precision Instruments). After infection, slices were returned to the incubator and checked for infection at 18–24 hours before use in experiments. Slices were infected with an attenuated Sindbis viruses expressing Kv4.2g produced using the SINrep(nsP2S726) viral vector and DH-BB(tRNA/ TE12) helper plasmid as described (Kim et al., 2004).
FRAP
24–36 hours after viral infection, slices were placed in a recording chamber and perfused with ACSF containing the following: 125 mM NaCl, 2.5 mM KCl, 25 mM NaHCO3, 1.25 mM NaH2PO4, 25 mM glucose, 2 mM CaCl2, 1 mM MgCl2, 20 mM HEPES (pH 7.4), the antioxidant Trolox 0.2 mM, (to reduce photo-damage), and bubbled with 5% CO2/95% O2. ACSF was phenol-red free to decrease non-specific fluorescence.
Proximal and distal dendrites from CA1 pyramidal neurons expressing the Kv4.2g construct were selected. Selection criteria included: proximal apical dendrites (50–150 µm from the soma) and distal apical dendrites (>200 µm from the soma). Both primary and oblique dendrites were selected, as we observed no significant difference in cycling rates between these groups at ether proximal or distal regions of the dendritic tree. Dendritic size was controlled for by selecting small proximal dendrites (2–3 µm) and large distal dendrites (~ 2 µm) for FRAP. The FRAP technique was then used to determine basal cycling rates of Kv4.2g. FRAP combined with live imaging is an established method to study the mobility of proteins (Lippincott-Schwartz et al., 2001).
FRAP was carried out at the NICHD Microscopy and Imaging Core using a Zeiss LSM 510 laser scanning confocal microscope with a 20X 1.0 N.A. water-immersion objective and the pinhole diameter set to 1 Airy Unit. The 488-nm laser line was used for both imaging and bleaching. During imaging, laser power was set to 8%, and during bleaching power was set to 100%.
To maximize scan-time and prevent photobleaching, images were acquired using the maximal frame scan rate for 512 X 512 resolution at a 4.5X optical zoom (1 frame every 1.1 seconds). Time-lapse images were captured every 1 sec for 60 sec using Zeiss LSM Image Browser software. After 5 baseline images were taken, Kv4.2g was bleached to 50% of baseline by 15–25 laser iterations over the bleach region of interest (ROI) which was fixed to a 2 µm X 2 µm area on both proximal and distal dendrites. Images in which the dendrite of interest was bleached to more than 50% of baseline were removed from the analysis.
Images were then background subtracted and change in fluorescence intensity was normalized to the baseline (prebleach intensity) for each time point and analyzed. Data was analyzed by graphically plotting normalized fluorescence intensities and by fitting a double exponential to the average data and computing a time-constant (half-recovery time, reported as τ1/2):
where Ai is the amplitude of each component, t is time and τi is the time constant of each component. Activation and recovery curves were generated for distal and proximal dendrites and the mobile fraction of the recovery kinetics was calculated as:
where F∞ is the fluorescence intensity at the end of the FRAP experiment, Fi is the initial fluorescence intensity prior to bleach and F0 is the intensity immediately postbleach (Axelrod et al., 1976; Lippincott-Schwartz et al., 2001).
Drugs
Myristolyated dynamin based inhibitory peptide (myrs-QVPSRPNRAP) and scrambled DYN (myrs-QPPASNPRVR) were synthesized and purified by Tocris Bioscience and used according to (Kim et al., 2007). Peptides (50 µM) were applied 10 minutes prior to FRAP or AMPA stimulation ((S)-AMPA) 100 µM, 5 minutes (Tocris).
Statistical analysis
Significance was assessed using the non-paired t-test and one way-ANOVA with Tukey’s post-hoc and mean values ± s.e.m. are presented.
Results
Genetic regulation of Kv4.2 expression level alters dendritic Ca2+ influx during the back-propagation of APs, suggesting that dynamic regulation of Kv4.2 channel expression may define physiologically relevant microdomains in dendrites (Chen et al., 2006; Kim et al., 2005). For instance, the application of 4-AP increases the amplitude of back propagating APs and the occurrence of Ca2+ spikes in dendrites, particularly in oblique apical dendrites (Frick et al 2003). These Ca2+ signals are compartmentalized at distal dendrites by branch points, which are important for establishing dendritic microdomains (Cai et al, 2004). The physiological relevance of activity-dependent Kv4.2 channel down-regulation, combined with observations that Kv4.2-dependent A-type K+ current amplitude increases in a distant-dependent manner lead us to hypothesize that Kv4.2 channels are differentially cycled at different regions along the apical dendrite in CA1 pyramidal neurons.
In order to test this hypothesis we used the FRAP technique to bleach primary apical and primary oblique dendrites at various regions in neurons expressing Kv4.2g construct in organotypic hippocampal slice cultures. Slice cultures were used because they offer excellent optical properties, ease of infection, and good control of the experimental conditions while preserving much of the cytoarchitecture of intact tissue (Gähwiler, 1984; Gähwiler et al., 1997). The FRAP technique is an established optical method for quantifying the two dimensional lateral diffusion of lipid bound proteins is mammalian cells (Axelrod et al., 1976). Our measurements of FRAP recovery curves in dendrites were divided into a mobile fraction and an immobile fraction (Star et al., 2002; Sprague and McNally, 2005).
It has been shown that Kv channels have a stable immobile fraction, and a highly mobile fraction (O'Connell et al., 2006). Because we have observed that Kv4.2 trafficking is coincident with GluR1 receptor trafficking (Kim et al., 2007), which has a recovery time constant on the order of ~ 50 seconds in dendrites (Sharma et al., 2006), we decided to measure the mobile component of the FRAP recovery curve for Kv4.2 channels over a 1 minute period. Although it is possible that further, slower fluorescence recovery continues after this time-point, we wanted to measure Kv4.2 cycling during the time at which GluR1 receptors cycle in dendrites. Furthermore, in our system, using our scan and bleach parameters, we observed substantial photobleaching of the fluorescence recovery after 1 minute of imaging. We were able to observe the maximal amount of fluorescence recovery before photobleaching in this interval.
Our recovery curves consisted of an initial fast decay followed by slow exponential decay. Based on modeling studies fitting the FRAP recovery curve with a two-phase decay exponential function (Carrero et al., 2003, Matsumoto et al., 2005), we suggest that Kv4.2g channels transiently interact with immobile cellular structures (Phair & Misteli, 2001). This is consistent with data showing that Kv4.2 interacts with the cytoskeletal protein filamin and large adaptor proteins like AKAPs and PSD-95 (Lin et al., 2010; Petrecca et al., 2000; Wong and Schlichter, 2004). Thus, in our experiments, the total mobile fraction is comprised of both fast and slow mobile components of the recovery curve and is the total normalized fluorescence recovery recorded in 1 minute, fit with a two-phase decay exponential function (Zheng et al., 2010)
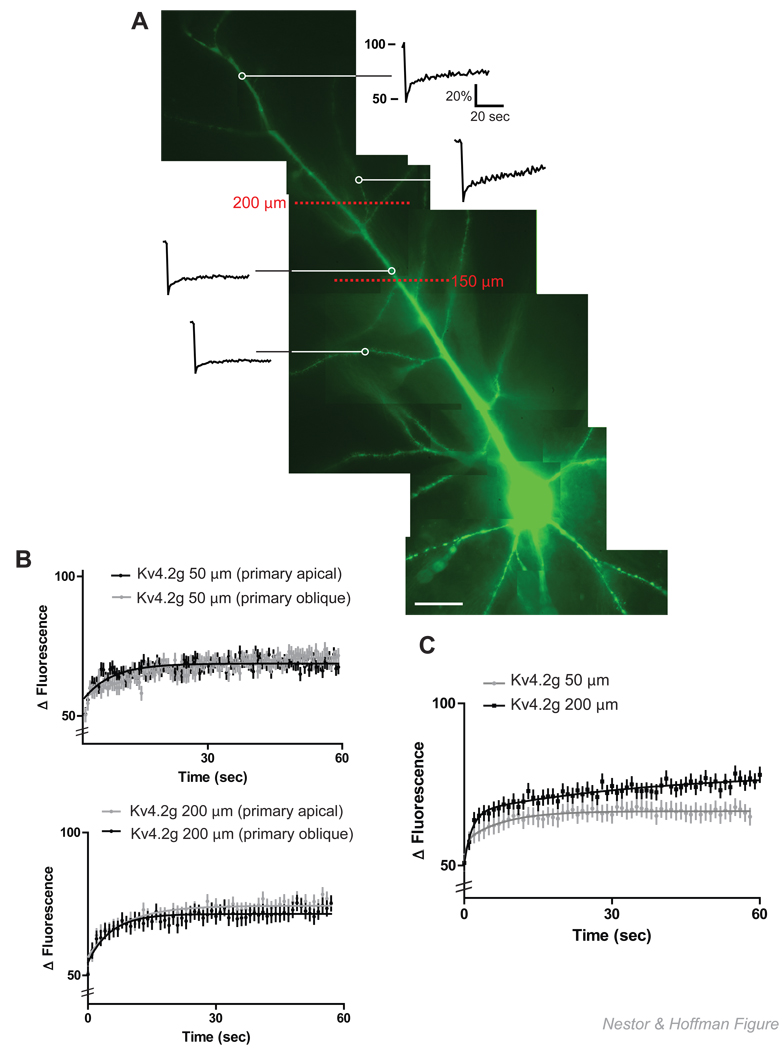
Kv4.2g constitutive cycling rate is faster at distal dendrites
CA1 pyramidal neurons were bleached at 50 µm increments along primary apical and primary oblique dendrites. After averaging, we found that, in our system, we could divide the main apical dendrite into a proximal (< 150 µm) and a distal (>200 µm) region (Figure 1A.) Within these general categories, we performed FRAP on both apical and oblique dendrites. In both groupings the total mobile fraction, measured as the plateau of the recovery curve, was not significantly different: 76.2% ± 1.6 for distal dendrites and 77.2% ± 1.7 for proximal dendrites (N = 20 dendrites/5 neurons/group; p >0.05, unpaired t-test). We also did not observe a significant difference in the recovery rate of Kv4.2g channels within these groups (N = 20 dendrites/5 neurons/group; τ½ = 21.6 s ± 0.8 for distal main apical vs. 28.5 s ± 0.7 for distal obliques; p >0.05, unpaired t-test, and τ½ = 43.2 s ± 0.4 for proximal main apical vs. 44.4 s ± 0.4 for proximal obliques; p >0.05, unpaired t-test)(Figure 1B).
Figure 1. Constitutive cycling of Kv4.2g is slower in proximal dendrites as compared to distal dendrites.
(A) Representative single normalized FRAP recovery curves at proximal (<150 µm) and distal (>200 µm) regions of the dendritic tree in a CA1 pyramidal neuron expressing Kv4.2 g, scale bar = 25 µm. (B) FRAP analysis of averaged, normalized recovery curves revealed no significant difference between primary and oblique dendrites at either proximal or distal regions (N = 20 dendrites/5 neurons/group; p > 0.05, t-test). (C) When proximal and distal FRAP recovery curves were compared, Kv4.2g was observed to recover at a significantly faster rate in distal dendrites (N = 20 dendrites/5 neurons/group; p < 0.05, t-test).
However, when the average normalized FRAP recovery curves were compared between groups, we observed a significant increase in the recovery rate at distal primary and oblique dendrites as compared to proximal primary and oblique dendrites (N = 20 dendrites/5 neurons/group; τ½ = 24.5 s ± 0.2 for distal vs. 51.4 s ± 0.3 for proximal; p < 0.05, unpaired t-test) (Figure 1C).
When we analyzed the recovery curves for fast mobile fractions (first exponential of the double exponential fit) of Kv4.2g at distal and proximal dendrites we observed no significant difference (τ½ = 1.1s ± 0.02 for distal vs. 1.6 s ± 0.01 for proximal; p > 0.05, unpaired t-test). This likely represents freely diffusible Kv4.2g since this recovery time constant is similar to that for neurons that express GFP alone (τ½ = 0.8 s) (Star et al., 2002) and is a small fraction of the total mobile Kv4.2g we observed in dendrites (35% in proximal dendrites and 37 % in distal dendrites). Thus, Kv4.2g channels cycle at a slower rate in proximal dendrites, primarily due to a differences in a slower mobile fraction which may represent channels transiently interacting with immobile structures.
Activity-dependent Kv4.2g cycling rate is faster at distal dendrites
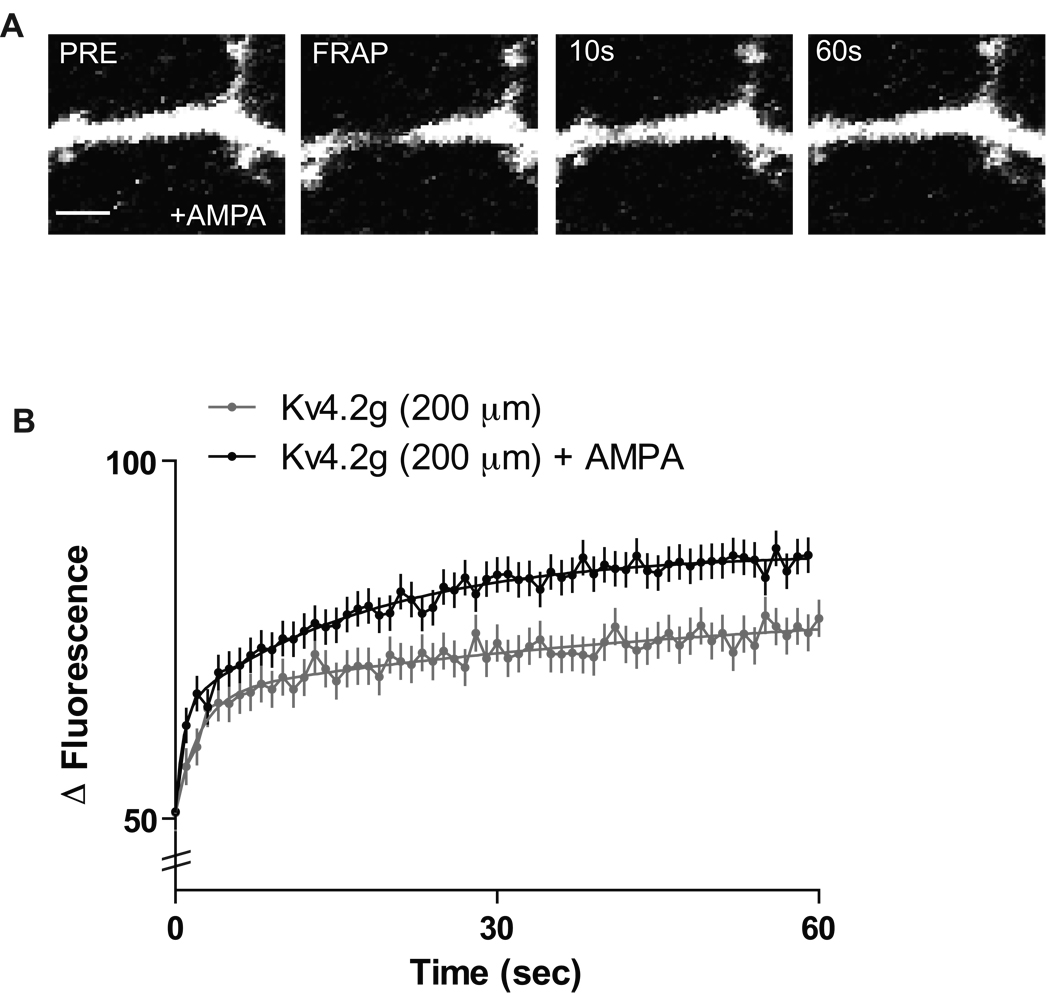
In addition to basal Kv4.2 turnover rates, Kv4.2 channels can mobilize in response to neuronal activity. Recently, it has been shown that stimulation of AMPA receptors in primary neuronal cultures expressing Kv4.2 can induce internalization of the channel in an NMDAR-dependent manner (Kim et al., 2007). The application of AMPA increases network activity activating NMDA receptors which results in a Ca2+-dependent internalization of Kv4.2 channels out of spines, thus presumably increasing Kv4.2 concentration in dendritic compartments. We therefore asked whether AMPA application to neurons expressing Kv4.2g in slice cultures would affect activity-dependent cycling rates. Application of 100 µM AMPA for 5 minutes induced a significant increase in the recovery rate of Kv4.2g channels in distal dendrites as compared to proximal dendrites, where the application of AMPA had no significant effect (N = 15 dendrites/5 neurons/group; τ½ = 24.5 s ± 0.2 for distal vs. 17.9 s ± 0.5 for distal + AMPA; p < 0.05, unpaired t-test) (Figure 2A, B). This suggests that activity-dependent Kv4.2g cycling preferentially occurs at distal apical dendrites and is partially dependent on NMDAR activity. Kv4.2 channels are constituents of a synaptic complex that regulates Ca2+ influx through NMDARs (Jung et al. 2008; Kim et al., 2007), suggesting that activity-dependent regulation of Kv4.2 expression in distal dendrites contributes to dendritic excitability and synaptic plasticity (Shah et al, 2010).
Figure 2. Kv4.2g cycling rate increases at distal dendrites after AMPA stimulation.
(A) Representative experiment showing a distal dendrite expressing Kv4.2g and bleached in the presence of AMPA, different recovery time points are shown. Scale bar = 2 µm. (B) 100 µm AMPA stimulation for 5 minutes induced a significant increase in Kv4.2 channel cycling rate at distal dendrites (N = 15 dendrites/5 neurons/group; p < 0.05, t-test).
Kv4.2g cycling at distal dendrites is dependent on PKA site Ser552
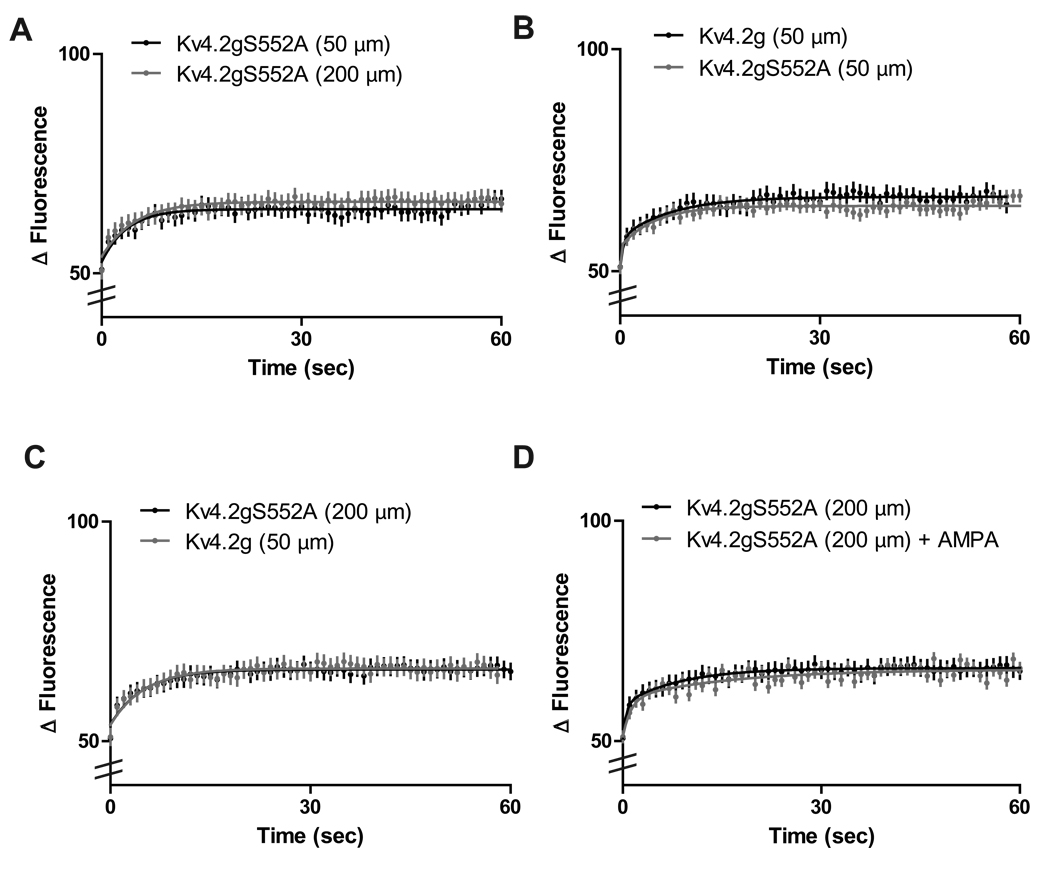
Hammond et al., (2008) found that AMPA application in primary neuronal cultures induced Kv4.2 internalization, which was blocked with a Kv4.2-PKA mutant lacking a phosphorylation site at Ser552 (Kv4.2gS552A). It is possible that neuronal stimulation and subsequent NMDA receptor activation leads to increased PKA levels (Roberson and Sweatt, 1996) and results in membrane-bound Kv4.2 channels being internalized to enhance the excitability of dendrites. Therefore, we asked whether the dynamic cycling of Kv4.2 channels at distal dendrites was dependent on PKA phosphorylation. In neurons expressing the PKA mutant Kv4.2gS552A, the rapid constitutive cycling of Kv4.2g channels at distal dendrites was diminished, and the recovery rate after FRAP was not significantly different than what was observed at proximal dendrites expressing Kv4.2gS552A (N = 10 dendrites/5 neurons/group; τ½ = 48.1 s ± 0.4 for proximal + Kv4.2gS552A vs. 49.8 s ± 0.5 for distal + Kv4.2gS552A; p > 0.05, unpaired t-test) (Figure 3A). Thus, the bifurcation of dynamic Kv4.2g cycling we observed above was abolished by the expression of channels that lack the PKA phosphorylation site at Ser552. In addition, Kv4.2gS552A expression had no effect on the cycling rate of channels at proximal dendrites compared with Kv4.2g, suggesting that PKA activity at distal dendrites is primarily responsible for enhanced constitutive cycling rates of Kv4.2g channels at these sites (Figure 3B). In fact, expression of Kv4.2gS552A resulted in cycling rates in distal dendritic regions equivalent to those found for Kv4.2g in proximal regions (N = 10 dendrites/5 neurons/group; τ½ = 51.4 s ± 0.3 for proximal vs. 49.8 s ± 0.5 for distal + Kv4.2gS552A; p > 0.05, unpaired t-test) (Figure 3C).
Figure 3. Distance-dependent Kv4.2 cycling requires Kv4.2 PKA phosphorylation site Ser552.
(A) Expression of the PKA mutant Kv4.2gS552A at distal dendrites abolished the rapid constitutive cycling of Kv4.2g (N = 10 dendrites/5 neurons/group; p > 0.05, t-test). (B) Expression of Kv4.2gS552A had no significant effect on proximal dendrites (N = 10 dendrites/5 neurons/group > 0.05, t-test). (C) Distal dendrites expressing the Kv4.2 phospho-mutant Kv4.2gS552A showed Kv4.2g cycling rates that were not significantly different than cycling rates observed in proximal dendrites indicating the PKA phosphorylation of Kv4.2 channels is necessary for the increase observed in distal dendrites (N = 10 dendrites/5 neurons/group > 0.05, t-test) (D) AMPA-induced increases in Kv4.2g cycling rates at distal dendrites are abolished by expression of Kv4.2gS552A. There is no significant difference in Kv4.2g cycling rates observed in distal dendrites expressing Kv4.2gS552A before or after treatment with 100 µM AMPA (N = 10 dendrites/5 neurons/group; p > 0.05, t-test). We note that these results show that the changes in Kv4.2g cycling we report here are not due to GFP labeling of the channel as these constructs differ only by a single amino acid point mutation in the pore of the channel.
PKA modulates hippocampal synaptic plasticity (Lin et al., 2008,Otmakhov et al., 2004). It has been shown that PKA activation induces a downregulation of Kv4.2 currents in Xenopus oocytes (Schrader et al., 2002). In addition, activation of PKA increases the amplitude of back propagating action potentials in hippocampal dendrites (Hoffman and Johnston, 1998). Therefore, we hypothesized that activity-dependent changes in Kv4.2g channels were dependent on PKA phosphorylation of the channel. To test this, we expressed Kv4.2gS552A in CA1 pyramidal neurons and then applied 100 µM AMPA for 5 minutes. The application of AMPA had no significant affect on the cycling rate of Kv4.2gS552A at distal dendrites. This suggests that the distance-dependent cycling of Kv4.2g that we have observed is regulated by activity-dependent phosphorylation of Kv4.2 channels by PKA (N = 10 dendrites/5 neurons/group; τ½ = 49.8 s ± 0.5 for distal + Kv4.2S552A vs. 50.6 s ± 0.5 for distal + Kv4.2gS552A + AMPA; p > 0.05, unpaired t-test) (Figure 3D).
Kv4.2g cycling rate is affected by clathrin-mediated endocytosis
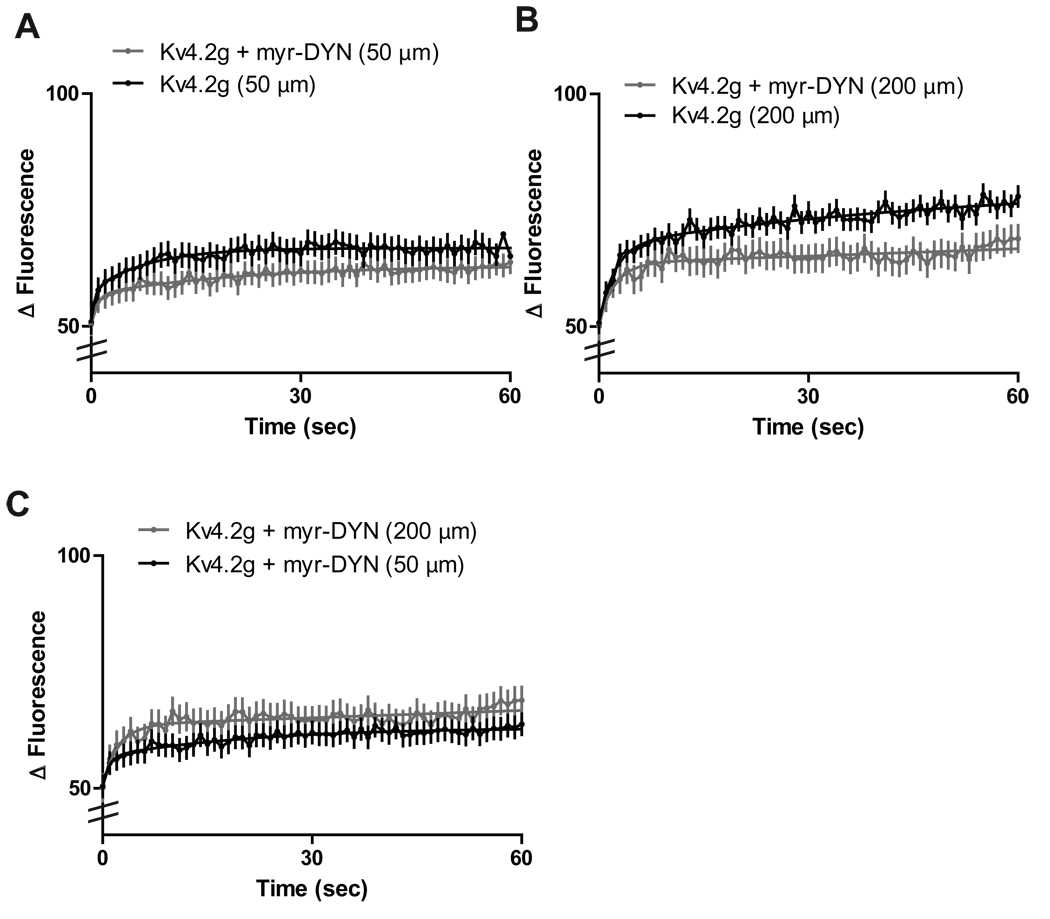
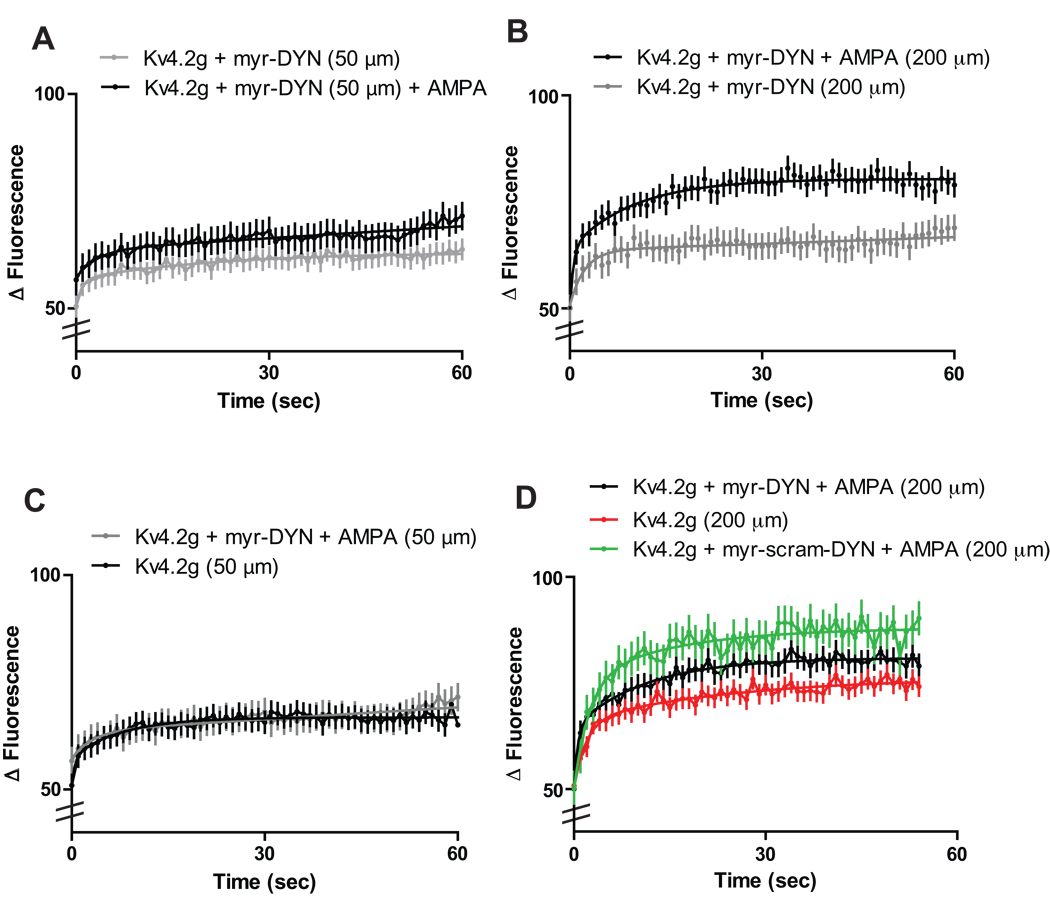
AMPA mediated endocytosis of Kv4.2 channels in primary hippocampal neurons is a clathrin-regulated process, under the control of dynamin (Kim et al., 2007). In order to isolate Kv4.2 channels inserted in the plasma membrane, we incubated slice cultures in a membrane-permeable dynamin-derived peptide (Kv4.2g + myr-DYN) or a non-functional scrambled peptide (Kv4.2g + myr-scram-DYN) for 10 minutes before applying 100 µm AMPA and performing FRAP at distal dendrites. The myr-DYN peptide blocks the recruitment of dynamin to clathrin-coated pits and thus inhibits clathrin mediated endocytosis of Kv4.2 channels at the dendritic membrane (Jung et al., 2009; Kim et al., 2007; Nong et al., 2003,).
Incubation of slice cultures with myr-DYN resulted in a significant decrease in the recovery rate of Kv4.2g at both distal and proximal dendrites (N = 10 dendrites/5 neurons/group; τ½= 46.9 s ± .6 for Kv4.2g + myr-DYN + distal vs. 24.5 s ± 0.2 for distal; τ½= 61.6 s ± 0.5 for Kv4.2g + myr-DYN + proximal vs. 53.7 s ± 0.6 for proximal; p < 0.05, unpaired t-test)(Figure 4A, B). In addition, myr-DYN significantly decreased the distance-dependent differences in Kv4.2g cycling rate we observed in distal dendrites. Thus, cycling rates at distal dendrites were not significantly different than those at proximal dendrites (N = 10 dendrites/5 neurons/group; τ½= 46.9 s ± .6 for Kv4.2g + myr-DYN + distal vs. 61.6 s ± 0.5 for Kv4.2g + myr-DYN + proximal; p > 0.05, unpaired t-test) (Figure 4C). Taken together, these results suggest that constitutive distance-dependent Kv4.2g cycling in dendrites is mediated by both PKA phosphorylation of the Kv4.2 channel and subsequent clathrin-mediated endocytosis of the channel.
Figure 4. Clathrin-mediated endocytosis regulates distance-dependent Kv4.2 channel cycling rates.
(A) Proximal dendrites treated with a dynamin-based inhibitory peptide (myr-DYN) show a significant decrease in Kv4.2 cycling rate compared to those expressing Kv4.2g alone (AMPA (N = 10 dendrites/5 neurons/group p < 0.05, t-test). (B) Distal dendrites treated with myr-DYN show a significant decrease in Kv4.2 cycling rate compared to those expressing Kv4.2g alone (N = 10 dendrites/5 neurons/group; p < 0.05, t-test). (C) Treatment of slice cultures expressing Kv4.2g with myr-DYN abolished distance-dependent differences in Kv4.2g cycling rate (N = 10 dendrites/5 neurons/group; p < 0.05, t-test).
Interestingly, recovery rates at both distal and proximal dendrites treated with myr-DYN increased significantly after the application of AMPA when compared to those treated with myr-DYN alone (N = 10 dendrites/5 neurons/group; τ½ = 22.4 s ± 0.7 for Kv4.2g + myr-DYN + AMPA + distal vs. 46.9 s ± .6 for Kv4.2g + myr-DYN + distal; τ½ = 49.3 s ± 0.7 for Kv4.2g + myr-DYN + AMPA + proximal vs. 61.6 s ± 0.5 for Kv4.2g + myr-DYN + proximal ; p > 0.05, unpaired t-test) (Figure 5A, B). However, application of AMPA to proximal dendrites treated with myr-DYN resulted in Kv4.2g cycling rates that were not significantly different than dendrites expressing Kv4.2g without myr-DYN treatment (N = 10 dendrites/5 neurons/group; τ½= 49.3 s ± 0.7 for Kv4.2g + myr-DYN + proximal + AMPA vs. 53.7 s ± 0.6 for proximal; p > 0.05, unpaired t-test) (Figure 5C).
Figure 5. Activity-dependent changes in Kv4.2 cycling rate are modulated by clathrin-mediated endocytosis.
Fluorescence recovery rates increased significantly at proximal (A) and distal (B) dendrites incubated with myr-DYN (50 µM, 10 minutes) and treated with AMPA (100µM, 5 minutes) (N = 10 dendrites/5 neurons/group; p < 0.05, t-test). (C) Cycling rates at proximal dendrites expressing Kv4.2g and treated with myr-DYN and AMPA were not significantly different than dendrites expressing Kv4.2g alone (N = 10 dendrites/5 neurons/group; p > 0.05, t-test). (D) Distal dendrites treated with Kv4.2g + myr-DYN (50 µM, 10 minutes) and 100 µM AMPA showed a significant decrease in Kv4.2g cycling rate as compared to those treated with myr-scram-DYN (N = 10 dendrites/5 neurons/group; p < 0.05 ANOVA and Tukey's test). (E) Cycling rates at distal dendrites expressing Kv4.2g alone were not significantly different than those expressing Kv4.2g and treated with both myr-DYN and AMPA (N = 10 dendrites/5 neurons/group; p > 0.05 ANOVA and Tukey's test).
Distal dendrites from neurons treated with myr-DYN and 100 µM AMPA showed a significant decrease in fluorescence recovery as compared to those treated with myr-scram-DYN (N = 10 dendrites/5 neurons/group; τ½ = 17.9 s ± 0.5 for distal + AMPA vs. 15.6 s ± 0.4 for Kv4.2g + myr-scram-DYN + AMPA + distal vs. 19.0 s ± 0.3 for Kv4.2g + myr-DYN + AMPA + distal; p < 0.05 ANOVA and Tukey's test) (Figure 5D). However, when distal dendrites expressing Kv4.2g were compared to dendrites treated with myr-DYN and AMPA, no significant difference was detected (N = 10 dendrites/5 neurons/group; τ½ = 19.3 s ± 0.5 for Kv4.2g + distal vs. 19.0 s ± 0.3 for Kv4.2g + myr-DYN + AMPA + distal; p > 0.05, ANOVA and Tukey's test) (Figure 5D). These results suggest that during synaptic activity, Kv4.2 is driven out of spines into the dendrite in a clathrin-mediated process. When clathrin-mediated endocytosis is blocked activity-mediated increases in Kv4.2 cycling rate at distal dendrites is inhibited.
Discussion
Kv4.2 channels are emerging as important transmembrane proteins that modulate dendritic excitability (Kim and Hoffman, 2008; Shah, et al., 2010). It has been shown that they undergo activity-dependent internalization in primary neuronal cultures (Kim et al., 2007). However, an investigation of the dynamic cycling of these channels in intact neurons has not been carried out. We have demonstrated using FRAP that Kv4.2 channel localization is dynamic and has different cycling rates at different distances from the soma. Specifically, FRAP experiments in slice cultures reveal that Kv4.2 channels constitutively cycle at faster rates at distal dendritic locations as compared to proximal ones. At distal sites, Kv4.2 cycling rate is increased by neuronal stimulation, which has been shown to be NMDAR-dependent (Kim et al., 2007). Furthermore, distant-dependent changes in constitutive Kv4.2 cycling rates are dependent on PKA phosphorylation of the channel. Neurons that expressed a Kv4.2-PKA phospho-mutant had no significant difference in cycling rate between proximal and distal dendrites. Activity-dependent increases in Kv4.2 channel cycling rate are also dependent on PKA phosphorylation site S552 on the Kv4.2 C-terminus. The distal dendrites of neurons expressing a Kv4.2-PKA phospho-mutant did not see an increase in the cycling rate of Kv4.2 after the application of AMPA, as was observed in neurons expressing Kv4.2 alone. Finally, the use of membrane-permeable dynamin-derived peptide revealed that constitutive Kv4.2 cycling is a clathrin-mediated process in dendrites and the distance-dependent differences in Kv4.2g cycling rate we observed are dependent on clathrin-mediated endocytosis.
Kv4.2 channel mobility and dendritic plasticity
Many reports have demonstrated that synaptic plasticity is due in part to the dynamic insertion and removal of glutamate receptors from synapses in the hippocampus (Barry and Ziff, 2002; Collingridge et al., 2004), but little work has been done on dendritic plasticity. For instance, it has been shown that K+ transient currents regulate the back-propagation of APs, plateau potentials, and frequency-dependent AP broadening (Cai et al., 2004; Hoffman et al., 1997; Kim et al., 2005; Kim et al., 2007). Kv4.2 channels help regulate Ca2+ influx through NMDARs (Jung et al. 2008; Kim et al., 2007). The regulation of intracellular Ca2+ and CAMKII kinase is critical for the induction of long-term potentiation (Malenka and Bear, 2004) and for local dendritic protein synthesis (Sutton et al., 2004). Expression of constitutively active CAMKII in CA1 pyramidal neurons results in an increase in A-type K+ currents, suggesting that the surface expression of Kv4.2 channels is also regulated in part by CAMKII phosphorylation (Varga et al., 2004). Interestingly, Ca2+-transients measured in dendrites show a heterogeneous distribution with larger transients occurring at distal dendrites and branch-points (Cai et al., 2004). For instance, the application of 4-AP to block A-type channels, increases the amplitude of bAPs and the occurrence of Ca2+ spikes in dendrites, particularly in oblique apical dendrites (Frick et al., 2004). Recent work from our group shows that NMDARs regulate the expression of Kv4.2 in a Ca2+ -dependent manner and that this activity may be reciprocal as Kv4.2 functional expression level controls synaptic NR2B expression (Kim et al., 2007; Jung et al., 2008). In addition, it has been demonstrated that NR2B receptors are required for LTP (Barria and Malinow, 2005), suggesting that the cycling and expression of Kv4.2 in neurons may play a role in potentiating different groups of synapses in different dendritic zones. Thus, Kv4.2 expression and trafficking can have extensive physiological effects on dendritic excitability and plasticity.
It is known that functional glutamate receptors exist outside of synapses in extrasynapic zones and in dendrites. For instance, membrane patches isolated from the dendrites of hippocampal neurons reveal AMPA receptor-mediated currents (Jonas and Sakmann, 1992; Spruston et al., 1995; Andrasfalvy and Magee, 2001). NMDARs have also been reported to exist within dendritic shafts (Petralia and Wenthold, 1999). The bifurcation of Kv4.2 channel cycling rates we report in dendrites may have functional relevance for how dendrites process information. For example, a recent study suggests that some forms of dendritic plasticity are only observed at proximal dendrites and that this plasticity is dependent on Kv4.2 trafficking in the perisomatic region of neurons (Losonczy et al., 2008). In this light, our results support growing evidence that dendrites are not passive structures, but are complex computational units that serve to isolate different signaling domains within a neuron.
It has been suggested that dendritic geometry strongly influences protein diffusion and therefore, protein concentration at different sites within a neuron (Bloodgood and Sabatini, 2007). Proteins must diffuse long distances along dendrites before insertion into synapses. Freely diffusible proteins encounter a number of diffusion traps as they move to distal regions where they interact with machinery at dendritic spines (Santamaria et al., 2006). The “somatic source model” of this pattern of diffusion predicts a proximal-to-distal gradient with a steep decay profile in the constitutive concentration of proteins-including receptors and channels-along the dendrite. This is based on a biophysical model of the diffusion of receptors that are inserted into the soma and allowed free diffusion from proximal to distal sites (Bressloff and Earnshaw, 2007).
Our observation that cycling changes with distance from the soma cannot be a function of this somatic source model of protein trafficking. We observed that rates are faster at distal dendrites, indicating that for a given bleached area, there are more mobile Kv4.2 channels available distally for lateral diffusion. The observation that there are increased A-type K+ currents in distal dendrites supports this finding (Hoffman et al., 1997). This has also been observed for AMPARs. AMPARs in CA1 pyramidal neurons display a reverse proximal-to-distal increasing gradient (Magee and Cook, 2000; Andrasfalvy and Magee, 2001; Smith et al., 2003). This result combined with our observation that neurons expressing Kv4.2S552A show no significant difference in Kv4.2 channel cycling rates at proximal or distal dendrites suggests a central role for PKA phosphorylation of the channel and directed intracellular membrane trafficking in regulating constitutive Kv4.2 dynamics at distal dendrites. Our observation that neurons expressing Kv4.2S552A show no significant increase in cycling after stimulation indicates that PKA phosphorylation is also required for activity-dependent Kv4.2 cycling at these sites, which has been observed in neuronal culture (Hammond et al., 2008; Kim et al., 2007). Indeed, LTP can be blocked by inhibition of PKA with a PKA inhibitory peptide fragment (Rosenkranz et al., 2009), suggesting that PKA regulation of Kv4.2 channel cycling may play a role in synaptic plasticity.
Distance-dependent dendritic Kv4.2 channel mobility
Protein synthesis is slow, taking hours to days in the case of glutamate receptors. Accordingly, the acute effects of neuronal stimulation or dynamin-based inhibitory peptide treatment that we observe here is unlikely due to protein synthesis at distal dendrites, but rather caused by channel turnover. We consider two potential models for enhanced rates of Kv4.2 cycling in distal dendrites compared to proximal. First, Kv4.2 channels are expressed at a higher concentration at distal dendrites. Therefore, when an area of distal dendrite is bleached, there are more channels available to diffuse into the bleached area. Our observation that constitutive cycling of the channel is faster at distal dendrites can possibly be explained by a difference in concentration gradient between proximal and distal pools.
A difference in Kv4.2 channel concentration is also supported by our report (Fig. 2) that that Kv4.2 channel turnover increased in distal dendrites after AMPA application. In spines, AMPA induces Kv4.2 channel internalization (Kim et al., 2007). It is possible then, that activity-dependent increases in Kv4.2 turnover at distal dendrites are due to Kv4.2 being driven out of spines and into the dendrites, which already contain a high concentration of the channel during basal conditions. In addition, our results showing that increased cycling in distal dendrites requires PKA activity indicates that PKA activity is more dynamic at distal dendrites, perhaps due to increased spine density.
A second proposal is that the enhanced mobility in distal dendrites is due to expression of a second, additional pool of Kv4.2 channels, which is preferentially modulated by PKA. This is supported by data showing that distal dendritic A-current properties are modulated by PKA activation to mimic those found in the soma and proximal dendrites (Hoffman and Johnston, 1998). In addition, the PKA activation regulates Kv4.2 channel expression in neurons (Hammond et al., 2008). PKA anchoring to Kv4.2 channels through A-kinase anchoring proteins (AKAPs) has been recently demonstrated (Lin et al., 2010). AKAPs are differentially expressed in different neuronal domains and are dynamically regulated (Smith et al., 2006).
Taken together, the bifurcation of Kv4.2 turnover rates we observe can be explained by changes in Kv4.2 concentration or PKA activation of a second pool of channels in dendrites. The increase in concentration of Kv4.2 channels at distal dendrites may be due to an increase in the number of synapses or an increase in signaling proteins like PKA, or both. Functionally, proximal CA1 dendrites receive afferents from hippocampal subregions CA3a and b and distal dendrites are the primary terminals for the Schaffer collateral pathway (Ishizuka et al., 1990, Thompson et al., 2006). Thus proximal and distal dendrites may receive two disparate sets of inputs, necessitating changes in Kv4.2 channel concentration in order to fine tune synaptic inputs.
Effect of endocytosis on dendritic Kv4.2 channel mobility
Our finding that treatment with a dynamin-based inhibitory peptide (myr-DYN) slows constitutive cycling at distal dendrites and abrogates distance-dependent Kv4.2 cycling in CA1 neurons suggests that dendritic concentrations of the channel are depleted during constitutive activity and are not replaced at distal zones because myr-DYN prevents internalization of Kv4.2 channels (Kim et al., 2007). Thus, myr-DYN may block available slots for new channels to be inserted in dendritic membranes and abolish any channel concentration-dependent effect observed at distal dendrites. During basal activity, we observed a decrease in fluorescence recovery at both proximal and distal dendrites, supporting the idea that myr-DYN block of the internalization of Kv4.2 channels loads the dendritic membrane with immobile Kv4.2 channels. Our model of increased Kv4.2 channel concentration at distal dendrites explains the greater decrease in fluorescence recovery we observed at distal dendrites after myr-DYN treatment. Because Kv4.2 concentrations are higher at distal dendrites, more membrane is taken up by immobile Kv4.2 channels than at proximal dendrites.
The results of this study indicate that Kv4.2 channels are dynamic components of the dendrite and cycling of these channels may play an important role in regulating synaptic plasticity. Although some of the mechanisms underlying Kv4.2 channel internalization have been reported, more work is required to further characterize the mechanisms involved in our finding that Kv4.2 is trafficked differentially at proximal and distal regions. These results pose some interesting questions about Kv4.2 function. For instance, how do changes in channel trafficking rates affect dendritic excitability and plasticity? Do neurons differentially regulate the dynamic trafficking of Kv4.2 channels at different zones along the dendrite in order locally shape excitability? How are Ca2+ signals affected by Kv4.2 channel expression levels? Although dendritic spines have received much of the attention, our results show that dendrites perform complex calculations using many of the same mechanisms seen in spines. The dynamic turnover of potassium channels in dendrites has important implications for understanding how somatodendritic signals are processed in neurons.
Acknowledgements
We thank Vincent Schram in NICHD imaging core for technical assistance.
Sponsorship: This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
References
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75(6):2277–2287. doi: 10.1046/j.1471-4159.2000.0752277.x. [DOI] [PubMed] [Google Scholar]
- Anderson AE, Adams JP, Qian Y, Cook RG, Pfaffinger PJ, Sweatt JD. Kv4.2 phosphorylation by cyclic AMP-dependent protein kinase. J Biol Chem. 2000;275(8):5337–5346. doi: 10.1074/jbc.275.8.5337. [DOI] [PubMed] [Google Scholar]
- Andrasfalvy BK, Magee JC. Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons. J Neurosci. 2001;21(23):9151–9159. doi: 10.1523/JNEUROSCI.21-23-09151.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Ertunc M, Moulder KL, Blackwell J, Chung C, Su J, Kavalali ET. Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J Neurosci. 2008;28(40):10151–10166. doi: 10.1523/JNEUROSCI.2432-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D, Koppel DE, Schlessinger J, Elson E, Webb WW. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12(3):279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18(24):10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Ca(2+) signaling in dendritic spines. Curr Opin Neurobiol. 2007;17(3):345–351. doi: 10.1016/j.conb.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Bressloff PC, Earnshaw BA. Diffusion-trapping model of receptor trafficking in dendrites. Phys Rev E Stat Nonlin Soft Matter Phys. 2007;75(4 Pt 1):041915. doi: 10.1103/PhysRevE.75.041915. [DOI] [PubMed] [Google Scholar]
- Carrero G, McDonald D, Crawford E, de Vries G, Hendzel MJ. Using FRAP and mathematical modeling to determine the in vivo kinetics of nuclear proteins. Methods. 2003;29(1):14–28. doi: 10.1016/s1046-2023(02)00288-8. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5(12):952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH. Slice cultures of cerebellar, hippocampal and hypothalamic tissue. Experientia. 1984;40(3):235–243. doi: 10.1007/BF01947561. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20(10):471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Lin L, Sidorov MS, Wikenheiser AM, Hoffman DA. Protein kinase A mediates activity-dependent Kv4.2 channel trafficking. J Neurosci. 2008;28(30):7513–7519. doi: 10.1523/JNEUROSCI.1951-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Johnston D. Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. J Neurosci. 1998;18(10):3521–3528. doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295(4):580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jerng HH, Kunjilwar K, Pfaffinger PJ. Multiprotein assembly of Kv4.2, KChIP3 and DPP10 produces ternary channel complexes with ISA-like properties. J Physiol. 2005;568(Pt 3):767–788. doi: 10.1113/jphysiol.2005.087858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng HH, Qian Y, Pfaffinger PJ. Modulation of Kv4.2 channel expression and gating by dipeptidyl peptidase 10 (DPP10) Biophys J. 2004;87(4):2380–2396. doi: 10.1529/biophysj.104.042358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Sakmann B. Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. J Physiol. 1992;455:143–171. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SC, Hoffman DA. Biphasic somatic A-type K channel downregulation mediates intrinsic plasticity in hippocampal CA1 pyramidal neurons. PLoS One. 2009;4(8):e6549. doi: 10.1371/journal.pone.0006549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SC, Kim J, Hoffman DA. Rapid, bidirectional remodeling of synaptic NMDA receptor subunit composition by A-type K+ channel activity in hippocampal CA1 pyramidal neurons. Neuron. 2008;60(4):657–671. doi: 10.1016/j.neuron.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron. 2007;54(6):933–947. doi: 10.1016/j.neuron.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Nadal MS, Clemens AM, Baron M, Jung SC, Misumi Y, Rudy B, Hoffman DA. Kv4 accessory protein DPPX (DPP6) is a critical regulator of membrane excitability in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2008;100(4):1835–1847. doi: 10.1152/jn.90261.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci. 2008;11(2):170–177. doi: 10.1038/nn2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Sun W, Wikenheiser AM, Kung F, Hoffman DA. KChIP4a regulates Kv4.2 channel trafficking through PKA phosphorylation. Mol Cell Neurosci. 2010;43(3):315–325. doi: 10.1016/j.mcn.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Snapp E, Kenworthy A. Studying protein dynamics in living cells. Nat Rev Mol Cell Biol. 2001;2(6):444–456. doi: 10.1038/35073068. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Llinas R. Electrophysiology of mammalian tectal neurons in vitro. I. Transient ionic conductances. J Neurophysiol. 1988;60(3):853–868. doi: 10.1152/jn.1988.60.3.853. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452(7186):436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- Magee JC, Cook EP. Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nat Neurosci. 2000;3(9):895–903. doi: 10.1038/78800. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malin SA, Nerbonne JM. Molecular heterogeneity of the voltage-gated fast transient outward K+ current, I(Af), in mammalian neurons. J Neurosci. 2001;21(20):8004–8014. doi: 10.1523/JNEUROSCI.21-20-08004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto G, Stojanovic A, Holmberg CI, Kim S, Morimoto RI. Structural properties and neuronal toxicity of amyotrophic lateral sclerosis-associated Cu/Zn superoxide dismutase 1 aggregates. J Cell Biol. 2005;171(1):75–85. doi: 10.1083/jcb.200504050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429(6993):761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal MS, Ozaita A, Amarillo Y, Vega-Saenz de Miera E, Ma Y, Mo W, Goldberg EM, Misumi Y, Ikehara Y, Neubert TA, et al. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron. 2003;37(3):449–461. doi: 10.1016/s0896-6273(02)01185-6. [DOI] [PubMed] [Google Scholar]
- Nestor MW, Mok LP, Tulapurkar ME, Thompson SM. Plasticity of neuron-glial interactions mediated by astrocytic EphARs. J Neurosci. 2007;27(47):12817–12828. doi: 10.1523/JNEUROSCI.2442-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell KM, Rolig AS, Whitesell JD, Tamkun MM. Kv2.1 potassium channels are retained within dynamic cell surface microdomains that are defined by a perimeter fence. J Neurosci. 2006;26(38):9609–9618. doi: 10.1523/JNEUROSCI.1825-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Immunocytochemistry of NMDA receptors. Methods Mol Biol. 1999;128:73–92. doi: 10.1385/1-59259-683-5:73. [DOI] [PubMed] [Google Scholar]
- Petrecca K, Miller DM, Shrier A. Localization and enhanced current density of the Kv4.2 potassium channel by interaction with the actin-binding protein filamin. J Neurosci. 2000;20(23):8736–8744. doi: 10.1523/JNEUROSCI.20-23-08736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phair RD, Misteli T. Kinetic modelling approaches to in vivo imaging. Nat Rev Mol Cell Biol. 2001;2(12):898–907. doi: 10.1038/35103000. [DOI] [PubMed] [Google Scholar]
- Ren X, Hayashi Y, Yoshimura N, Takimoto K. Transmembrane interaction mediates complex formation between peptidase homologues and Kv4 channels. Mol Cell Neurosci. 2005;29(2):320–332. doi: 10.1016/j.mcn.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Sweatt JD. Transient activation of cyclic AMP-dependent protein kinase during hippocampal long-term potentiation. J Biol Chem. 1996;271(48):30436–30441. doi: 10.1074/jbc.271.48.30436. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Frick A, Johnston D. Kinase-dependent modification of dendritic excitability after long-term potentiation. J Physiol. 2009;587(Pt 1):115–125. doi: 10.1113/jphysiol.2008.158816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria F, Wils S, De Schutter E, Augustine GJ. Anomalous diffusion in Purkinje cell dendrites caused by spines. Neuron. 2006;52(4):635–648. doi: 10.1016/j.neuron.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serodio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol. 1998;79(2):1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- Shah MM, Hammond RS, Hoffman DA. Dendritic ion channel trafficking and plasticity. Trends Neurosci. doi: 10.1016/j.tins.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Fong DK, Craig AM. Postsynaptic protein mobility in dendritic spines: long-term regulation by synaptic NMDA receptor activation. Mol Cell Neurosci. 2006;31(4):702–712. doi: 10.1016/j.mcn.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Sheng M, Tsaur ML, Jan YN, Jan LY. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992;9(2):271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- Shibata R, Misonou H, Campomanes CR, Anderson AE, Schrader LA, Doliveira LC, Carroll KI, Sweatt JD, Rhodes KJ, Trimmer JS. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem. 2003;278(38):36445–36454. doi: 10.1074/jbc.M306142200. [DOI] [PubMed] [Google Scholar]
- Schrader LA, Anderson AE, Mayne A, Pfaffinger PJ, Sweatt JD. PKA modulation of Kv4.2-encoded A-type potassium channels requires formation of a supramolecular complex. J Neurosci. 2002;22(23):10123–10133. doi: 10.1523/JNEUROSCI.22-23-10123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ellis-Davies GC, Magee JC. Mechanism of the distance-dependent scaling of Schaffer collateral synapses in rat CA1 pyramidal neurons. J Physiol. 2003;548(Pt 1):245–258. doi: 10.1113/jphysiol.2002.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Gibson ES, Dell'Acqua ML. cAMP-dependent protein kinase postsynaptic localization regulated by NMDA receptor activation through translocation of an A-kinase anchoring protein scaffold protein. J Neurosci. 2006;26(9):2391–2402. doi: 10.1523/JNEUROSCI.3092-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague BL, McNally JG. FRAP analysis of binding: proper and fitting. Trends Cell Biol. 2005;15(2):84–91. doi: 10.1016/j.tcb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Spruston N, Jonas P, Sakmann B. Dendritic glutamate receptor channels in rat hippocampal CA3 and CA1 pyramidal neurons. J Physiol. 1995;482(Pt 2):325–352. doi: 10.1113/jphysiol.1995.sp020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Star EN, Kwiatkowski DJ, Murthy VN. Rapid turnover of actin in dendritic spines and its regulation by activity. Nat Neurosci. 2002;5(3):239–246. doi: 10.1038/nn811. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37(2):173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Varga AW, Yuan LL, Anderson AE, Schrader LA, Wu GY, Gatchel JR, Johnston D, Sweatt JD. Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J Neurosci. 2004;24(14):3643–3654. doi: 10.1523/JNEUROSCI.0154-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W, Schlichter LC. Differential recruitment of Kv1.4 and Kv4.2 to lipid rafts by PSD-95. J Biol Chem. 2004;279(1):444–452. doi: 10.1074/jbc.M304675200. [DOI] [PubMed] [Google Scholar]
- Zheng CY, Petralia RS, Wang YX, Kachar B, Wenthold RJ. SAP102 is a highly mobile MAGUK in spines. J Neurosci. 30(13):4757–4766. doi: 10.1523/JNEUROSCI.6108-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Qian Y, Kunjilwar K, Pfaffinger PJ, Choe S. Structural insights into the functional interaction of KChIP1 with Shal-type K(+) channels. Neuron. 2004;41(4):573–586. doi: 10.1016/s0896-6273(04)00045-5. [DOI] [PubMed] [Google Scholar]