Abstract
Background
Although it is recognized that caring for a child with type 1 diabetes (T1D) is stressful for parents, few interventions have been developed and tested for this population.
Objectives
To compare a group educational intervention for parents of children with T1D to a coping skills training intervention.
Method
Parents of children with T1D were randomized to the group educational (n = 106) or coping skills training (n = 75) conditions. Parents completed measures of family conflict, responsibility for treatment, coping, and quality of life at baseline and 3 months, 6 months, and 12 months postintervention. Clinical data (i.e., HbA1c) were collected from children’s medical records pre- and postintervention.
Results
There were no significant treatment effects 12 months postintervention, but parents in both groups reported improved coping (p < .001), less responsibility for treatment management (p < .001), and improved quality of life (p = .005). While children’s metabolic control worsened over time, mean values at 12 months were still within the recommended levels in this well-controlled sample (HbA1c < 8%).
Discussion
Group-based interventions for parents of children with T1D may lessen the impact of treatment management, improving coping and quality of life.
Keywords: coping skills, parents, type 1 diabetes mellitus
Type 1 diabetes (T1D) is one of the most common chronic childhood diseases, affecting 1.54 in 1,000 youth in the USA (Liese et al., 2006). While most often diagnosed in adolescents, the incidence of T1D among younger children appears to be rising (Gale, 2002; Liese et al., 2006). The treatment regimen is complex and demanding, requiring multiple injections and pump adjustments, frequent monitoring of blood glucose levels, diet tracking, and accounting for carbohydrate intake (Doyle & Grey, 2010). Parents take on the responsibility for treatment management, particularly for younger children; interventions are needed to help lessen the impact of T1D on parents and families.
Stress, Coping, and Family Functioning in Parents of Children with T1D
Parenting a child with T1D can be extremely stressful. Parents describe the need for constant vigilance, a sense of continuous responsibility to maintain metabolic control and prevent episodes of hypoglycemia (Sullivan-Bolyai, Deatrick, Gruppuso, Tamborlane, & Grey, 2003). Mothers of younger children may have particularly high levels of worry because their children do not have the cognitive ability to recognize and respond to symptoms of hypoglycemia (American Diabetes Association, 2008). Further, parents of children with T1D report elevated rates of depressive symptoms (Cameron, Northam, Ambler, & Daneman, 2007). Few interventions to ameliorate parents' difficulty in caring for a child with T1D have been developed and tested.
Parental coping with the stress of diabetes is likely to play an important role in child and family adjustment to the disease. The extent to which mothers find coping with diabetes upsetting has been related to maternal distress and to child’s reported quality of life (QOL) in school-age and older children (Whittemore, Urban, Tamborlane, & Grey, 2003). Maternal reports of coping with diabetes as difficult or upsetting have been shown also to mediate the relationship between maternal and child depressive symptoms in school-age children with T1D (Jaser, Whittemore, Ambrosino, Lindemann, & Grey, 2008). Few researchers have examined coping strategies in parents of children with T1D, but Blankfeld and Holahan (1996) found that greater use of approach coping (e.g., problem-solving) was related to fewer symptoms of depression in mothers. The ways that mothers cope with the stress of diabetes management, however, has not been related to children’s metabolic control (Stallwood, 2005). While more research is needed to examine the relationship between parental coping with diabetes-related stress and adjustment to the disease, preliminary findings suggest that parental coping may represent an important target for intervention to improve parental and child psychosocial adjustment to diabetes.
Family functioning, or parent-child relationship quality, has been linked to both metabolic control and psychosocial adjustment in youth with T1D (Whittemore, Kanner, & Grey, 2004). For example, family conflict over diabetes management has been related to poor metabolic control and poorer QOL in youth with T1D (Anderson et al., 2002; Laffel et al., 2003; Lewin et al., 2006). On the other hand, family support for treatment management has been related to better metabolic control in children and adolescents, mediated by adherence to treatment (Lewin et al., 2006). Higher levels of family cohesion also have been shown to predict better adherence to treatment (Cohen, Lumley, Naar-King, Partridge, & Cakan, 2004) and better metabolic control (Jacobson et al., 1994) in youth with T1D. For younger children in particular, family cohesion appears to be a strong predictor of adjustment to diabetes (Whittemore et al., 2004). These findings support the need for family-based interventions that reduce family conflict while promoting family cohesion and supportive involvement.
Family-based Interventions
Family-based interventions for T1D have been focused typically on families of adolescents rather than younger children (e.g., Anderson, Brackett, Ho, & Laffel, 1999; Harris, Harris, & Mertlich, 2005; Wysocki, Greco, Harris, Bubb, & White, 2001; Wysocki et al., 2008). For example, Anderson et al. (1999) found that an office-based intervention to promote parent-child teamwork improved parental involvement in diabetes management and was related to improved metabolic control. Similarly, a behavioral family therapy intervention for adolescents and their families was found to improve metabolic control and parent-child communication over time (Wysocki et al., 2007, 2008). These interventions have shown promise for improving family functioning and adolescent outcomes, but it is important to consider parental outcomes as well. In one of the only studies of interventions for parents of young children with T1D, Sullivan-Bolyai et al. (2010) found that mothers valued the support of experienced parent mentors shortly after diagnosis. A broader focus on coping skills may provide greater benefits for a larger number of families.
Coping Skills Training
Coping skills training (CST) is based on Bandura’s (1986) social cognitive theory, which posits that practicing and rehearsing a new behavior, such as learning how to cope successfully with a problem situation, can enhance self-efficacy and promote positive behaviors. The goal of CST is to increase competence and mastery by retraining nonconstructive coping styles and behaviors into more constructive behaviors. A CST protocol developed for school-aged children to prevent alcohol and drug use (Forman, 1993) was modified for youth with T1D by incorporating stress related to living with T1D (e.g., how to tell friends about diabetes; Davidson, Boland, & Grey, 1997). The CST protocol has been shown to improve family functioning and QOL in adolescents with T1D (Grey, Boland, Davidson, Li, & Tamborlane, 2000; Grey, Boland, Davidson, Sullivan-Bolyai, & Tamborlane, 1998). Parents of younger children with T1D may face additional challenges related to treatment management, and it is not known whether CST would have positive effects on parents of children with T1D.
Framework for Adaptation to Chronic Illness in Childhood
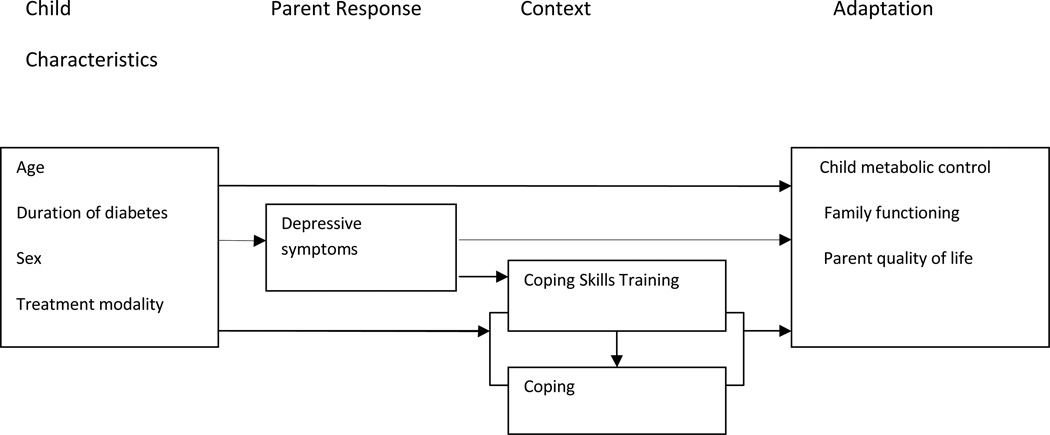
The conceptual framework for this study was a stress-adaptation model viewing adaptation as an active process whereby an individual or family adjusts to the challenges of a chronic illness (Grey & Thurber, 1991). The framework suggests that child characteristics such as age, sex, and health problems; parent responses (depressive symptoms); and context (parent coping) influence the level of child and family adaptation. In this model, adaptation has both physiologic (metabolic control) and psychosocial (family functioning, QOL) components (Figure 1).
Figure 1.
Conceptual Model
Purpose
The purpose of this randomized clinical trial was to determine the effects of a CST program conducted with parents of preadolescent children (ages 1–12 years) with T1D compared to an attention-control group on the outcomes of parental coping, family functioning (i.e., conflict, responsibility for treatment), QOL, and child metabolic control (i.e., HbA1c). The data in this analysis include primarily parent outcomes (see Grey et al., 2009 for a description of child outcomes). A secondary aim was to explore mediators (parental coping) and moderators (child age, sex, other health problems, duration of illness, and treatment modality) on intervention efficacy.
The following hypotheses were tested: (a) parents of children with T1D who participate in CST will demonstrate fewer issues in coping, better family functioning (less parent responsibility and family conflict), and better QOL compared to parents of children with T1D who participate in an education program; (b) children with T1D whose parents participate in CST will demonstrate better metabolic control (lower HbA1c) compared to children whose parents participate in an education program; (c) child’s age, sex, other health problems, duration of illness, and treatment modality will moderate the intervention effect on metabolic control and parent QOL; and (d) changes in parent coping will mediate the intervention effect on metabolic control, family functioning, and parent QOL.
Research Design and Methods
Data from two separate randomized clinical trials of CST interventions (one trial for parents and their school-age children (ages 8–12 years) and the other for parents of young children (< 8 years of age) were combined to achieve a sufficient sample. Two-group experimental designs were used in both trials, in which families were assigned randomly to a CST or a group diabetes education (GE) intervention. Eligible participants were recruited from the pediatric diabetes clinic of a large university-based medical center using a convenience approach. Families were eligible if the child had been diagnosed with T1D for at least 6 months and was in the school grade appropriate to within 1 year of age (if applicable). Data were collected at baseline and 3, 6, and 12 months post intervention for both trials by trained research assistants who were blinded to group assignment. Data collection for the School Age Child study occurred from February 2000 to August 2007 and for the Parents of Younger Children study from December 2002 to August 2007.
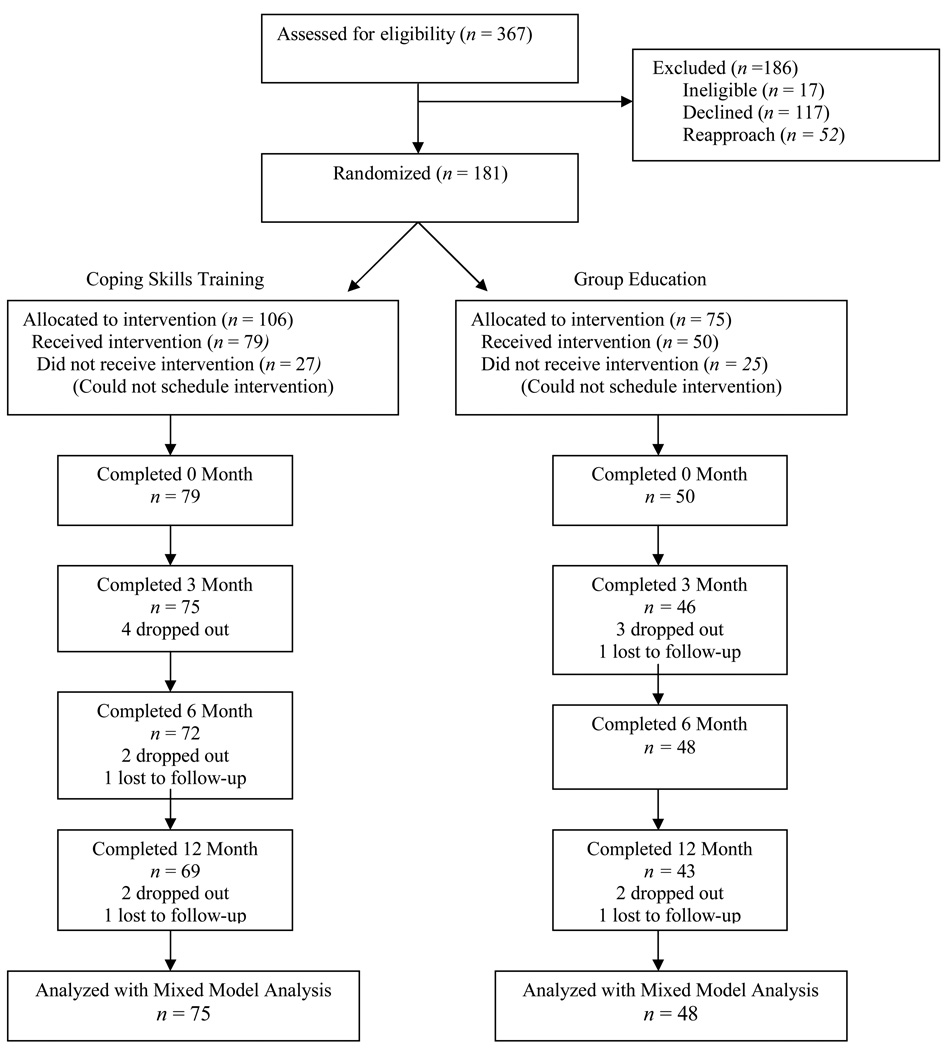
Parents completed the consent process approved by the university institutional review board. After completing baseline questionnaires, participants were randomized using a sealed envelope technique to either the CST or GE condition. Participants’ progress through the trial, in keeping with the Consolidated Standards of Reporting Trials (CONSORT) criteria for clinical trials (Schulz et al, 2010) are summarized in Figure 2. The most common reasons for refusal were that groups were too far away, families were too busy, and parents were not interested. On average, parents randomized to CST attended 4.6 sessions (range = 1–6; SD = 1.21), while those in GE attended 3.3 (range = 1–4; SD = .75).
Figure 2.
Consort Table
Interventions
Coping skills intervention and training
The primary goal of CST is to replace inappropriate or unconstructive coping styles with more positive and adaptive behaviors, thereby increasing children’s and parents’ sense of competence and mastery. Content was emphasized on coping with day-to-day problems, and managing the thoughts, feelings, and behaviors that arise from daily stress related to T1D management. Coping skills addressed were communication (including social skills and assertiveness), social problem-solving, cognitive restructuring (e.g., positive self-talk), stress management, and conflict resolution (Davidson, Boland, & Grey, 1997). These were taught in an interactive way through the use of role play techniques and discussion for maximal skill development. Six sessions (one per coping skill) lasting 1.5 hours were conducted in small groups of parents of 2–5 families. Although only one parent provided data, both parents were encouraged to attend. The content of sessions was tailored to the developmental level of the child (i.e., groups for parents of school-aged children used different examples than those for parents of younger children). Sessions were facilitated by a health professional (marriage and family therapist, clinical psychologist). All CST groups (n = 26) were audiotaped and reviewed for treatment fidelity.
Group education
Diabetes education is provided at diagnosis and as necessary in routine clinic appointments to assure adequate self and family management of the disease as the standard of care. Thus, all children and families had received diabetes education. An equal-attention GE program provided updates in T1D care as the control condition. The GE program was focused on intensive insulin regimens (multiple daily injections or pump), carbohydrate counting, managing sick days, and intake and insulin adjustments for sports. All GE groups (n = 11) were audiotaped to ensure fidelity. Four sessions (one for each topic) for small groups of parents (2–5 families) lasting 1.5 hours each were led by a nurse who was a certified diabetes educator (see Grey et al., 2009 for further description of interventions).
Instruments
Disease-related variables were collected from each child’s medical record including duration of T1D and treatment regimen (injection or pump).
Metabolic control was assessed via HbA1c using the Bayer Diagnostics DCA2000® (Tarrytown, NY), which has evidence of high reliability (normal range = 4.2–6.3%). The ADA recommendation for the treatment goal for children age 6–12 years is < 8%, and for children under 6 is 7.5–8.5% (Silverstein et al., 2005).
Demographic information was reported by parents and included the parent or guardian’s relationship to child, age, and sociodemographic data; and child’s gender, age, and presence of other health problems. Parents of children with other health problems were recruited if the problem was mild or controlled with medication (e.g., hypothyroidism).
The Issues in Coping with IDDM-Parent scale (ICC; Kovacs, Brent, Feinberg, Paulauskas, & Reid, 1986) was used to measure parents’ issues in coping with their child’s diabetes. The ICC is a self-report measure of how difficult (25 items; total score 0–100) and how upsetting (32 items; total score 0–128) parents find it to cope with issues related to the child’s T1D management. Cronbach’s alpha coefficients were 0.82 and 0.74 for the difficult and upsetting subscales, respectively. To limit multicollinearity, the mean of the two scales was used in all analyses as an overall coping score. Higher scores indicate coping with diabetes is more difficult and more upsetting.
The Center for Epidemiologic Studies - Depression Scale (CES-D; Radloff, 1977) is a brief, self-report screening measure of depressive symptoms developed by the National Institutes of Mental Health (Radloff, 1977). A total score is calculated from 20 items and ranges from 0 to 60. Higher scores indicate greater depressive symptoms; 16 serves as a clinical cut-off, indicating the need for further evaluation. This measure is used widely with clinical and community samples. Parents who exhibited high levels of depressive symptoms (CES-D score ≥ 16) were referred for follow-up, but not excluded from the intervention. Cronbach’s alpha coefficient was 0.77 for the total score.
The Diabetes Responsibility and Conflict Scale (DRC; Rubin, Young-Hyman, & Peyrot, 1989) is a self-report measure designed to evaluate the distribution of diabetes-related responsibilities for parent and child (15 items; total score 15–75) and the degree of diabetes-related conflict encountered by parent and child (15 items; total score 15–75). Higher scores reflect greater parent responsibility and more parent-child conflict, respectively. In these data, Cronbach’s alpha coefficients were 0.87 and 0.94 for the responsibility and conflict subscales.
The Parents Diabetes Quality of Life Questionnaire (PDQOL) was adapted by Vandagriff et al. (1992) to assess parents’ perceptions of the impact of diabetes treatment on their general satisfaction with life. The PDQOL has established reliability and validity and has been used in several previous studies of parents of children with T1D (e.g., Faulkner & Clarke, 1988). There are three subscales: Diabetes Life Satisfaction (18 items); Disease Impact (21 items); and Disease-related Worries (8 items). In the current analyses, only the Disease Impact subscale was used due to the high correlation between subscales (r = .84 with Worries subscale, r = −.55 with Satisfaction subscale). Scores range from 21 to 84, and higher scores indicate greater negative impact. Internal reliability for the present sample was 0.88 for the Impact scale.
Data Analyses
All data were double-entered and checked for accuracy. Analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC). Groups were compared on baseline characteristics using t-tests for continuous variables and chi-square analyses for categorical variables (Table 1).
Table 1.
Comparison of Baseline Characteristics
| Coping Skill Training (n = 79) |
Group Education (n = 50) |
p (chi-square Test) |
|
|---|---|---|---|
| Treatment Type | |||
| Injection | 24 (30.4%) | 10 (21.3%) | .266 |
| Insulin Pump | 55 (69.6%) | 37 (78.7%) | |
| Child Gender | |||
| Male | 36 (45.6%) | 17 (35.4%) | .260 |
| Female | 43 (54.4%) | 31 (64.6%) | |
| CES-D at baseline | |||
| <16 | 56 (71.8%) | 43 (86.0%) | .061 |
| ≥ 16 | 22 (28.2%) | 7 (14.0%) | |
| Mean (SD) | Mean (SD) | p (t-test) | |
| Child Age | 8.1 (2.9) | 7.9 (2.8) | .674 |
| HbA1C | 7.0 (1.2) | 7.0 (1.0) | .842 |
| CES-D | 11.6 (7.8) | 9.6 (7.1) | .136 |
| ICC | 39.5 (9.5) | 35.2 (7.5) | .008** |
| DRC Responsibility | 56.0 (10.3) | 55.2 (12.1) | .688 |
| DRC Conflict | 26.9 (11.4) | 22.6 (10.6) | .036* |
Notes. CES-D = Center for Epidemiologic Studies-Depression Scale; ICC = Issues in Coping with Diabetes Scale; DRC = Diabetes Responsibility and Conflict Scale
p < .05.
p < .01.
To determine the effect of CST for parents of children with T1D compared to the GE group, a random coefficient regression analysis was used with an intent-to-treat approach, in which all subjects were included in the data analysis, as randomized, regardless of whether they withdrew or deviated from the protocol (Fisher et al., 1990). The purpose of the approach is to preserve balance in the characteristics of groups achieved by randomization, and to guard against a potential bias in the outcomes from differential drop-outs. To control for multiple tests, alpha was set at p < .010.
The SAS Proc Mixed routine was used to perform the random coefficient regression analysis, in which missing outcome data are treated as missing at random (MAR; i.e., given the previous outcome values and covariables, the missingness is independent of unobserved outcomes; Rubin, 1976). Outcomes of interest included metabolic control (HbA1c), parent coping, family functioning, and parent QOL. Random coefficient models included intervention group, time, and the group-by-time interaction as fixed effects, along with random effects for subject-specific intercepts and slopes. This allowed each participant to have his or her own initial value of the outcome and the trajectory of change in the outcome. Differences in slopes (rates of change) between the two treatment groups, obtained from an interaction of treatment group-by-time in the regression model, were used to evaluate intervention efficacy. For an overall effect of time on each outcome of interest, regardless of group assignment, the group-by-time interaction was removed, and the main effect of time was evaluated. These analyses controlled for child gender, age, other health problems, treatment type (pump vs. injections), and parental depression at baseline. Results are presented as annual rates of change for each intervention group and combined across both groups.
Additional analyses were conducted to determine for whom and how the treatment may have worked by exploring potential mediators and moderators of intervention efficacy (Kraemer, Wilson, Fairburn, & Agras, 2002). Based on previous research and the conceptual framework (Grey & Thurber, 1991), the pre-existing characteristics of child age, gender, treatment type, and other health problems were evaluated as moderators of treatment by testing the interaction with treatment group and time. Following the recommendations of Kraemer et al. (2002), the proposed mediator of changes in outcomes (coping) was tested with partial correlations of change in coping with change in outcome variables at 6 months and 12 months, controlling for baseline coping.
Results
Preliminary Analyses
Demographic and clinical characteristics of the participants are shown in Table 1. Overall, parents reported moderate issues in coping related to diabetes (upset and difficulty), little negative impact on QOL, low levels of conflict related to diabetes care, and moderately high levels of responsibility for diabetes care. At baseline, 22% of parents reported elevated depressive symptoms. Parents in the CST group reported significantly greater issues related to coping, impact on QOL, and diabetes-related family conflict at baseline compared to parents in the GE group (Table 1). The baseline values were controlled for in subsequent analyses.
Intervention Efficacy
There were no significant treatment effects on any outcome variable (Table 2). As seen in Table 3, when rates of change over time were examined across both groups, there was improvement in parental coping (p < .001) and QOL (p = .005). Both groups also reported a significant decrease in parental responsibility for diabetes-related tasks over 12 months (p < .001), indicating that children were taking on more responsibility for their treatment management over time. There was also a significant increase over time in children’s HbA1c levels in both groups (p < .001). There were no significant changes in diabetes-related conflict. Number of sessions attended was not related significantly to changes in outcomes.
Table 2.
Means and Standard Deviations of Outcomes Between CST and GE Groups from Baseline to 12 Months
| Mean (SD) | ||||||
|---|---|---|---|---|---|---|
| Measure | Group | Baseline | 3 months | 6 months | 12 months | p |
| Issues in Coping | CST | 39.5 (9.7) | 37.3 (10.6) | 35.2 (9.8) | 36.5 (11.7) | .881 |
| GE | 35.2 (7.5) | 33.1 (7.6) | 31.3 (6.5) | 32.5 (6.6) | ||
| HbA1c | CST | 7.0 (1.2) | 7.2 (1.0) | 7.3 (1.1) | 7.5 (1.0) | .596 |
| GE | 7.0 (1.0) | 7.2 (1.1) | 7.3 (1.3) | 7.4 (1.2) | ||
| Quality of Life Impact | CST | 35.5 (9.4) | 33.7 (8.3) | 33.6 (7.2) | 33.6 (8.0) | .818 |
| GE | 32.0 (5.4) | 31.0 (4.9) | 30.2 (5.5) | 30.6 (5.7) | ||
| DRC Responsibility | CST | 56.0 (10.2) | 52.5 (11.0) | 52.8 (10.2) | 49.6 (11.6) | .298 |
| GE | 55.2 (12.1) | 52.4 (11.9) | 50.5 (12.3) | 49.6 (11.4) | ||
| DRC Conflict | CST | 26.9 (11.4) | 24.9 (9.4) | 25.2 (7.7) | 25.5 (10.1) | .581 |
| GE | 22.6 (10.6) | 22.3 (9.2) | 21.8 (7.8) | 22.2 (8.1) | ||
Notes. Values were produced from longitudinal mixed models for interaction between the intervention and time after controlling for child’s gender, age, other health problems, insulin treatment (insulin pump vs. injection) and parental depression (CES-D) at baseline. DRC = Diabetes Responsibility and Conflict
Table 3.
Means and Standard Deviations of Outcomes from Baseline to 12 Months in Combined Groups
| Mean (SD) | |||||
|---|---|---|---|---|---|
| Baseline (n = 129) |
3 months (n = 121) |
6 months (n = 120) |
12 months (n = 112) |
p | |
| Issues in Coping | 37.8 (9.1) | 35.7 (9.8) | 33.6 (8.8) | 34.9 (10.2) | < .001 |
| HbA1c | 7.0 (1.1) | 7.2 (1.1) | 7.3 (1.2) | 7.4 (1.1) | < .001 |
| Quality of Life Impact | 34.1 (8.3) | 32.7 (7.3) | 32.3 (6.8) | 32.5 (7.3) | .005 |
| DRC Responsibility | 55.7 (11.0) | 52.5 (11.3) | 51.8 (11.1) | 49.6 (11.5) | < .001 |
| DRC Conflict | 25.2 (11.2) | 23.9 (9.4) | 23.8 (7.9) | 24.2 (9.5) | .696 |
Notes. Values were produced from longitudinal mixed models for time effect after controlling for group, child’s gender, age, other health problem, treatment type (insulin pump vs. injection) and parental depression (CES-D) at baseline. DRC = Diabetes Responsibility and Conflict
Moderators
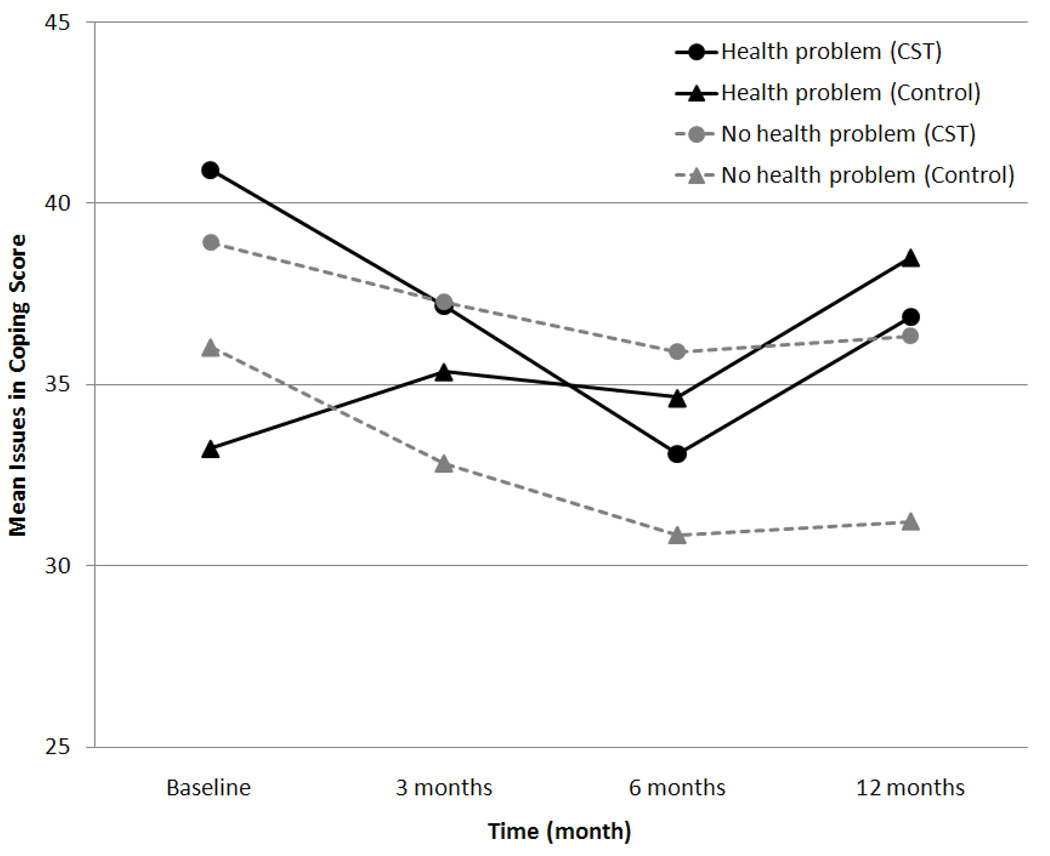
Child age, gender, treatment type, diabetes duration, and other health problems were included as interaction terms in the model to test for moderation. There was a significant treatment-group by health problems by time interaction for coping (p < .001). Among parents of children with other health problems (n = 24), parents in the CST group reported fewer issues in coping with diabetes over 12 months (Figure 3). There was also a significant health problems by time interaction for diabetes-related family conflict (p = .030), such that parents of children with no other health problems (n = 95) reported less conflict over time, and parents of children with other health problems reported no change in conflict over time (data not shown). Child age, gender, diabetes duration, and treatment type were not significant moderators of treatment effects on outcomes.
Figure 3.
Child health problems as a moderator of the effect of the intervention on coping. In the CST group, 16 had health problems, 53 had no health problems; in the Group Education group, 8 had health problems, 42 had no health problems.
Effects on Coping
Based on the conceptual model, we examined the effects of the intervention on coping. In line with Kraemer et al. (2002), changes in the proposed mediator (coping) were tested in relation to changes in outcomes (i.e., family conflict, responsibility for diabetes care, QOL, and HbA1c) across intervention groups. Results revealed that 3-month decreases in upset and difficulty in coping with diabetes were associated with 3-month improvements in HbA1c (r = .36, p = .005). Further, such decreases in issues with coping were related to decreases in parental responsibility for diabetes care over 3, 6, and 12 months (r = .45, .36, and .40, respectively; all p < .005).
Discussion
The purpose of this study was to examine the efficacy of a group-based CST intervention for parents of children with T1D compared to a GE group. The primary hypothesis that parents of the CST intervention would demonstrate better coping, family functioning, and QOL was not supported. In addition, there was no intervention effect on child metabolic control in this sample with excellent metabolic control at baseline.
Although significant effects for CST were not found, several significant time effects were demonstrated in this study. Parents of children who received either CST or GE reported significantly fewer issues in coping, better parental QOL, and less parental responsibility for diabetes management over time. Perceptions of family conflict remained stable over time. These are important findings, since psychosocial difficulties are common in parents of children with a chronic condition (Blankfeld & Holahan, 1996; Streisand, Swift, Wickmark, Chen, & Holmes, 2005). In addition, less parental responsibility over time with diabetes management is an interesting finding in this study with school-aged and younger children. While research supports the need for gradual transfer of responsibility from parent to child as developmentally appropriate, this typically occurs in the preteen and teen years (Anderson et al., 2002; Wiebe et al., 2005). It is possible that less parental responsibility over time is an indication of a change in treatment modality from multiple injections to a continuous infusion pump. Although there was a significant worsening of metabolic control over time across groups, as would be expected as children reach puberty (ADA, 2008), it is important to note that the mean levels at 12 months (7.3%) were still well within the goal range for children (HbA1c < 8.0%, ADA, 2008). The intervention might have had stronger effects on metabolic control if it was targeted to those families whose children had poorer metabolic control (e.g., Harris, Harris, & Mertlich, 2005). Collectively, these findings highlight the delicate balance between family functioning, parental coping with stress of T1D, child responsibility for diabetes care, and metabolic control.
While further research is indicated, the results suggest that regardless of content, group-based interventions for parents of children with T1D improved family functioning. Although most diabetes education is provided individually for children, providing education in a group might facilitate social support from peers. This social support has been shown to improve outcomes in parents of young children with T1D (Sullivan-Bolyai et al., 2004). Anecdotal reports from parents and the study interventionist for the GE sessions indicated that a supportive group process occurred within the context of providing diabetes-specific education. The findings suggest that a direction for future research may be to determine whether group-based diabetes education programs would be more clinically effective and cost-effective than education for individual families of children with T1D.
Results of the moderator analyses provide a beginning identification of underlying mechanisms of the potential benefit of CST. Parents of children with additional health problems had a greater intervention effect of CST compared to parents of children with T1D who reported no other child health problems. Notably, parents of children with major health disorders in addition to diabetes were not eligible to participate in the study, so the additional health problems were relatively minor or controlled with medication. Nonetheless, the data suggest that the presence of another health problem places increased stress and demands on parents, which may make them more responsive to the CST program.
In addition, improvements in parental coping were associated with decreased parental responsibility for diabetes management and improvement in metabolic control in the short term. Premature relinquishment of parental responsibility for diabetes management can lead to deterioration in metabolic control (Anderson et al., 2002). On the other hand, prolonged overmanagement by parents can lead to increased parent-child conflict (Anderson et al., 2002). Helping parents manage this delicate transition through training in coping skills may lead to a smoother transfer of responsibility for diabetes management and ultimately to better metabolic control. Further research targeting parental coping in families with a child with T1D is indicated.
Findings of this study must be interpreted in light of several limitations. The sample was primarily White, of middle to upper socioeconomic status, with parents reporting good QOL at baseline; thus, results may not be generalized to other populations. The majority of children used an insulin pump and demonstrated excellent metabolic control at baseline, creating a floor effect for that variable. Mean HbA1c levels in the sample are not reflective of other studies of youth with T1D (e.g., Valenzuela et al., 2006) and may reflect that pump therapy is strongly encouraged at the study clinic. Finally, data about child characteristics (e.g., temperament, behavior problems) was not available to test the potential pathway of child characteristics to parent response (e.g., depressive symptoms).
Despite these limitations, there are several important clinical and research implications. Parents of children with T1D were successful in carrying out the intense treatment regimen, as evidenced by excellent metabolic control in their children. Although parents in this sample reported good QOL, moderate issues in coping with diabetes were expressed, and 22% of parents demonstrated elevated depressive symptoms at baseline. Thus, these findings highlight the importance of screening for issues in coping with diabetes and depressive symptoms in parents of children with T1D. The ADA recommends annual screening of depression in youth with T1D (Silverstein et al., 2005); assessment of parental depression may be equally important (Cameron et al., 2007).
Group-based GE or CST may be helpful options also for parents of children with T1D. Ideally, parents of the child with T1D are equipped with the education and support to promote their own well-being and psychosocial adjustment. Better family functioning and positive psychosocial adjustment of parents has been shown to be predictive of better psychosocial adjustment of children with varied chronic conditions (Drotar, 1997). Indeed, to care effectively for a child with T1D, the health of parents must be maintained. Research with families of children with chronic conditions has shown that family caregivers often need social and professional support (Boling, 2005). In addition, assessment for parental coping and referral for supportive interventions appears particularly important when a child with T1D also has more than one chronic condition. Further research is needed to determine if CST may be beneficial for parents of children with suboptimal metabolic control.
Conclusion
Although CST did not have the expected effect on parent and family outcomes in this relatively well-adjusted sample of parents of children with T1D, the data suggest that supportive group-based educational and behavioral interventions may be associated with improved parental and diabetes outcomes. Such interventions need to be investigated further to determine if it is appropriate to provide group-based diabetes care programs for families coping with diabetes.
Acknowledgments
Funding for this study was provided by grant R01 NR04009 from the National Institute of Nursing Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Margaret Grey, Yale University School of Nursing, New Haven, Connecticut.
Sarah S. Jaser, Yale University School of Nursing, New Haven, Connecticut.
Robin Whittemore, Yale University School of Nursing, New Haven, Connecticut.
Sangchoon Jeon, Yale University School of Nursing, New Haven, Connecticut.
Evie Lindemann, Albertus Magnus College, New Haven, Connecticut.
References
- American Diabetes Association. Standards of medical care in diabetes - 2008. Diabetes Care. 2008;31:S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Brackett J, Ho J, Laffel LM. An office-based intervention to maintain parent-adolescent teamwork in diabetes management. Impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care. 1999;22(5):713–721. doi: 10.2337/diacare.22.5.713. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Vangsness L, Connell A, Butler D, Goebel-Fabbri A, Laffel LM. Family conflict, adherence, and glycaemic control in youth with short duration type 1 diabetes. Diabetic Medicine. 2002;19(8):635–642. doi: 10.1046/j.1464-5491.2002.00752.x. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: The exercise of control. New York, NY: W. H. Freeman; 1986. [Google Scholar]
- Blankfeld DF, Holahan CJ. Family support, coping strategies, and depressive symptoms among mothers of children with diabetes. Journal of Family Psychology. 1996;10:173–179. [Google Scholar]
- Boling W. The health of chronically ill children: Lessons learned from assessing family caregiver quality of life. Family & Community Health. 2005;28(2):176–183. doi: 10.1097/00003727-200504000-00009. [DOI] [PubMed] [Google Scholar]
- Cameron FJ, Northam EA, Ambler GR, Daneman D. Routine psychological screening in youth with type 1 diabetes and their parents: A notion whose time has come? Diabetes Care. 2007;30(10):2716–2724. doi: 10.2337/dc07-0603. [DOI] [PubMed] [Google Scholar]
- Cohen DM, Lumley MA, Naar-King S, Partridge T, Cakan N. Child behavior problems and family functioning as predictors of adherence and glycemic control in economically disadvantaged children with type I diabetes: A prospective study. Journal of Pediatric Psychology. 2004;29(3):171–184. doi: 10.1093/jpepsy/jsh019. [DOI] [PubMed] [Google Scholar]
- Davidson M, Boland EA, Grey M. Teaching teens to cope: Coping skills training for adolescents with insulin-dependent diabetes mellitus. Journal of the Society of Pediatric Nursing. 1997;2(2):65–72. doi: 10.1111/j.1744-6155.1997.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Doyle EB, Grey M. Diabetes mellitus (type 1 and type 2) In: Jackson Allen PL, Vessey J, editors. Primary care of the child with a chronic condition. 5th ed. St. Louis, MO: Mosby Elsevier; 2010. pp. 427–446. [Google Scholar]
- Drotar D. Relating parent and family functioning to the psychological adjustment of children and with chronic health conditions: What have we learned? What do we need to know? Journal of Pediatric Psychology. 1997;22(2):149–165. doi: 10.1093/jpepsy/22.2.149. [DOI] [PubMed] [Google Scholar]
- Faulkner MS, Clarke FS. Quality of life for parents with children and adolescents with type 1 diabetes. The Diabetes Educator. 1998;24(6):721–727. doi: 10.1177/014572179802400607. [DOI] [PubMed] [Google Scholar]
- Fisher L, Dixon D, Herson J, Frankowski RK, Hearron MS, Peace KE. Intention to treat in clinical trials. In: Peace KE, editor. Statistical issues in drug research and development. New York, NY: Marcel Dekker; 1990. [Google Scholar]
- Forman S. Coping skills interventions for children and adolescents. San Francisco, CA: Jossey-Bass; 1993. [Google Scholar]
- Gale EAM. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51(12):3353–3361. doi: 10.2337/diabetes.51.12.3353. [DOI] [PubMed] [Google Scholar]
- Grey M, Boland EA, Davidson M, Li J, Tamborlane WV. Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life. The Journal of Pediatrics. 2000;137(1):107–113. doi: 10.1067/mpd.2000.106568. [DOI] [PubMed] [Google Scholar]
- Grey M, Boland EA, Davidson M, Yu C, Sullivan-Bolyai S, Tamborlane WV. Short-term effects of coping skills training as adjunct to intensive therapy in adolescents. Diabetes Care. 1998;21(6):902–908. doi: 10.2337/diacare.21.6.902. [DOI] [PubMed] [Google Scholar]
- Grey M, Thurber FW. Adaptation to chronic illness in childhood: Diabetes mellitus. Journal of Pediatric Nursing. 1991;6(5):302–309. [PubMed] [Google Scholar]
- Grey M, Whittemore R, Jaser SS, Ambrosino J, Lindemann E, Liberti L, et al. Effects of coping skills training in school-age children with type 1 diabetes. Research in Nursing & Health. 2009;32(4):405–418. doi: 10.1002/nur.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MA, Harris BS, Mertlich D. Brief report: In-home family therapy for adolescents with poorly controlled diabetes: Failure to maintain benefits at 6-month follow-up. Journal of Pediatric Psychology. 2005;30(8):683–688. doi: 10.1093/jpepsy/jsi055. [DOI] [PubMed] [Google Scholar]
- Jacobson AM, Hauser ST, Lavori P, Willett JB, Cole CF, Wolfsdorf JI, et al. Family environment and glycemic control: A four-year prospective study of children and adolescents with insulin-dependent diabetes mellitus. Psychosomatic Medicine. 1994;56(5):401–409. doi: 10.1097/00006842-199409000-00004. [DOI] [PubMed] [Google Scholar]
- Jaser SS, Whittemore R, Ambrosino JM, Lindemann E, Grey M. Mediators of depressive symptoms in children with type 1 diabetes and their mothers. Journal of Pediatric Psychology. 2008;33(5):509–519. doi: 10.1093/jpepsy/jsm104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Brent D, Steinberg TF, Paulauskas S, Reid J. Children's self-reports of psychologic adjustment and coping strategies during first year of insulin-dependent diabetes mellitus. Diabetes Care. 1986;9(5):472–479. doi: 10.2337/diacare.9.5.472. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Wilson T, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry. 2002;59(10):877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- Laffel LM, Connell A, Vangness L, Goebel-Fabbri A, Mansfield A, Anderson BJ. General quality of life in youth with type 1 diabetes: Relationship to patient management and diabetes-specific family conflict. Diabetes Care. 2003;26(11):3067–3073. doi: 10.2337/diacare.26.11.3067. [DOI] [PubMed] [Google Scholar]
- Lewin AB, Heidgerken AD, Geffken GR, Williams LB, Storch EA, Gelfand KM, et al. The relation between family factors and metabolic control: The role of diabetes adherence. Journal of Pediatric Psychology. 2006;31(2):174–183. doi: 10.1093/jpepsy/jsj004. [DOI] [PubMed] [Google Scholar]
- Liese AD, D'Agostino RB, Jr, Hamman RF, Kilgo PD, Lawrence JM, Liu LL, et al. The burden of diabetes mellitus among US youth: Prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118(4):1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592. [Google Scholar]
- Rubin RR, Young-Hyman D, Peyrot M. Parent-child responsibility and conflict in diabetes care. Diabetes. 1989;38 Supplement 2:28A. [Google Scholar]
- Schulz KF, Altman DG, Moher D for the CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomized trials. British Medical Journal. 2010;340:c332. [Google Scholar]
- Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, et al. Care of children and adolescents with type 1 diabetes: A statement of the American Diabetes Association. Diabetes Care. 2005;28(1):184–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- Stallwood L. Influence of caregiver stress and coping on glycemic control of young children with diabetes. Journal of Pediatric Health Care. 2005;19(5):293–300. doi: 10.1016/j.pedhc.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Streisand R, Swift E, Wickmark T, Chen R, Holmes CS. Pediatric parenting stress among parents of children with type 1 diabetes: The role of self-efficacy, responsibility, and fear. Journal of Pediatric Psychology. 2005;30(6):513–521. doi: 10.1093/jpepsy/jsi076. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Bova C, Leung K, Trudeau T, Lee M, Gruppuso P. Social Support to Empower Parents (STEP): An intervention for parents of young children newly diagnosed with type 1 diabetes. The Diabetes Educator. 2010;36(1):88–97. doi: 10.1177/0145721709352384. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Deatrick J, Gruppuso P, Tamborlane W, Grey M. Constant vigilance: Mothers' work parenting young children with type 1 diabetes. Journal of Pediatric Nursing. 2003;18(1):21–29. doi: 10.1053/jpdn.2003.4. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Grey M, Deatrick J, Gruppuso P, Giraitis P, Tamborlane W. Helping other mothers effectively work at raising young children with type 1 diabetes. The Diabetes Educator. 2004;30(3):476–484. doi: 10.1177/014572170403000319. [DOI] [PubMed] [Google Scholar]
- Valenzuela JM, Patino AM, McCullough J, Ring C, Sanchez J, Eidson M, et al. Insulin pump therapy and health-related quality of life in children and adolescents with type 1 diabetes. Journal of Pediatric Psychology. 2006;31(6):650–660. doi: 10.1093/jpepsy/jsj088. [DOI] [PubMed] [Google Scholar]
- Vandagriff JL, Marrero DG, Ingersoll GM, Fineberg NS. Parents of children with diabetes: What are they worried about? The Diabetes Educator. 1992;18(4):299–302. doi: 10.1177/014572179201800407. [DOI] [PubMed] [Google Scholar]
- Whittemore R, Kanner S, Grey M. The influence of family on physiological and psychosocial health in youth with type 1 diabetes: A systematic review. In: Melnyk B, Fineat-Overholt E, editors. Evidence-Based Practice in Nursing and Healthcare: A Guide to Best Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. CD22–73–CD22–87. [Google Scholar]
- Whittemore R, Urban AD, Tamborlane WV, Grey M. Quality of life in school-aged children with type 1 diabetes on intensive treatment and their parents. The Diabetes Educator. 2003;29(5):847–854. doi: 10.1177/014572170302900514. [DOI] [PubMed] [Google Scholar]
- Wiebe DJ, Berg CA, Korbel C, Palmer DL, Beveridge RM, Upchurch R, et al. Children's appraisals of maternal involvement in coping with diabetes: Enhancing our understanding of adherence, metabolic control, and quality of life across adolescence. Journal of Pediatric Psychology. 2005;30(2):167–178. doi: 10.1093/jpepsy/jsi004. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Greco P, Harris MA, Bubb J, White NH. Behavior therapy for families of adolescents with diabetes: Maintenance of treatment effects. Diabetes Care. 2001;24(3):441–446. doi: 10.2337/diacare.24.3.441. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Mauras N, et al. Randomized trial of behavioral family systems therapy for diabetes: Maintenance of effects on diabetes outcomes in adolescents. Diabetes Care. 2007;30(3):555–560. doi: 10.2337/dc06-1613. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Taylor A, et al. Randomized, controlled trial of Behavioral Family Systems Therapy for diabetes: Maintenance and generalization of effects on parent-adolescent communication. Behavior Therapy. 2008;39(1):33–46. doi: 10.1016/j.beth.2007.04.001. [DOI] [PubMed] [Google Scholar]