Abstract
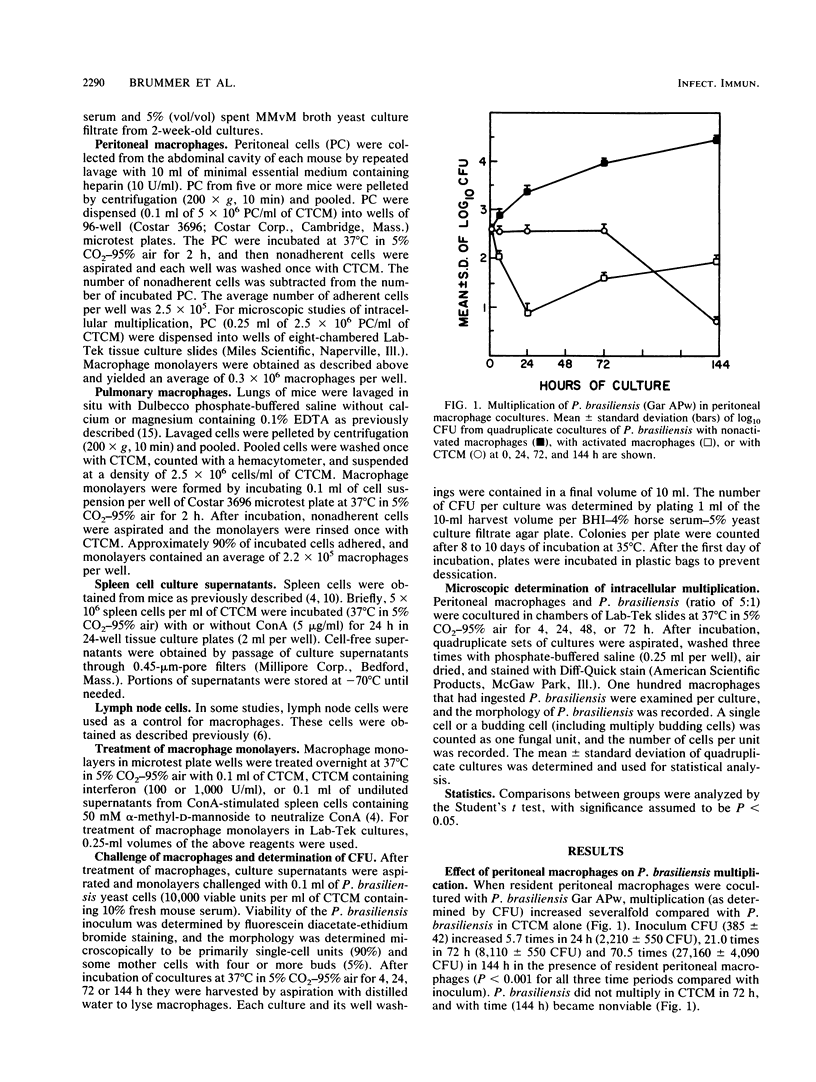
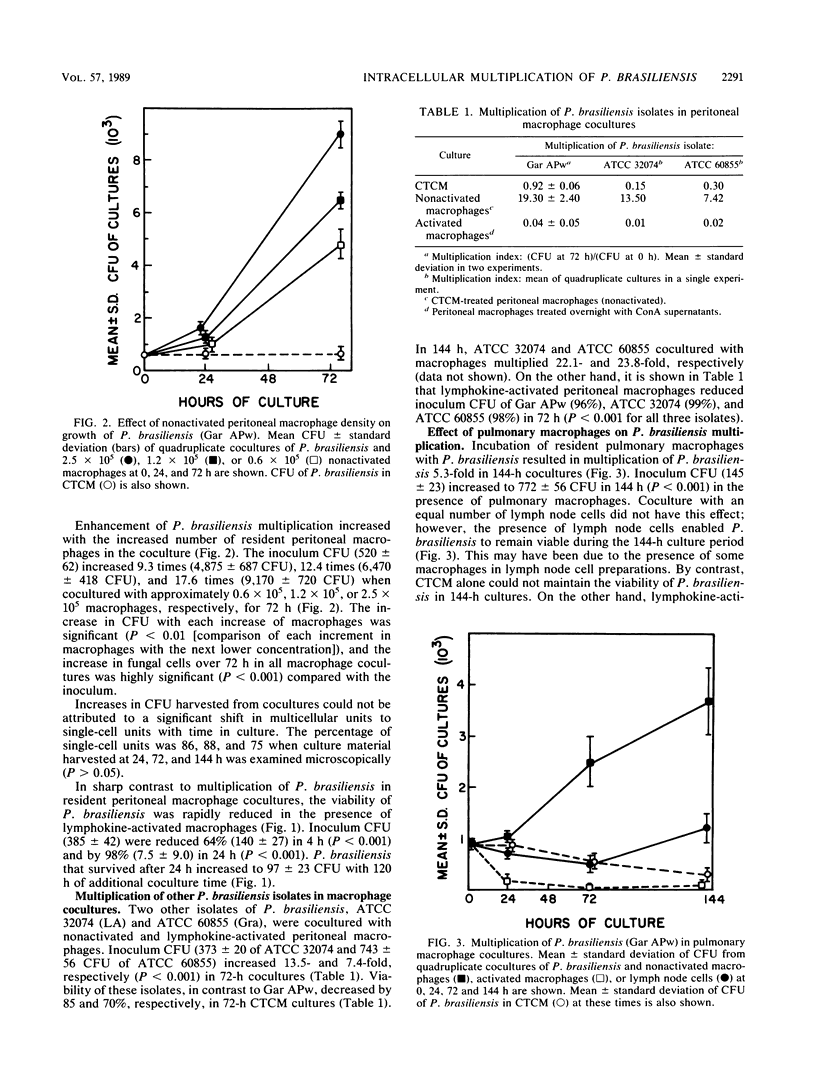
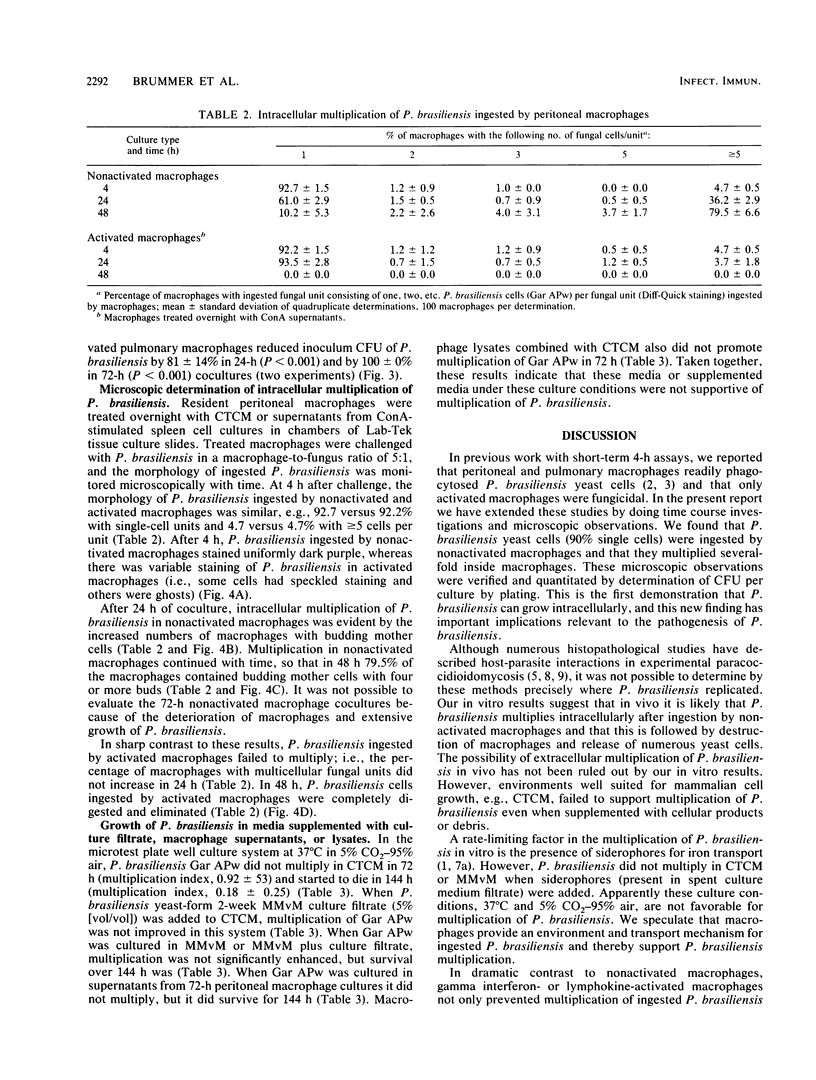
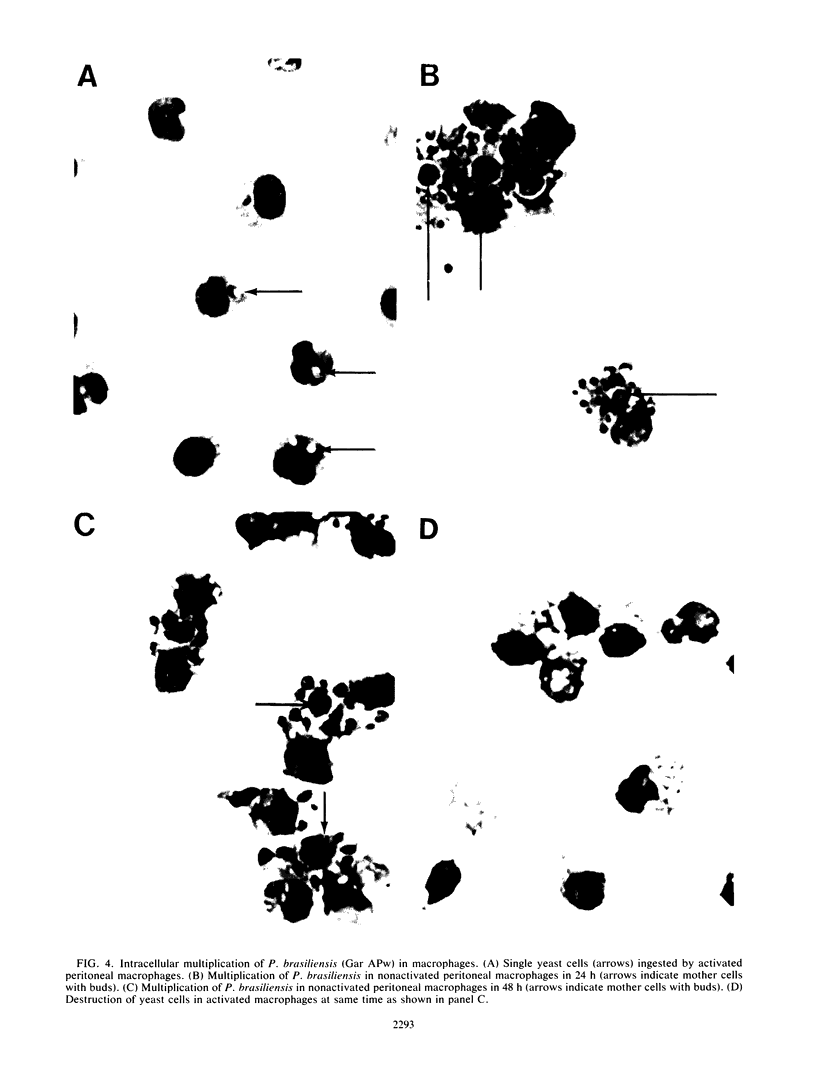
The effect of coculturing yeast-form Paracoccidioides brasiliensis with murine cells was studied. Coculture of resident peritoneal or pulmonary macrophages with P. brasiliensis for 72 h dramatically enhanced fungal multiplication 19.3 +/- 2.4- and 4.7 +/- 0.8-fold, respectively, compared with cocultures with lymph node cells or complete tissue culture medium alone. Support of P. brasiliensis multiplication by resident peritoneal macrophages was macrophage dose dependent. Lysates of macrophages, supernatants from macrophage cultures, or McVeigh-Morton broth, like complete tissue culture medium, did not support multiplication of P. brasiliensis in 72-h cultures. Time course microscopic studies of cocultures in slide wells showed that macrophages ingested P. brasiliensis cells and that the ingested cells multiplied intracellularly. In sharp contrast to resident macrophages, lymphokine-activated peritoneal and pulmonary macrophages not only prevented multiplication but reduced inoculum CFU by 96 and 100%, respectively, in 72 h. Microscopic studies confirmed killing and digestion of P. brasiliensis ingested by activated macrophages in 48 h. These findings indicate that resident macrophages are permissive for intracellular multiplication of P. brasiliensis and that this could be a factor in pathogenicity. By contrast, activated macrophages are fungicidal for P. brasiliensis.
Full text
PDF

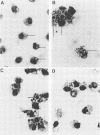
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arango R., Restrepo A. Growth and production of iron chelants by Paracoccidioides brasiliensis mycelial and yeast forms. J Med Vet Mycol. 1988 Apr;26(2):113–118. [PubMed] [Google Scholar]
- Brummer E., Hanson L. H., Restrepo A., Stevens D. A. In vivo and in vitro activation of pulmonary macrophages by IFN-gamma for enhanced killing of Paracoccidioides brasiliensis or Blastomyces dermatitidis. J Immunol. 1988 Apr 15;140(8):2786–2789. [PubMed] [Google Scholar]
- Brummer E., Hanson L. H., Stevens D. A. Gamma-interferon activation of macrophages for killing of Paracoccidioides brasiliensis and evidence for nonoxidative mechanisms. Int J Immunopharmacol. 1988;10(8):945–952. doi: 10.1016/0192-0561(88)90041-0. [DOI] [PubMed] [Google Scholar]
- Brummer E., Morrison C. J., Stevens D. A. Recombinant and natural gamma-interferon activation of macrophages in vitro: different dose requirements for induction of killing activity against phagocytizable and nonphagocytizable fungi. Infect Immun. 1985 Sep;49(3):724–730. doi: 10.1128/iai.49.3.724-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., Restrepo A., Stevens D. A., Azzi R., Gomez A. M., Hoyos G. L., McEwen J. G., Cano L. E., de Bedout C. Murine model of paracoccidioidomycosis. Production of fatal acute pulmonary or chronic pulmonary and disseminated disease: immunological and pathological observations. J Exp Pathol. 1984 Summer;1(3):241–255. [PubMed] [Google Scholar]
- Brummer E., Vris T. W., Lawrence H. S. A microculture system for the measurement of antigen-induced murine lymphocyte proliferation: advantages of 5% horse serum and 5 X 10(-5) M mercaptoethanol. J Immunol Methods. 1977;17(3-4):319–327. doi: 10.1016/0022-1759(77)90114-4. [DOI] [PubMed] [Google Scholar]
- Castaneda E., Brummer E., Pappagianis D., Stevens D. A. Chronic pulmonary and disseminated paracoccidioidomycosis in mice: quantitation of progression and chronicity. J Med Vet Mycol. 1987 Dec;25(6):377–387. doi: 10.1080/02681218780000461. [DOI] [PubMed] [Google Scholar]
- Castaneda E., Brummer E., Perlman A. M., McEwen J. G., Stevens D. A. A culture medium for Paracoccidioides brasiliensis with high plating efficiency, and the effect of siderophores. J Med Vet Mycol. 1988;26(6):351–358. [PubMed] [Google Scholar]
- Defaveri J., Rezkallah-Iwasso M. T., de Franco M. F. Experimental pulmonary paracoccidioidomycosis in mice: morphology and correlation of lesions with humoral and cellular immune response. Mycopathologia. 1982 Jan 15;77(1):3–11. doi: 10.1007/BF00588649. [DOI] [PubMed] [Google Scholar]
- McEwen J. G., Bedoya V., Patiño M. M., Salazar M. E., Restrepo A. Experimental murine paracoccidiodomycosis induced by the inhalation of conidia. J Med Vet Mycol. 1987 Jun;25(3):165–175. doi: 10.1080/02681218780000231. [DOI] [PubMed] [Google Scholar]
- Morozumi P. A., Brummer E., Stevens D. A. Protection against pulmonary blastomycosis: correlation with cellular and humoral immunity in mice after subcutaneous nonlethal infection. Infect Immun. 1982 Aug;37(2):670–678. doi: 10.1128/iai.37.2.670-678.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo A., Cano L. E., de Bedout C., Brummer E., Stevens D. A. Comparison of various techniques for determining viability of Paracoccidioides brasiliensis yeast-form cells. J Clin Microbiol. 1982 Jul;16(1):209–211. doi: 10.1128/jcm.16.1.209-211.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo A., Jiménez B. E. Growth of Paracoccidioides brasiliensis yeast phase in a chemically defined culture medium. J Clin Microbiol. 1980 Aug;12(2):279–281. doi: 10.1128/jcm.12.2.279-281.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Davis C. E., Schaffner T., Markert M., Douglas H., Braude A. I. In vitro susceptibility of fungi to killing by neutrophil granulocytes discriminates between primary pathogenicity and opportunism. J Clin Invest. 1986 Aug;78(2):511–524. doi: 10.1172/JCI112603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar A. M., Brummer E., Stevens D. A. Murine pulmonary macrophages: evaluation of lung lavage fluids, miniaturized monolayers, and candidacidal activity. Am Rev Respir Dis. 1983 Jan;127(1):110–112. doi: 10.1164/arrd.1983.127.1.110. [DOI] [PubMed] [Google Scholar]
- Svedersky L. P., Benton C. V., Berger W. H., Rinderknecht E., Harkins R. N., Palladino M. A. Biological and antigenic similarities of murine interferon-gamma and macrophage-activating factor. J Exp Med. 1984 Mar 1;159(3):812–827. doi: 10.1084/jem.159.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]