Abstract
There are more cases of tuberculosis in the world today than at anytime in history. The global epidemic has generated intense interest into the immunological mechanisms that control infection. While CD4+ T cells play a critical role in host immunity to Mycobacterium tuberculosis, there is considerable interest in understanding the role of other T cell subsets in preventing disease development following infection. CD8+ T cells are required for optimum host defense following M. tuberculosis infection, which has led to investigation of how this protective effect is mediated. A critical review of recent literature regarding the role of CD8+ T cells in protective immunity to M. tuberculosis infection is now required to address the strengths and weaknesses of these studies. In this review, we evaluate the evidence that CD8+ T cells are critical in immunity to M. tuberculosis infection. We discuss the specific mycobacterial proteins that are recognized by CD8+ T cells elicited during infection. Finally, we examine the effector mechanisms of CD8+ T cells generated during infection and synthesize recent studies to consider the protective roles that these T cells serve in vivo.
Keywords: Bacterial infection, T cells
I. Introduction
The human pathogen M. tuberculosis is responsible for more deaths than any other infectious disease. The continuing HIV/AIDS* epidemic and the spread of multi-drug resistant M. tuberculosis has contributed to the perpetuation of the global epidemic of tuberculosis. While M. bovis BCG is used universally as a vaccine, its efficacy in preventing pulmonary tuberculosis in adults is controversial (1). To combat this ongoing worldwide scourge, vaccine development for tuberculosis is an international priority. Clinically, only 5–10% of M. tuberculosis infected people, defined by their positive skin test to purified protein derivative (i.e., TST+), go on to develop the disease tuberculosis which must mean that most infected individuals develop long-lived protective immunity. Cellular immunity to M. tuberculosis plays a critical role in controlling infection and an effective T cell response determines whether the infection resolves or develops into clinically evident disease. Consequently, there is considerable interest in determining which T cell subsets mediate anti-mycobacterial immunity, delineating their effector functions, and evaluating whether vaccination can elicit these various T cell subsets and induce protective immunity. Such information will provide a more rational basis for vaccine design. It is widely appreciated that class II MHC-restricted CD4+ T cells are critical for resistance to M. tuberculosis in both humans and rodent models. CD4+ T cells are required to control the initial infection as well as to prevent recrudescence in both humans and animal models (2). On the other hand, the relative contribution of CD8+ T cells still requires definition. Some early experimental work using avirulent mycobacterial strains may have underestimated the role of CD8+ T cells. In contrast, investigators using antibody-mediated depletion of T cell subsets in vivo, adoptive transfer of purified T cells, and the use of knockout mice that lack certain T cell subsets, have generated convincing experimental evidence that CD8+ T cells are required for optimum immunity to tuberculosis (see below for details) (3;4). To activate CD8+ T cells, mycobacterial antigens need to enter the class I MHC pathway before they can be processed and presented by class I MHC on the cell surface. DNA vaccines, recombinant viruses, and live attenuated intracellular bacteria can target antigens to the class I MHC pathways. Unfortunately, evaluating whether these strategies successfully activate CD8+ T cells has been hampered because of the paucity of mycobacterial antigens known to be presented by class I MHC. Even basic questions concerning the function of CD8+ T cells during M. tuberculosis infection remain unanswered. Recently, substantial progress has been made in the identification of mycobacterial proteins that elicit antigen-specific CD8+ T cells following infection. These advances now provide investigators with tools to track antigen-specific CD8+ T cells and measure their function. With this new approach, a better understanding of CD8+ T cell biology during M. tuberculosis infection is emerging.
The focus of this review will be the biology of CD8+ T cells elicited by M. tuberculosis infection. First we will review the data that CD8+ T cells are an essential component of host immunity. Second, recent progress on the identification of mycobacterial antigens that are recognized by CD8+ T cells elicited during infection will be summarized. Finally, we will discuss studies on the function of CD8+ T cells and their role in host resistance to disease.
II. CD8+ T cells are an integral part of the immune response to M. tuberculosis
A. CD8+ T cells specific for mycobacterial antigens are detected in BCG vaccinated and M. tuberculosis infected people
Historically, identifying mycobacterial specific CD8+ T cells from human tuberculosis patients has been difficult. This difficulty, which was likely due to technical problems such as the use of nonviable bacteria or soluble antigens to stimulate antigen-specific CD8+ T cells, led to the general impression that CD8+ T cells were not an important component of the human immune response against M. tuberculosis (5;6). This impression was reinforced by the developing paradigm of how CD4+ and CD8+ T cells recognize antigens. CD4+ T cells recognize exogenous antigens that professional APC (e.g., class II MHC+ B cells, macrophages and DC) take up by endocytosis. Antigen processing takes place in the specialized endocytic compartments. Proteins are cleaved into peptides that bind class II MHC proteins, and the resulting complex traffics to the cell surface where it can be recognized by CD4+ T cells. In contrast, CD8+ T cells recognize cytosolic antigens, which after processing by the proteasome associate with class I MHC proteins that are expressed by all nucleated somatic cells. Viral proteins are the prototypical class I MHC-presented foreign antigens and the primary immune response to viral infection is mediated by CD8+ T cells. As a pathogen that resides in the phagosome of macrophages, it made sense that antigens of M. tuberculosis would be preferentially presented by the class II MHC pathway. Furthermore, human class II MHC-restricted CD4+ T cell lines had significant cytolytic activity against M. tuberculosis infected macrophages in vitro, which “obviated” the need for CD8+ CTL (7;8). A role for CD8+ T cells in host resistance against tuberculosis emerged from studies using the murine tuberculosis model (discussed below), which eventually forced a re-evaluation of the role of CD8+ T cells in the human immune response to M. tuberculosis.
CD8+ T cells were identified as an integral component of granulomas and were found in the pleural exudates of patients with tuberculosis, sometime at a frequency exceeding that observed for CD4+ T cells (9–11). Cloning T cells from the pleural fluid of patients with tuberculosis demonstrated that CD8+ T cells could be specific for M. tuberculosis antigens (12). One of the earliest indications that CD8+ T cells may have a role in immunity to tuberculosis was the clinical observation that an HIV-seronegative patient with recurrent refractory tuberculosis had an absolute reduction in peripheral CD8+ T cells (13). Bothamley et al suggested that chemotherapy was insufficient to cure the infection – CD8+ T cells were required as well. Further efforts led to success in obtaining antigen-specific CD8+ T cells from BCG-vaccinated subjects (14), people with latent tuberculosis (15–17), and patients with active disease (18;19). Interestingly, these human CD8+ T cells fell into several categories. While many of the CD8+ T cells were restricted by class I MHC (i.e. HLA-A and HLA-B), others were restricted by non-classical antigen-presenting molecules such as class Ib MHC (e.g., HLA-E), or by the CD1 family of antigen presenting molecules (15;20–23).
The AIDS epidemic and the proclivity of HIV to destroy CD4+ T cells has established a critical role for CD4+ T cells in immunity to tuberculosis. The rates of both primary and secondary (i.e., reactivation) tuberculosis are both increased in patients’ co-infected with HIV. This clinical data is supported by a clear and substantial role of CD4+ T cells in immunity to tuberculosis in experimental animal models. To date, there have been no “experiments of nature” to prove whether CD8+ T cells are critical for immunity to tuberculosis in people. However, the existence of human antigen-specific CD8+ T cells that recognize class I MHC-presented mycobacterial antigens indicates that mycobacterial antigens enter the class I MHC processing pathway and lead to priming of CD8+ T cells in vivo. Furthermore, by deriving CD8+ T cell lines, several studies have shown that M. tuberculosis infected macrophages, the preferred host cell for M. tuberculosis, can be recognized and killed by human CD8+ T cells. Finally, as will be detailed in Section III, the definition of the minimal epitopes presented by class I MHC now makes possible the development of research tools that can track antigen-specific CD8+ T cells and should provide a deeper understanding of their function during infection.
B. CD8+ T cells mediate host protection against M. tuberculosis in experimental animal models
1. Antibody depletion and adoptive transfer studies show a modest role for CD8+ T cells in resistance against M. tuberculosis
The use of monoclonal antibodies that deplete T cell subsets in vivo provide another method to address the role of CD8+ T cells in immunity to tuberculosis. Müller et al observed that depletion of CD8+ T cells in thymectomized mice prior to infection impaired control of H37Rv and BCG replication in the spleen when measured 3 weeks after IV infection (24). In contrast, two other studies found that depletion of CD8+ T cells led to only a minor exacerbation of bacterial replication following IV BCG infection using a similar approach (25;26). However, the outcome of these later studies may have been affected by the use of less virulent bacteria. Depletion of CD4+ T cells led to a exacerbation of bacterial replication in all three of these studies.
Depletion of a T cell subset can only determine whether it is required for host resistance – to determine whether a particular T cell subset is sufficient to mediate host resistance against M. tuberculosis, immune T cells obtained from infected donor mice or T cell clones propagated in vitro can be adoptively transferred into recipient mice, which are challenged with M. tuberculosis. Adoptive transfer studies in the murine tuberculosis model demonstrated that immune CD8+ T cells could transfer resistance as measured by a reduction of splenic CFU in M. tuberculosis and BCG challenged recipient mice (27–29). CD8+ T cell clones specific for M. leprae heat-shock protein (hsp) 65 also protected mice against virulent M. tuberculosis challenge (30–32). In 1987, Ian Orme showed that both CD4+ and CD8+ T cells purified from the spleens of IV infected mice could provide protection to sublethally irradiated mice against M. tuberculosis infection. In this study, CD8+ T cells had a significant role in protection following high dose aerosol challenge; however, CD4+ T cells had a greater capacity to protect mice against IV and low dose aerosol challenge (3). Using T cell clones specific for the related bacteria M. lepraemurium, Hussein et al dissociated DTH from resistance to infection (33). While the majority of CD4+ T cell clones transferred DTH, none significantly mediated resistance. Instead, a CD8+ T cell clone, which did not transfer DTH was best at transferring resistance.
2. Studies with knockout mice show an essential role for CD8+ T cells in resistance against M. tuberculosis
In 1992, Flynn and colleagues showed that mice lacking β2-microglobulin (β2m) succumb rapidly following IV infection (34). Because β2m is required for assembly and trafficking of the class I heavy chain, no class I MHC is expressed on the cell surface in the absence of β2m. Consequently, class I MHC-restricted CD8+ T cells fail to be positively selected during thymic development, leading to a developmental deficiency of CD8+ T cells. Since data from previous studies predicted only a modest role for CD8+ T cells in immunity to M. tuberculosis, it was a remarkable result that mice genetically deficient in β2m that consequently lack CD8+ T cells, were unable to control infection, particularly in the lung, and succumbed prematurely to tuberculosis (34). These data were the first evidence that CD8+ T cells were required for survival of M. tuberculosis infected mice.
However, the interpretation that the β2m −/− phenotype is entirely due to a deficiency of class I MHC-restricted CD8+ T cells is complicated by the fact that other β2m associated proteins exist and some, including CD1 and nonclassical (class Ib) MHC proteins, function as antigen presenting molecules (35–37). Based on the discovery that CD1 presents mycobacterial lipid antigens to T cells, Behar et al tested the hypothesis that the susceptibility of the β2m −/− is secondary to the absence of CD1-restricted T cells (38). The susceptibility of CD1 −/− mice was compared to transporter associated with antigen processing (TAP)-1 −/− mice after high-dose IV infection with virulent M. tuberculosis. TAP-1 is required for the transport of small peptides from the cytosol into the ER where the class I MHC/β2m/peptide complex is assembled. In the absence of TAP-1, class I MHC can not be stably assembled and consequently, cell surface expression of class I MHC is impaired (39). Presumably, this global deficiency of class I MHC expression impairs positive selection of CD8+ T cells during thymic maturation (40). TAP-1 −/− mice but not CD1d−/− mice, had impaired resistance to M. tuberculosis, demonstrating an important role for the class I antigen processing pathway and confirming the requirement for conventional (i.e., class I MHC-restricted) CD8+ T cells (38).
We now appreciate that murine CD1 is an orthologue of human CD1d and that while mice have two CD1d-othologues, they lack group I CD1 genes (i.e., CD1A, CD1B, and CD1C). Thus, while CD1d is not required in the murine model for optimum host resistance following high dose IV or low dose aerosol M. tuberculosis infection (38;41;42), the possibility exists that group I CD1-restricted T cells contribute to anti-mycobacterial immunity in humans and other species (discussed below). Mice with disruptions in the β2m or TAP1 genes are unable to control M. tuberculosis replication in the lung and die prematurely compared to normal mice following large inoculums administered intravenously and after low-dose aerosol infection (34;38;41–43). The increased susceptibility of CD8α −/− mice and the class I MHC heavy chain knockout (KbDb −/−) also confirm the requirement for CD8+ T cells following primary infection (44–46). Interestingly, the susceptibility of TAP1 −/−, KbDb −/−, and CD8α −/− mice to tuberculosis is less than that of β2m −/− mice. Although this data argues that class I MHC-restricted CD8+ T cells serve a critical role in immunity to virulent M. tuberculosis, other data questions their relative contribution. The susceptibility of various knockout mice and antibody-treated mice is determined following low dose aerosol infection with M. tuberculosis in a well-controlled study by Mogues et al (47). While CD8+ T cells (as shown by antibody depletion) and β2m-dependent T cells (using knockout mice) contribute to protective immunity, their effect is quantitatively smaller than the contribution of class II MHC-restricted CD4+ T cells.
3. Studies using knockout mice require cautious interpretation
The ability to selectively delete or inactivate specific genes in mice has provided enormous insight into nearly every field of biology. Nevertheless, the literature is full of cautionary tales related to the use of knockout mice in research. It is important to critically assess all of these studies as there can be unanticipated developmental abnormalities and compensatory effects. Some of the most important caveats related to the experiments described above are discussed here.
a. β2m −/− mice
The finding that β2m −/− mice are more susceptible to M. tuberculosis than TAP1 −/− and KbDb −/− mice raises the possibility that CD8+ T cells other than ones that are class I MHC-restricted may be important in immunity to tuberculosis. Although the contribution of CD1d has been excluded as discussed above, β2m is also a component of the presenting molecules H2-M3, TL, Qa-1, and Qa-2. The non-classical class Ib MHC molecule Qa-2 molecule binds peptide antigens in a TAP-dependent fashion and CTL recognition of Qa-2 has been recently demonstrated (29;48–51). On the other hand, TL expression is TAP independent although it is still controversial whether it can bind or present peptides to T cells (52–55). Transport of formylated peptides that bind H2-M3 are transported into the ER by both TAP dependent and independent mechanisms (36;56;57). Antigen-specific H2-M3-restricted CD8+ T cells are elicited following infection to M. tuberculosis and other intracellular bacteria and these T cells appear to mediate protection against infection under certain conditions (58–61). The class Ib MHC protein Qa-1 has many similarities to human HLA-E, including its binding to a peptide derived from the signal leader sequence of class Ia MHC proteins, although it can also bind other self-peptides (62;63). The Qa-1/leader peptide sequence and the analogous HLA-E complex both bind to the CD94/NKG2A and CD94/NKG2C inhibitory receptors expressed by NK cells. The recognition of the class I MHC leader peptide bound to Qa-1 and HLA-E is thought to allow NK cells to monitor the amount of class I MHC expression by different cells. Although the recognition of the leader peptide sequence is TAP and tapasin dependent, presentation of other self-peptides is independent of TAP and tapasin (64). Whether Qa-1 could present mycobacterial peptides to CD8+ T cells during infection has not been addressed, but it is particularly interesting that human CD8+ T cell clones have been established from PPD+ donors that recognize mycobacterial antigens presented by HLA-E (21). Although KbDb −/− mice are more susceptible than WT control mice, which indicates an indispensable role for class I MHC-restricted CD8+ T cells, Urdhal et al observed that following M. tuberculosis infection of KbDb −/− mice, the residual CD8+ T cells become activated, are recruited to the lung, and can produce IFNγ. While this suggests that class Ib MHC-restricted CD8+ T cells participate in the immune response to M. tuberculosis, it is not clear whether these CD8+ T cells can mediate protection.
The increased susceptibility of β2m −/− mice relative to KbDb −/− mice could also be explained by defects in β2m-associated proteins other than those known to be involved in antigen presentation. In addition to the antigen presenting molecules discussed above, β2m also associates with FcRn, the neonatal Fc receptor, and HFE, a class I MHC homolog, which regulates iron metabolism (65). Because of defective HFE expression, the β2m −/− mouse suffers from iron overload (66). The growth of M. tuberculosis is dependent on iron acquisition from the host, which led Schaible et al to hypothesize that increased tissue iron levels in β2m −/− mice increase the severity of infection (67). Lactoferrin therapy, which binds iron, reduced bacterial growth in infected β2m −/− mice but not in C57BL/6 or KbDb −/− mice. Although iron overload is primarily observed in the liver, β2m −/− mice have impaired bacterial control in the lung, spleen, and liver (66;67). HFE is expressed in macrophages and interacts with the transferrin receptor to regulate the iron balance of the cell. Growth of M. tuberculosis in WT and β2m −/− macrophages is similar, and in vitro treatment with lactoferrin leads to a reduction in bacterial replication (67). Macrophages from β2m −/− mice do not upregulate the transferrin receptor following M. tuberculosis infection, and anti-transferrin mAb inhibits bacterial growth in WT but not β2m −/− macrophages. These results raise a number of questions. For example, if M. tuberculosis grows similarly and is inhibited by treatment with lactoferrin in WT and β2m −/− macrophages, the increased susceptibility of β2m −/− mice is still likely to arise from a defect in adaptive immunity. In light of this data, one needs to consider the possibility that iron metabolism could affect T cell mediated immunity. Examination of the HFE −/− mouse may be particularly enlightening, especially with the appreciation that b2m-associated proteins other than HFE may affect iron metabolism (68).
Class I MHC has other functions distinct from antigen presentation such as acting as an inhibitory ligand for NK cell receptors and CD8+ class I MHC restricted T cells are not the only cellular subset that is abnormal in β2m and TAP1 −/− mice (69;70). The repertoire of NK cells may be altered secondary to a change in the peptides bound by the class I MHC molecules and the overall decreased surface expression of class I MHC (71). Indeed, NK cells from β2m −/− mice are abnormal since they do not recognize class I MHC-deficient target cells and are tolerant of bone marrow grafts (72). These multiple consequences of β2m-deficiency emphasizes the need to employ several independent experimental strategies to demonstrate with certainty the requirement for CD8+ T cells during M. tuberculosis infection.
b. CD8α −/− mice
CD8+ T cells express the CD8αβ heterodimer and mice lacking CD8α (CD8α −/− mice) fail to select CD8αβ+ T cells (73). Thus, the finding that CD8α −/− mice are more susceptible to tuberculosis is consistent with a role for class I MHC-restricted CD8+ T cells in host defense, although it is interesting that CD8α −/− mice are more resistant than β2m and TAP1 −/− mice (42;44). Furthermore, while β2m, KbDb, and TAP1 −/− mice all have abnormal granuloma formation, granuloma formation in CD8α −/− mice is relatively unimpaired. Similarly, there is no impairment of innate immunity in CD8α −/− mice while there is in β2m and KbDb mice (74). On possibility is that M. tuberculosis-specific class I MHC-restricted T cells that are CD8αβ− are elicited in CD8α −/− mice. Such T cells have been described in other models (75;76). Thus, CD8α −/− mice should not be thought of as deficient in class I MHC-restricted T cells.
c. CD4 −/− mice
The increased susceptibility of CD4 −/− mice to M. tuberculosis is cited as evidence for the importance of CD4+ T cells for immunity (77). Consequently, the ability of DNA vaccination to prolong survival and decrease bacterial load after aerosol M. tuberculosis infection in CD4 −/− mice is cited as evidence that class I MHC-restricted CD8+ T cells elicited by DNA immunization can mediate host protection (78). However, CD4 −/− mice have class II MHC-restricted CD4− T cells (79;80). These class II MHC-restricted T cells make up a large fraction of the residual T cells and many of these class II MHC-restricted T cells express CD8 (81;82). Thus, even depletion of CD8+ T cells cannot establish that immunity is mediated by class I MHC-restricted T cells. Although vaccination with DNA vectors may preferentially elicit class I MHC-restricted T cells, they also elicit class II MHC-restricted CD4+ T cells. Furthermore, optimum generation of antigen-specific CD8+ T cells may require interaction with elicited class II MHC-restricted CD4+ T cells (see below) (83). Either way, DNA vaccination of CD4 −/− mice is unlikely to reveal the full potential of CD8+ T cells to protect mice from tuberculosis.
d. The generation of antigen-specific CD8+ T cells may be dependent upon CD4+ T cells
CD4+ T cells are perceived to be more important than CD8+ T cells in host immunity to M. tuberculosis based in part on the finding that mice lacking CD8+ T cells survive longer following infection compared to mice deficient in class II MHC-restricted CD4+ T cells (47). Although, class II MHC-deficient mice may be more susceptible than mice lacking CD8+ T cells, it is now understood that CD4+ T cells make a critical contribution to the initiation and perpetuation of the CD8+ T cell response and the development of CD8+ memory T cells (84). Therefore, mice lacking class II MHC are more susceptible to tuberculosis because they lack CD4+ T cells, but also because they are unable to generate an optimal CD8+ T cell response. Furthermore, CD4+ T cell help may also affect the expression of effector functions by CD8+ T cells. For example, Serbina et al found that the activation, IFNγ production and infiltration into the lung of CD8+ T cells in CD4 −/− mice was comparable to WT control mice; however, the cytotoxic activity of the CD8+ T cells was diminished (83). As discussed above, CD4 −/− mice may not be completely devoid of class II MHC-restricted T cells, but nevertheless, and the antigen-specific CD8+ T cell response may be even more impaired in the complete absence of T cell help. Importantly, all of these results emphasize the difficulty in comparing class II MHC −/− and β2m −/− mice, or in vivo depletion of CD4+ and CD8+ T cells: mice lacking CD4+ T cells are likely to have an abnormal CD8+ T cell response.
4. When are CD8+ T cells important during the natural history of M. tuberculosis infection?
All of the studies discussed above address the role of CD8+ T cells in the primary immune response to M. tuberculosis. It is more difficult to address the possibility that CD8+ T cells are critical in other phases of the infection. For example, van Pinxteren established a model of latency induced by antibiotics (85). When the antibiotics were discontinued, reactivation occurred. They found that during the acute phase of the infection, treatment with anti-CD4 but not anti-CD8 mAb exacerbated the pulmonary disease. The reverse was found during the latent phase of the infection: treatment with anti-CD8 mAb led to a greater increase in bacterial numbers in the lung than did administration of anti-CD4 mAb. This suggests that during the primary or acute phase of disease, CD4+ T cells may be more important in controlling bacterial replication. In contrast, CD8+ T cells may have a greater role in maintaining control of the infection during latency, possibly via immunosurveillance of heavily infected cells that have lost the capacity to inhibit bacterial replication (85;86). Similarly, little is known about the relative role of CD4+ and CD8+ T cells during the memory T cell response. Serbina and Flynn demonstrated that CD8+ T cell with a memory phenotype do develop in the lungs of memory-immune mice, and these CD8+ T cells undergo rapid memory immune response following rechallenge with M. tuberculosis (87). Recently, using tetramers to track antigen-specific CD8+ T cells, we have found that T cells resembling central memory T cells develop even during chronic infection. Following resolution of infection induced by antibiotic treatment, there is a contraction of the antigen-specific CD8+ T cell pool, which is accompanied by a shift towards a greater predominance of central memory T cells especially in the spleen and LN (Arati Kamath, J.W. and S.M.B, manuscript submitted). If CD8+ T cells indeed play a critical role during latency, it will be interesting to determine whether CD8+ T cells acquire certain functions as they differentiate into memory cells and perhaps play a more critical role in control of infection.
5. Is the mouse a suitable model to study CD8+ T cells?
The murine model of tuberculosis has been useful for drug and vaccine testing, and for identifying many components of the immune system that are required for host resistance. However, limitations of the murine model include the inability to study latent infection as one of its greatest drawbacks. Furthermore, while there are extraordinary similarities between the human and murine immune system, there are also fundamental differences and several of these could affect immunity to M. tuberculosis.
a. Granulysin
One of these differences is that human CD8+ T cells and NK cells produce granulysin, a protein found in cytotoxic granules that has direct microbicidal action against a variety of microorganisms (88). The microbicidal activity of granulysin against intracellular bacteria requires that it can gain access to the appropriate compartment via a pore forming molecule such as perforin (88). No murine orthologue of granulysin has been reported. Thus, one must consider the possibility that human CD8+ T cells have a greater capacity to limit bacterial replication compared to murine human CD8+ T cells.
b. Group I CD1
Another potentially important difference between the murine and human immune system is that humans have both group I (CD1a, CD1b, and CD1c) and group II (CD1d) CD1, whereas mice only have group II CD1 (89;90). While human T cells can recognize mycobacterial lipids presented by group I CD1, this has not been well-documented for group II CD1 (91). Initially of unknown significance, human CD1-restricted T cells appear to play an active role in immunity to M. tuberculosis. Mycobacterial lipids enter the CD1 antigen processing pathway following infection of macrophages with M. tuberculosis, and the lipid-specific CD1-restricted T cells have been shown to recognize infected macrophages. Furthermore, there is now evidence that CD1-restricted and lipid-specific T cell responses can be detected in the peripheral blood of patients with tuberculosis (92). Although mice are an excellent model for group II CD1 (e.g., CD1d), they can not serve as a model for the study of group 1 CD1-restricted T cells. However, there exist other animal species that have maintained CD1 in their genome and can be used for research on tuberculosis including guinea pigs, rabbits, and cattle (93–97).
III. M. tuberculosis antigens recognized by CD8+ T cells
Class II MHC-restricted CD4+ T cells primarily recognize “exogenous” antigens that enter the endocytic pathway of professional APC such as macrophages, DC, and B cells, which express class II MHC (98). Phagocytosis of microbes represents the predominant way that microbial antigens enter endocytic compartments. Acidification of the phagosome and the action of hydrolytic enzymes can destroy many microorganisms leading to the release of proteins. These proteins become denatured, disulfide bonds undergo reduction, and further processing by proteases leads to the generation of peptide fragments, which can be loaded onto class II MHC molecules. In the case of intracellular pathogens that survive in the phagosome such as M. tuberculosis, proteins secreted or shed by the bacteria are more likely to enter the class II MHC pathway because of the dynamic nature of vesicular transport to and from the phagosome. The class II MHC/peptide complex is ultimately transported to the cell surface where it can be recognized by CD4+ T cells. Consequently, the identification of antigens that are presented by class II MHC is relatively straightforward. Determining whether a mycobacterial protein is recognized by immune CD4+ T cells can be determined by measuring CD4+ T cell activation during the coculture of immune CD4+ T cells with APC and the candidate protein antigen.
Defining antigens recognized by CD8+ T cells is more difficult. The class I MHC pathway has evolved to process and present intracellular antigens, such as viral proteins produced within the cell’s cytoplasm. Both self and foreign proteins in the cytosol are cleaved by the proteosome and the resulting peptides are translocated into the endoplasmic reticulum (ER) through the action of tapasin and the TAP1/TAP2 heterodimer (99). Once in the ER, the peptides assemble with the class I MHC heavy chain and β2 microglobulin (β2m) to form a trimeric complex, which is transported to the cell surface (100). Most CD8+ T cells recognize short peptides of 8–11 amino acids derived from cytosolic proteins that are presented by class I MHC molecules. Class I MHC-restricted CD8+ T cells are critical for host resistance to viral infections, and they also play a critical role in immunity to certain intracellular bacterial infections (101). Exogenous antigens taken up by APC do not, in general, gain access to the class I MHC pathway, although this occurs under certain conditions and is known as cross-presentation (102;103).
The potential for certain mycobacterial antigens to be recognized by CD8+ T cells is sometimes tested by immunizing mice with DNA vaccines encoding a M. tuberculosis antigen or immunizing with transfected tumor cells that over-express a M. tuberculosis antigen. While this approach can provide information about the potential for M. tuberculosis antigens to be recognized by CD8+ cells, the actual antigens presented by the class I MHC pathway in infected cells or in an infected host are likely to be very different. The cellular location of the proteins, their access to the different antigen processing pathways, and competition with other antigens, are important variables that will be affected by the infection and ensuing inflammatory process. For example, although T cells specific for multiple Ag85A epitopes are elicited following DNA vaccination, only a few of these epitopes prime T cells during M. tuberculosis infection (104). This same phenomenon has been observed for other mycobacterial proteins and other pathogens such as HIV (105;106). Consequently, a microbial vaccine has the potential to elicit CD8+ T cells that recognize antigen epitopes that may not be processed and presented by class I MHC on the surface of M. tuberculosis infected cells (107). Therefore, the failure of certain CD8+ T cell vaccination strategies to induce protective immunity requires cautious interpretation because of the potential incongruence between the antigen epitopes that elicit CD8+ T cells following vaccination and the epitopes presented by infected APC. Alternately, the hierarchy of immunodominant epitopes following vaccination may differ from the hierarchy established during M. tuberculosis infection. A vaccine that elicits a potent CD8+ T cell response to an epitope that is subdominant during infection, may still mediate protection if subdominance arises from limitations in the T cell response and not from a failure to efficiently process and present the epitope. Clearly, if one wishes to identify mycobacterial antigens and their dominant epitopes that actually prime CD8+ T cells following infection and that are presented by infected macrophages, one needs to use CD8+ T cells obtained from infected individuals.
Bulk CD8+ T cells obtained from infected experimental animals or short-term human CD8+ T cell lines or clones can be shown to be M. tuberculosis-specific by determining whether they recognize M. tuberculosis infected macrophages or DC. Although it is important to show that the CD8+ T cells recognize M. tuberculosis infected macrophages and DC in vitro, this approach can not be used to identify which antigens are recognized. To identify mycobacterial antigens that are recognized by M. tuberculosis-specific CD8+ T cells, a variety of strategies have been used. For example, cultured cell-lines transfected with DNA encoding M. tuberculosis antigens or transduced with recombinant viruses expressing mycobacterial antigens can be tested for their recognition by M. tuberculosis-specific CD8+ T cells. Such approaches lead to the expression of M. tuberculosis antigens in the cytosol which facilitates protein entry into the class I MHC processing pathway. As will be discusses in greater detail below, these approaches have shown that various M. tuberculosis antigens including Ag85A, Ag85B, Ag85C, 38 kD, 19 kD, CFP10, ESAT-6 and MPT64, can be processed by the class I MHC antigen processing pathway and presented to CD8+ T cells (18;19;22;108–113).
A. Approaches to antigen identification
There are two general strategies to identify antigens recognized by CD8+ T cells. The traditional strategy is the candidate antigen approach. This requires testing individual mycobacterial proteins by expressing them in the cytosol of APC or using a library of synthetic overlapping peptides that span the protein sequence. Now that the sequence of M. tuberculosis genome has been ascertained, a second method is the bioinformatic approach. This method screens the M. tuberculosis genomic sequence using computer algorithms to identify peptide epitopes that are likely to bind to class I MHC proteins, followed by synthesis of these specific epitopes and experimental confirmation of their recognition by immune CD8+ T cells.
1. The Candidate Antigen discovery approach
The basis for selecting proteins to analyze by the candidate antigen strategy varies from investigator to investigator. A particular protein may be chosen for evaluation as a candidate antigen recognized by CD8+ T cells based on certain biological properties. For example, small MW secreted proteins may preferentially enter the class I MHC processing pathway. Alternately, preliminary immunological characterization such as finding that DNA vaccination elicits antigen-specific CD8+ T cell can suggest an antigen to pursue. Often the choice may be driven by the available reagents (e.g., cloned genes, recombinant proteins, etc.). Once a candidate antigen is selected, an appropriate experimental strategy is designed. Although there are well-described examples of exogenous antigens presented by class I MHC, presumably via the vacuolar pathway, antigen generally need to be expressed in the cytosol in order to enter the class I MHC pathway. This can be achieved by osmotic loading of the protein, antigen delivery using liposomes, infecting APC with a recombinant virus that produces the protein, or by establishing stable cell lines transfected with DNA that encoded the mycobacterial antigen (111;114–116). Although establishing transfected cell lines is often the simplest and most cost-effective approach, it is also the most restrictive because of the need to match the MHC type of the donor CD8+ T cells with that of the APC. While this may not be a great impediment for studies using inbred mouse strains, it is a significant limitation for studies involving human T cells or outbred experimental animals.
An alternative to expressing the antigen in the cytoplasm of the APC is to use overlapping synthetic peptides that span the length of the candidate protein. Just as there are many innovative ways to express proteins in the cytoplasm of APC, many novel techniques exist for the design and screening of peptide libraries (117). Cost-effective strategies vary the length of each individual peptide and the amount of overlap in order to optimize the library for the detection of epitopes that bind to class I MHC and are recognized by CD8+ T cells (118). Alternately, comprehensive approaches synthesize every 9mer peptide in the protein such that every peptide overlaps by 8 amino acids. The creation of peptide pools is used when large numbers of peptides require screening and sophisticated pooling protocols exist so that unique epitopes can identified after deconvolution of the data from the initial screen (119).
As alluded to above, an important consideration is the source of CD8+ T cells and the assay conditions since T cell activation is used to determine whether a protein or peptide is an antigen. To identify epitopes recognized by human CD8+ T cells, T cells are generally purified from the peripheral blood of PPD+ individuals or patients with active tuberculosis. Alternately, CD8+ T cell clones can be established and prescreened for the recognition of infected macrophages or DC, which will increase the sensitivity of the final screen (22;120). Short-term T cell lines can be established by stimulating T cells from PPD+ individuals with the antigen of interest (121). Although this obviously introduces bias into the experimental design, the tradeoff is the potential enrichment of T cells specific for the antigen. Animal models allow access to large numbers of bulk CD8+ cells. The frequency of antigen-specific T cells in the lung is particularly high and using mononuclear cells from the lungs of experimentally infected animals as a source of T cells will increase the sensitivity of the screen, although it is more difficult to obtain highly purified CD8+ T cells from the lung. This is especially a concern if class II MHC-restricted epitopes are represented in the peptide library since even small numbers of contaminating CD4+ T cells can produce significant false-positive results. The assays used to measure T cell activation are standard immunological assays. Cell culture supernatants are often assayed for IFNγ production by ELISA since most CD8+ T cells produce this cytokine. The IFNγ ELISPOT may be more sensitive technique for detecting recognition of synthetic peptides by CD8+ T cells and provides information about the frequency of antigen-specific T cells (19;22;120). Alternately, T cell activation can be detected by measuring proliferation or cytolytic activity (121;122). While measuring the cytolytic action of CD8+ T cells is more cumbersome, this approach may be particularly important given the discrepancy between the total number of antigen-specific CD8+ T cells and the fraction that produce cytokines.
2. The Bioinformatic Antigen discovery approach
Now that the genome of M. tuberculosis has been sequenced and made available in databases, a novel strategy to identify class I MHC-restricted epitopes has emerged. Research on antigen presentation by the MHC has provided a wealth of information on the molecular basis of how class I MHC proteins binds peptides. A peptide binding groove is formed between the α-helices that sit on the β-sheet platform. There are generally six “pockets” (A–F) in the groove, which accommodate the amino acid side chains of the antigenic peptide. The unique binding specificity of each MHC allele is due to polymorphic residues in the groove which alter the exact configuration of these pockets, and thus leads to the preferential binding of peptides with varied length and amino acid sequence. The anchor residues, typically at positions 2 and 9 of the peptide, are particularly important in determining the affinity of the peptide for MHC. Careful inspection of all peptides known to bind to a particular class I MHC protein reveals a pattern or motif that is required for optimum binding. Based on these motifs, one can predict portions of proteins that are likely to bind to various MHC alleles. Computer algorithms have been developed that can rapidly make these predictions (117). Some algorithms are able to predict where proteosome cleavage sites are located in the protein. Thus, the entire genome of a pathogen, or more selectively, it’s open-reading frames (ORF), can now be rapidly analyzed using these algorithms to identify peptide epitopes that are predicted to bind to class I MHC proteins (122;123). While some studies have found a good correlation between predicted binding and experimentally determined binding, this is not always the case (124;125). As these programs only predict MHC binding, most of the predicted peptide epitopes that bind MHC will fail to prime T cells. Why one MHC-binding peptide makes a good antigen and another fails to stimulate T cells is not completely understood. Some possible explanations include relative abundance of the peptide, half-life of the peptide, and competition among peptides for transport into the ER and MHC-binding. Therefore, once predictions have been made, they still need to be experimentally verified. Ultimately, synthetic peptides corresponding to the predicted epitopes require testing for recognition by CD8+ T cells as described above (121).
Majlessi et al applied this approach and analyzed 400 ORFs with the SYPEITHI program (122). One hundred and fifty 9mers were identified with the H-2 Kd binding motif; 84 of these had highly predictive scores (≥23). Eleven were synthesized, and of these, one was recognized by CD8+ T cell obtained from infected mice. The epitope, TB10.3/10.420–28 is a 9mer found in the homologous TB10.3 (Rv3804c) and TB10.4 (Rv0288) proteins. Hammond et al used a similar approach to perform a genome-wide screen of all 3924 ORF of M. tuberculosis for epitopes presented by HLA-B*3501 (123). The algorithm HLA_BIND predicted 479 epitopes, of which the 13 with the highest score were synthesized and used to screen BCG-vaccinated individuals. Several of the peptides were clearly recognized by CD8+ T cells from BCG-vaccinated donors. Clearly, this is a very efficient way to screen large numbers of proteins for their recognition by CD8+ T cells. However, this approach may not be very comprehensive since algorithms are not available for all MHC alleles. Furthermore, most investigators select only the highest scoring epitopes for further evaluation, which biases the analysis. This introduces an important source of bias into an approach designed to avoid bias in the selection of the antigens for study. For example, there are instances where epitopes that are antigens are not highly predicted by MHC-binding algorithms. The CFP1032–39 H-2 Kk epitope is one of these, which has a SYPEITHI score of only 13, yet is an immunodominant epitope following infection with M. tuberculosis (126). The low frequencies of antigen-specific CD8+ T cells identified, the diversity of antigens recognized, and the lack of hierarchal organization, may indicate that the strategy used by Hammond et al failed to detect any immunodominant epitopes. Interestingly, data exist that suggest an inverse correlation between immunodominance and MHC/peptide affinity (127;128).
B. Mycobacterial antigens elicit class I MHC-restricted CD8+ T cells following infection
Just how bacterial antigens traffic from the phagosome to the cytoplasm where they can enter the class I MHC processing pathway is a matter of controversy. Although several mechanisms have been proposed (129;130), it is clear that ultimately mycobacterial antigens do enter the class I MHC pathway, since class I MHC-restricted CD8+ T cells are elicited by infection in both people and experimental animals. During the past several years a number of M. tuberculosis proteins have been identified that are recognized by CD8+ T cells. Work using human CD8+ T cell clones has provided important insight into the antigens that are recognized and the how these proteins are processed by APC. Work using experimental animal models has provided crucial details about the evolution of the CD8+ T cell response and ultimately has the potential to determine whether these CD8+ T cells mediate protection against M. tuberculosis infection. We have attempted to catalog the different antigens that are recognized by either human T cells or murine T cells following infection (see Tables I and II, respectively).
Table I.
Mycobacterial antigens recognized by human CD8+ T cells
| Antigena | Rv numberb | Restrictionc | Epitoped | Sequencee | Frequencyf | Ref |
|---|---|---|---|---|---|---|
| CFP10 | Rv3874 | a) HLA-B14 | a) CFP1085–94 | a) RADEEQQQAL | a) 1/2,100h; LTBIk | (22) |
| b) HLA-B44 | b) CFP102–11 | b) AEMKTDAATL | b) 1/700h; LTBI | |||
| CFP10 | Rv3874 | a) HLA-A*02 | a) CFP1076–85 | a) IRQAGVQYSR | ND | (147) |
| b) HLA-B*35 | b) CFP1071–79 | b) EISTNIRQA | ||||
| ESAT6 | Rv3875 | a) HLA-A2 | a) ESAT682–90 | a) AMASTEGNV | a) 1/50,000h; TB | (19) |
| b) HLA-B52 | b) ESAT669–76 | b) LQNLARTI | b) 1/23,000h; TB | |||
| ESAT6 | Rv3875 | HLA-A68.02 | ESAT621–29 | NVTSIHSLL | 1/2,500h; LTBI | (148) |
| Ag85A | Rv3804c | HLA-A2 | a) Ag85A48–56 | a) GLPVEYLQV | a) 1/3,500g,i; BCG | (108) |
| b) Ag85A242–250 | a) KLIANNTRV | b) 1/3,500g,i; BCG | ||||
| Ag85B | Rv1886c | HLA-A2 | a) Ag85B143–152 | a) FIYAGSLSAL | ND | (109) |
| b) Ag85B126–135 | b) SMAGSSAMIL | |||||
| c) Ag85B199–207 | c) KLVANNTRL | |||||
| d) Ag85B158–166 | d) GMGPSLIGL | |||||
| Ag85B | Rv1886c | HLA-A*0201 | Ag85B15–23 | LMIGTAAAV | ND | (196) |
| Ag85C | Rv0129c | HLA-B*3501 | Ag85C204–212 | WPTIIGLAM | 1/13,700–22,222h; BCG | (110) |
| Ag85ABC | HLA-B*35 | Ag85A110–118 | MPVGGQSSF | ND | (110) | |
| 19 kDa | Rv3763 | HLA-A*0201 | 19 kDa88–97 | VLTDGNPPEV | ND | (18) |
| 19 kDa | Rv3763 | HLA-A*0201 | 19 kDa88–97 | VLTDGNPPEV | 1/123–769g,i | (153) |
| 16 kDa | Rv2031c | HLA-A*0201 | a) 16-kDa21–29 | a) LFAAFPSFA | ~ 1/1000g,j; TB | (156) |
| b) 16-kDa120–128 | b) GILTVSVA | |||||
| SodA | Rv3846 | HLA-A2 | SodA160–168 | DMWEHAFYL | ND | (227) |
| AlaDH | Rv0512 | HLA-A2 | AlaDH160–169 | VLMGGVPGVE | ND | (227) |
| GlnS | Rv2220 | HLA-A2 | GlnS308–316 | GLLHHAPSL | ND | (227) |
| Mtb39 | Rv1196 | HLA-B44 | a) Mtb39144–153 | a) MWAQDAAAMF | ND | (116) |
| b) Mtb39346–355 | b) AAERGPGQML | |||||
| ThyA | Rv2764c | HLA-A*0201 | ThyA30–38 | RLPLVLPAV | ND | (196) |
| PstA1 | Rv0930 | HLA-A*0201 | PstA175–83 | SLYFGGICV | ND | (196) |
| Hbha | Rv0475 | ND | ND | ND | ND | (182) |
Common name for antigen.
The Rv designation for the mycobacterial protein.
The restricting MHC protein and allele, if known.
The location of the epitope within protein sequence.
The amino acid sequence of the epitope.
The frequency of antigen-specific T cells in PBMC unless otherwise stated.
The frequency of antigen-specific T cells among CD8+ T cells.
Determined using an Elispot assay.
Determined by flow cytometric analysis using peptide-loaded class I MHC-tetramers.
Determined by flow cytometric analysis using intracellular cytokine staining.
Infection status of subjects: LTBI, latent tuberculosis infection; TB, active tuberculosis; BCG, BCG vaccinated.
ND, not determined
Table II.
Mycobacterial antigens recognized by murine CD8+ T cells
| Antigena | Rv numberb | Restrictionc | Epitoped | Sequencee | Frequencyf | Tissueg | Ref |
|---|---|---|---|---|---|---|---|
| CFP10 | Rv3874 | H-2 Kk | CFP1032–39 | VESTAGSL | 1/3i | lung | (126) |
| TB10.3 | Rv0288 | H-2 Kd | TB10.320–28 | GYAGTLQSL | 1/3i | lung | (122;126) |
| TB10.4 | Rv3019c | TB10.420–28 | |||||
| Mtb32 | Rv0125 | H-2 Db | 32C93–102 | GAPINSATAM | 1/13i | lung | (158;159) |
| MPT51 | Rv3803c | H-2 Dd | MPT5124–32 | GGPHAVYLL | 1/91j | spleen | (149) |
| PstS3 | Rv0928 | H-2 Db | PstS3285–293 | SGVGNDLVL | 1/5650h | spleen | (203) |
| MPT64 | Rv1980c | H-2 Db | MPT64190–198 | FAVTNDGVI | 1/6250i,k | spleen | (157) |
| Ag85A | Rv3804c | H-2 Kd | Ag85A144–152 | VYAGAMSGL | ND | (122) | |
| 38 kD | Rv0934 | H-2b | 38225–234 | ALGENGNGGM | ND | (106;111;157) |
Common name for antigen.
The Rv designation for the mycobacterial protein.
The restricting MHC protein, if known; if not, the H-2 haplotype of the mouse strain.
The location of the epitope within protein sequence.
The amino acid sequence of the epitope.
The frequency of antigen-specific T cells among CD8+ T cells.
The tissue used to determine the T cell frequency.
Determined using an Elispot assay
Determined by flow cytometric analysis using peptide-loaded class I MHC-tetramers.
Determined by flow cytometric analysis using intracellular cytokine staining
Mice infected with BCG ND, not done
Although it is certain that greater insight into how mycobacterial proteins enter the class I MHC processing pathway will be gained as additional antigens are described, inspection of Table I and II reveals certain commonalities between the antigens. The most obvious trend is that nearly all of the antigens are secreted proteins. Secreted M. tuberculosis protein antigens have been extensively studied in part because they are targets of T cell mediated immunity (131). Antigens such as Ag85, 6-kDa early secretory antigen target (ESAT6), 10-kDa culture filtrate protein (CFP10) and others elicit strong CD4+ T cells responses in both mice and humans (108;132). Another feature is that most of the antigens are small MW proteins. Although there are many sources of bias in the data, these observations are consistent with the hypothesis that small MW proteins can cross the phagosomal membrane. One possible mechanism involves the host protein Sec61, which normally mediates retrograde protein translocation as a way to transport misfolded proteins back into the cytosol where they can be targeted for degradation (133). Proteins secreted by M. tuberculosis into the phagosomal compartment might be translocated by Sec61 across the phagosomal membrane into the cytosol where they could enter the class I MHC processing pathway. Alternately, a bacterial secretion system could mediate transport of bacterial proteins across the phagosomal membrane. These are two possible mechanisms to explain how mycobacterial antigens in infected APC enter the class I MHC processing pathway. Alternately, cross presentation of mycobacterial antigens by uninfected DC could lead to cross-priming of CD8+ T cells. One way uninfected DC could prime CD8+ T cells is by taking up apoptotic vesicles derived from M. tuberculosis infected macrophages. This mechanism, which has been referred to as the “Detour Pathway,” has been shown to activate both human and murine M. tuberculosis-specific CD8+ T cells (134;135). How the antigens get out of the apoptotic vesicles, which are contained within phagosomes, and into the cytosol, still remains a formidable problem to solve.
1. ESAT6-related proteins
Through careful genomic mapping of different BCG strains from around the world, Marcel Behr defined several regions that had been deleted from M. bovis during the derivation and propagation of BCG, the widely used vaccine strain (136–141). Using these “regions of difference” (RD), a family tree of the different BCG strains was created and led to the conclusion that the deletion of RD-1 was likely to be the original event that led to the attenuation of M. bovis. The same area is absent from M. microti, which is closely related to M. tuberculosis, but is avirulent. Through targeted mutation, it is now confirmed that the RD-1 locus is critical for the virulence of M. tuberculosis. Two of the proteins encoded within the RD-1 locus are CFP10 and ESAT6, which are secreted as a heterodimer (142), and were already known to be a major antigens recognized by M. tuberculosis-specific human T and B cells (131;143;144). The cfp10 gene is in the same operon as the esat-6 gene, and the two genes share 40% sequence homology and belong to the ESAT6 family of small proteins (136;140;145). In addition to RD-1, M. tuberculosis H37Rv has 11 distinct loci that encodes 23 ESAT6-related proteins (reviewed in (9)). This extended family of ESAT6-related proteins have been given the names EsxA through EsxW, with the best characterized ESAT6 and CFP10 proteins named EsxA and EsxB. Other proteins in the RD-1 locus, adjacent to ESAT6 and CFP10, are required for the secretion of the CFP10 and ESAT6, and together, the locus appears to encode a specialized secretory apparatus, which is referred to as ESX-1. Four other related loci exist.
Although some data indicates that ESAT6 can disrupt planar membranes, the exact function of ESAT6 and CFP10 are unknown (146). As CFP10 and ESAT6 are expressed by pathogenic mycobacterium species but not by BCG, CFP10 and ESAT6 may be virulence factors. This of course begs the question of whether the secretion of CFP10 and ESAT6 are required for virulence or whether the RD-1 dependent secretion of other proteins is the critical factor.
Interestingly, the ESAT6-related proteins appear to frequently be targets of the host immune response and recent evidence suggests that specific immunity to ESAT6 enhance host resistance against M. tuberculosis infection (141). It is well documented that ESAT6 and CFP10 elicit T cells in humans as well as experimentally infected animals. In addition, T cells to other ESAT6-related proteins are generated following infection (9). It is quite remarkable that a number of ESAT6-related proteins including ESAT6 (EsxA), CFP10 (EsxB), and TB10.3/10.4 (EsxR/H) generate CD8+ T cell responses in infected people and mice.
In 2000, Lewinsohn et al. cloned CD8+ T cells from a PPD+ individual using autologous M. tuberculosis infected DC (120). Four of the CD8+ T cell clones are class I MHC-restricted and two recognize distinct peptide epitopes derived from the CFP10 protein (22). This data is significant for several reasons. First, it demonstrates that CFP10 has access to the class I MHC processing pathway and shows that CFP10-specific CD8+ T cells are primed following infection. Second, CFP10-specific CD8+ T cell clones recognize infected macrophages, which indicates their potential to act as effector cells (22;147). Finally, CFP10-specific CD8+ T cells were detected at frequencies as high as 1/700 total CD8+ T cells, suggesting that CFP10 can be an immunodominant antigen in people. A follow-up study by Shams et al defined a 15mer peptide (CFP1071–85), which contains at least two distinct epitopes, each one that can be presented by class I and II MHC to both CD8+ and CD4+ T cells, respectively (147). The promiscuity of these epitopes for different alleles of class I and class II MHC proteins may explain why nearly 100% of people with latent M. tuberculosis infection recognize CFP10.
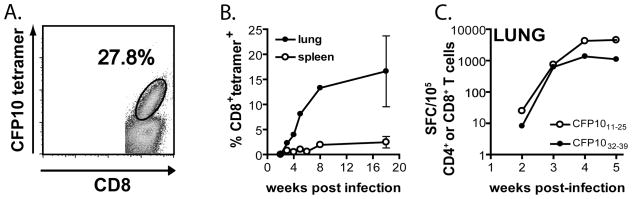
These observations prompted my own lab to determine whether CFP10 was a target of the CD8+ T cell response in the mouse model. We identified two epitopes from the CFP10 protein: CFP1011–25 and CFP1032–39, which are recognized by CD4+ and CD8+ T cells, respectively. The CFP1032–39-specific CD8+ T cells were H-2 Kk-restricted and were elicited following infection with virulent M. tuberculosis (Erdman and H37Rv) but not H37Ra or BCG. CFP1032–39-loaded Kk-tetramers identified CFP10-specific CD8+ T cells in the lung, spleen and LN (see Figure 1A). Surprisingly, nearly 30% of pulmonary CD8+ T cells recognize CFP1032–39, demonstrating the CFP10 is an immunodominant antigen in mice of the H-2k haplotype (see Figure 1) (126).
FIGURE 1. Frequency of CFP10-specific T cells elicited following aerosol infection with M. tuberculosis.
A. A representative FACS plot shows the percentage of pulmonary CD8+ T cells from M. tuberculosis infected C3H mice that are specific for CFP1032–39, as determined using CFP1032–39-loaded H-2 Kk tetramers.
B. Nearly 30% of the pulmonary CD8+ T cells from M. tuberculosis infected C3H mice are specific for CFP1032–39 as determined by staining with the CFP1032–39-loaded H-2 K tetramer. Lung mononuclear cells and splenocytes were gated on lymphocytes based on forward and side scatter and then gated on CD8+ events.
C. The kinetics of the CD4+ and CD8+ T cell response to the CFP1011–25 and CFP1032–39 epitopes, respectively. C3H mice were infected by the respiratory route and the frequency of CFP10-specific CD4+ and CD8+ T cells in the lung was determined by Elispot at the indicated time points.
As mentioned above, the ESAT6-related proteins TB10.3 and TB10.4 both contain an identical epitope (TB10.3/10.420–28), which is recognized by H-2 Kd-restricted CD8+ T cells following respiratory M. tuberculosis infection (126). As many as 30–40% of the CD8+ T cells in the lungs of BALB/c mice recognize this epitope and these CD8+ T cells have cytolytic activity both in vitro and in vivo (Arati Kamath, J.S.M.W., and S.M.B., manuscript submitted)(122;126). The identification of immunodominant epitopes such as CFP1032–39 and TB10.3/10.420–28 that are recognized by CD8+ T cells following infection is critical for studies elucidating the key elements of protective immunity. In contrast to CD8+ T cells that recognize subdominant epitopes, the high frequency of CD8+ T cells that are specific for dominant epitopes can facilitate studies on the activation, differentiation, trafficking, and effector functions of these T cells. These epitopes will make possible further insight into how other host and microbial factors modulate the antigen-specific CD8+ T cell response and allow comparison of different vaccination strategies that elicit CD8+ T cell responses. Thus, identification of the dominant epitopes recognized by CD8+ T cells is critical to understanding how these T cells mediate host resistance to tuberculosis.
Just as CFP10 elicits antigen-specific CD8+ T cells following M. tuberculosis infection, specific epitopes within the ESAT6 protein are recognized by CD8+ T cells obtained from the blood of infected individuals. Lalvani et al identified two class I MHC-restricted epitopes from the ESAT6 protein in an individual with active tuberculosis (see Table I) (19). In a second study, CD8+ T cells specific for ESAT6 were detected in two unrelated individuals, one a PPD+ household contact and a second with untreated healed tuberculosis (148). In both cases the CD8+ T cells recognized ESAT621–29 presented by HLA-A68.02 and these T cells were present at a frequency of up to 1/2500 peripheral blood lymphocytes. The frequency of T cells recognizing ESAT621–29 was similar to the frequency of T cells specific for PPD. Furthermore, the frequency of ESAT621–29-specific CD8+ T cells was stable during 21 months of follow-up. Pathan et al argue that the persistent high frequency of these T cells suggest that ESAT6-specific CD8+ T cells have a role in protection (148).
Why this group of proteins should be so frequently recognized by T cells is unknown, but as they are all small secreted proteins, they may more efficiently enter the class I and class II MHC antigen processing pathways. Just as CFP10 and ESAT6 are potential virulence factors as defined by there absence from BCG, it is interesting to speculate on whether they are potentially important immunological targets required for control of the M. tuberculosis by the immune system. Could it be that BCG in an ineffective vaccine because it fails to stimulate an immune response to CFP10 and ESAT6?
2. Ag85 complex
Another protein secreted by M. tuberculosis is the Ag85 complex (Ag85A, Ag85B, and Ag85C), which is a family of homologous proteins that is conserved among Mycobacterium species. Each of these has a MW of 30–32 kDa and is associated with mycolyltransferase activity. Furthermore, Ag85 is a well-known antigen that elicits a strong IFNγ response both in tuberculosis patients, BCG-vaccinated individuals, and in animals experimentally infected with M. tuberculosis or BCG.
Using computer algorithms, Klein et al identified three epitopes from the Ag85 complex that were recognized by BCG-vaccinated HLA-B*3501 individuals: a) an epitope shared by Ag85ABC (Ag85110–118) b) Ag85C268–276; and Ag85B204–212 (110). An interesting strategy for the identification of mycobacterial epitopes that are presented by human class I MHC is to use transgenic mice that express HLA-A*0201 (109). Thirty candidate peptides were synthesized and 16 were found to bind to HLA-A*0201 with high or intermediate affinity. DNA vaccination of mice elicited CTL activity against four of the peptides. Two of the peptides (Ag85B143–152 and Ag85B199–207) were able to stimulate short-term antigen-specific CD8+ T cell lines from BCG-vaccinated individuals. In the case of Ag85B199–207, tetramers were made and CD8+ T cells that stained with the tetramers were enriched in the short-term cell lines. Disappointingly, tetramer+ cells could not be detected directly in the peripheral blood of BCG-responsive donors suggesting that their frequency in the circulation is <1/1000. While these two examples indicate that BCG elicits CD8+ T cells that recognize members of the Ag85 complex, we can not ascertain from these studies whether Ag85 is an important target of CD8+ T cell response in M. tuberculosis infection.
Using a different strategy, Smith et al established short-term T cell lines from BCG-vaccinated HLA-A*0201 donors and tested the CD8+ T cells against an overlapping peptide library corresponding to Ag85A (108). Three peptides were recognized and two of these contained HLA-A*0201 binding motifs (Ag85A48–56 and Ag85A242–250). Using CD8+ T cells purified from the peripheral blood of BCG-vaccinated individuals, specific tetramers and an IFNγ Elispot was used to determine the frequency of Ag85A-specific T cells (see Table I). Studies with the tetramers revealed that ~1/3500 peripheral blood CD8+ T cells were specific for Ag85A48–56 and Ag85A242–250. Interestingly, this study also found that the Elispot assay underestimated the frequency of antigen-specific CD8+ T cells by approximately 6-fold. Short-term CD8+ T cell lines derived by stimulating PBMC with these peptides recognized autologous M. tuberculosis infected macrophages. Thus, BCG vaccination elicits Ag85A-specific CD8+ T cells that can be detected in peripheral blood, and are able to recognize M. tuberculosis infected macrophages.
Although not formally part of the Ag85 complex, MPT51 is another major secreted protein that has some structural and immunological similarity to Ag85A, -B, and C. H-2 Kb-restricted CD8+ T cells specific for epitopes have been identified following MPT51 DNA vaccination (149).
3. 19-kDa protein
The 19-kDa lipoprotein is found in mycobacterial species within the M. tuberculosis complex including the BCG vaccine strain (150). Although it is cell wall associated, it is also secreted into the culture media (150). The 19-kDa lipoprotein has a variety of effects on the immune system. Not only does it stimulate both an antibody and a T cell response, but it also activates the innate immune system, probably by signaling through toll-like receptors (151). Both CD4+ and CD8+ T cells responses are made against the 19-kDa lipoprotein. The 19-kDa antigen is an excellent candidate protein to elicit CD8+ T cells since not only is it secreted by the bacterium, but it is also able to gain access into the class I MHC presentation pathway both in M. tuberculosis infected macrophages and DC (152). Interestingly, entry into the class I MHC pathway appears to be independent of the TAP1 transporter. Mahagheghpour et al measured the relative affinity of 28 peptides that were predicted to bind HLA-A*0201. Five peptides stabilized HLA-A*0201 expression on T2 cells. One of these peptides, 19-kDa88–97, was recognized by CD8+ T cells in the peripheral blood of two PPD+ healthy subjects (18). The19-kDa88–97 peptide was used to derive CD8+ T cell lines from these individuals and these CD8+ T cell lines had CTL activity that was specific for the 19-kDa88–97 peptide, and was blocked by antibodies to class I MHC or to CD8. Similar CD8+ T cell lines specific for the 19-kDa88–97 peptide were obtained from patients with active tuberculosis but not from PPD− donors, suggesting that M. tuberculosis infection leads to in vivo priming of CD8+ T cells specific for the 19-kDa protein. Importantly, CD8+ T cell lines derived in vitro using the 19-kDa88–97 peptide recognized autologous monocytes infected with M. tuberculosis, establishing that the 19-kDa88–97 peptide is presented by infected cells (18). Using HLA-A*0201 tetramers loaded with the 19-kDa88–97 peptide, Hohn et al report that between 0.13–0.81% of the peripheral blood CD8+ T cells stained with the tetramer (153). Interestingly, the frequency of 19-kDa88–97-specific CD8+ T cells steadily declined in seven out ten patients treated with triple antibiotic therapy for active tuberculosis (154). This high frequency of antigen-specific CD8+ T cells detected in peripheral blood independently confirms that M. tuberculosis infection leads to priming of human CD8+ T cells specific for the 19-kDa antigen.
4. 16-kDa protein
The 16-kDa protein [hspX, acr, Rv2031c] has homology to proteins belonging to the α-crystallin family of the small heat-shock proteins (155). Five peptides from this protein were highly predicted to bind to HLA-A*0201 and these were used to establish short-term T cell lines using peripheral blood CD8+ T cells from 8 children with pulmonary tuberculosis (156). CD8+ T cell lines that recognized the 16-kDa21–29 and 16-kDa120–128 epitopes were successfully established from all 8 patients. These CD8+ T cell lines produced both TNF and IFNγ, and their recognition of peptide pulsed cells was abrogated by antibodies to CD8 or HLA-A2. These CD8+ T cell lines also expressed granulysin and perforin, and lysed THP-1 cells (which express HLA-A*0201) when infected in vitro with H37Ra or with BCG. Finally, by stimulating fresh PBMC with the 16-kDa21–29 and 16-kDa120–128 peptides and measuring the frequency of IFNγ-producing T cells, Caccamo et al show that between 0.1–0.2% of the CD8+ T cells in peripheral blood of patients with tuberculosis recognize the 16-kDa protein (156).
5. 38-kDa protein
The 38-kDa protein is a glycolipoprotein which is secreted but remains attached to the cell surface of M. tuberculosis by its lipid tail. Following DNA vaccination or immunization with transfected tumor cells expressing the 38-kDa protein, CTL activity specific for the 38-kDa protein was elicited in C57BL/6 mice (111). CTL derived from these vaccinated mice were used to screen a library of overlapping peptides, as well as 12 additional peptides corresponding to epitopes that were predicted using algorithms. Several peptides were specifically recognized by CTL and some were shown to bind to H-2 Kb or Db. One peptide, 38-kDa225–234, was recognized by CTL derived from M. tuberculosis infected mice. The repertoire of IFNγ-producing CD8+ T cells specific for 38-kDa protein was compared following DNA vaccination and after M. tuberculosis infection (106). DNA vaccination elicited CD8+ T cells that recognized three distinct epitopes in the 38-kDa antigen. Interestingly, these three epitopes were completely nonoverlapping with the one epitope (38-kDa225–23) that was recognized by CD8+ T cells after M. tuberculosis infection. This emphasizes that epitopes that prime CD8+ T cell following vaccination do not always elicit CD8+ T cells following infection. Other investigators have been unable to detect 38-kDa225–234-specific CD8+ T cells using an IFNγ Elispot [(157) and S.M.B, unpublished observations]. This may indicate that these CD8+ T cells are present at a very low frequency.
6. Mtb32c protein
The Mtb32c antigen contains an epitope that is recognized by CD8+ T cells from infected C57BL/6 mice (158). This epitope was initially identified during the characterization of the 72F vaccine which is a fusion protein of Mtb32C and Mtb39 (159). CD8+ T cells that recognize the Mtb32c93–102 epitope are elicited following M. tuberculosis infection. After low dose infection, up to 7.5% of the CD8+ T cells in the lung of C57BL/6 mice are specific for the Mtb32c93–102 epitope (158). These antigen-specific CD8+ T cells express IFNγ and during the chronic phase of infection, a subset down regulates CD45RB and upregulates IL-7 receptor-α, consistent with emergence of memory CD8+ T cells. This epitope will likely be important for the study of CD8+ T cells since it is the only one described to date that elicits a high frequency of antigen-specific CD8+ T cells in C57BL/6 mice. Furthermore, these T cells may contribute to the protective efficacy of the 72F vaccine.
B. Mycobacterial antigens elicit CD1-restricted CD8+ T cells
The CD1 family of antigen presenting molecules is a distinct lineage of molecules that may have coevolved with the MHC. They all form a heterodimer with β2 microglobulin and have an overall topological structure similar to class I MHC proteins. However, their precise tertiary dimensional structure is unique and includes a hydrophobic antigen binding pocket, which makes these molecules well-suited to present lipid and glycolipid molecules to T cells. Group 1 CD1-restricted T cells include several distinct T cell subsets, which have been described to use both the αβ-TCR and the γδ-TCR (160;161). While most group I CD1-restricted T cells described in the literature are CD4−8−, some are CD4+ or CD8+ (15;20). This serves to remind us that not all CD8+ T cells are restricted by class I MHC; some are restricted by non-classical class Ib MHC molecules and some are restricted by CD1. CD1-restricted CD8+ T cells recognize mycobacterial lipids (20) and these T cells express many of the molecules associated with classical CD8+ T cells, such as granulysin, and kill target cells using the cytotoxic granule pathway (15). Furthermore, because these T cells do express granulysin, they have been shown to have the capacity to kill M. tuberculosis. Work from the Sugita lab has shown that a majority of the CD8+ T cells found in the peripheral blood of BCG-vaccinated individuals are CD1-restricted (23). Thus, mycobacterial infection in people elicits lipid-specific group I CD1-restricted CD8+ T cells that have the potential to participate in host defense against M. tuberculosis infection.
C. Protective antigens
There is an enormous emphasis on defining protective antigens as these are perceived to be a critical step in designing a vaccine against tuberculosis. Clearly an important component of this definition is that the antigen needs to be processed and presented by the infected macrophage. It is not enough to show that the protein is presented by transfectant or virally transduced cell lines. Even the most potent immune response will be ineffective if the target epitope is not processed and presented by infected cells. Once class I MHC epitopes are identified that are naturally presented by infected macrophages, one needs to demonstrate that the vaccination strategy elicits CD8+ T cells specific for epitope that is being studied. At a minimum this requires a methodology that can assess the frequency of antigen-specific CD8+ T cells. Such methodologies include Elispot, tetramers staining, or intracellular cytokine staining. Such immunological evaluation has the potential to compare different vaccination strategies for their immunological efficacy before empirically testing the vaccine’s protective efficacy. Once such preliminary studies are complete, one can assay the capacity of vaccination to protect experimental animals from challenge with M. tuberculosis. In selected examples, passive transfer of antigen-specific CD8+ T cell clones may also provide evidence that the target is a protective antigen.
IV. CD8+ T cells potentially mediate protection against M. tuberculosis infection by several mechanisms
How class I MHC-restricted CD8+ T cells mediate their protection in vivo, either during active or latent infection is unknown. Several defined immunological functions attributed to M. tuberculosis-specific CD8+ T cells may be important in host resistance to M. tuberculosis. However, few of these functions have been experimentally tested to establish the requirements for protection by CD8+ T cells. Another question is whether any of these biological activities are uniquely important for protection mediated by CD8+ T cells, since there is overlap in the effector functions between CD4+ and CD8+ T cells. Although the discussion below focuses on the protective capacity of CD8+ T cells, tuberculosis is a disease caused in large part by an over-exuberant immune response and the tissue inflammation is out of proportion to the number of bacteria present in the tissue. CD8+ T cells can potentially be detrimental. Situations have been observed in which recruitment of immune CD8+ T cells elicited by vaccination are associated with pulmonary injury when the host is challenged with M. tuberculosis (162;163). Thus, while the effector functions of CD8+ T cells may play a role in host defense, it is also possible that these same mechanisms mediate immunopathology.
A. M. tuberculosis-specific CD8+ T cells produce pro-inflammatory cytokines
Perhaps the most studied effector mechanism of T cells in M. tuberculosis infection is the secretion of type 1 cytokines, which have various anti-microbial effects. In particular, IFNγ and TNF act synergistically to activate macrophages, which increase their microbicidal activity. This is mediated in large part though the induction of nitric oxide synthase and phagocyte oxidase, which are critical for the production of nitrogen and oxygen radicals (i.e., NO and O3) capable of killing M. tuberculosis. These cytokines also mediate a variety of immunological functions including inducing class I and II MHC, upregulating CD1d, and modulating the expression of many genes (164;165). As described below, these cytokines are essential for the host response to M. tuberculosis.
1. IFNγ production may be an important protective function of CD8+ T cells
The importance of IFNγ in a productive immune response to M. tuberculosis is well appreciated. IFNγ −/− and IFNγR −/− are unable to control bacterial replication and die rapidly after M. tuberculosis infection (47;166–168). Furthermore, individual people with defective IFNγ signaling pathways, including those with anti-IFNγ autoantibodies, appear to have increased susceptibility to intracellular pathogens including mycobacterial species (169–171). Furthermore, there is also a suggestion that reduced IFNγ production by both CD4+ and CD8+ T cells in M. tuberculosis infected patients is correlated with increased disease severity (172).
The major defined effect of IFNγ is the activation of infected macrophages to increase their bactericidal capacity via the production of nitrogen and oxygen radicals and LRG47 (173–175). More recently, IFNγ has also been associated with inducing autophagy of M. tuberculosis-infected macrophages, a process that decreases the viability of intracellular bacteria (176). Finally, IFNγ is an antagonist of a Th2-type immune response, which is non-protective against M. tuberculosis infection.
The production of IFNγ is a key mediator of the protection afforded by CD4+ T cells (2;177). However, activated M. tuberculosis-specific CD8+ T cells isolated from infected people and experimental animals also produce IFNγ (22;122;126;149;173;175;178). Moreover, IFNγ production by stimulated CD8+ T cells is used regularly as a functional positive output for the screening of responses to M. tuberculosis-derived antigens, as well as a biological correlate of the efficacy of vaccination strategies for protective responses against M. tuberculosis (179;180). In this respect, the ability of CD8+ T cells to produce IFNγ upon antigenic stimulation appears to be independent of specificity to any particular group of M. tuberculosis-derived antigen, and may therefore be a general property of the CD8+ cell response.
Not all M. tuberculosis-specific CD8+ T cells produce IFNγ. In studies that enumerate M. tuberculosis antigen-specific CD8+ T cells, there is often a discrepancy between the total number of antigen-specific CD8+ T cells and those that produce IFNγ upon stimulation. This disparity is especially noted when the frequency of CD8+ T cells specific for a single peptide antigen is determine using tetramers by flow cytometry or by IFNγ-based Elispot assays (compare Figure 1B and 1C)(108;181). While there are technical issues such as the difficulty of efficiently activating CD8+ T cells in vitro, differences in how dead cells are or are not discriminated, and variability in whether whole PBMC or purified CD8+ T cells are analyzed, the differing results obtained with these assays may also reflect heterogeneity in the function of M. tuberculosis-specific CD8+ T cells. Supporting this possibility, Temmerman et al identified distinct populations of IFNγ-producing and perforin-expressing human CD8+ T cells specific for heparin-binding hemagglutinin (182).
Although IFNγ is essential for immunity to tuberculosis and CD8+ T cells clearly have the potential to produce IFNγ, it is more difficult to determine whether IFNγ production by CD8+ T cells have a protective role during M. tuberculosis infection. To address this question, Tascon et al showed that naïve WT CD8+ T cells but not IFNγ −/− CD8+ T cells could protect nude mice from M. tuberculosis when adoptively transferred prior to infection (27). This result shows that IFNγ production by CD8+ T cells can mediate protection. However, in a normal response in mice with an intact immune system, the production of IFNγ is also provided by CD4+ T cells (absent in nude mice). Whether IFNγ production by CD8+ T cells is an important function in hosts with intact immunity, where CD4+ T cells are fulfilling their natural role as abundant IFNγ producers, is unknown. Nevertheless, these findings support that CD8+ T cells can be appropriately activated in vivo by infected cells during M. tuberculosis infection and their IFNγ production is required for protection under these circumstances.
2. M. tuberculosis-specific CD8+ T cells frequently produce TNF
TNF is another potent effector cytokine during M. tuberculosis infection and studies using knockout mice, transgenic mice, blocking antibodies, and soluble TNF receptors, have all demonstrated its importance in controlling the M. tuberculosis infection (183–186). Furthermore, the increased incidence of clinical tuberculosis in patients treated with TNF blocking agents for autoimmune disease corroborates that TNF is essential for control of M. tuberculosis in people (187–189). TNF activates bactericidal activity in M. tuberculosis-infected macrophages and these actions are synergistic with IFNγ (184–186). Additionally, TNF appears to be important in chemokine production by macrophages, macrophage and lymphocyte recruitment to the lung, and granuloma formation (185;186;190). Both infected macrophages and CD4+ T cells isolated from infected people and experimental animals produce TNF (191;192). As with IFNγ, antigen-specific CD8+ T cells obtained from infected people and experimental animals also produce TNF in M. tuberculosis infection although, the relative importance of TNF-production by M. tuberculosis-specific CD8+ T cells has not been addressed (156;193)(Arati Kamath, J.S.M.W. and S.M.B., manuscript submitted).
3. Other Cytokines
M. tuberculosis-specific CD8+ T cells also produce other cytokines. While a Tc1-cytokine profile, including IFNγ production is most often reported, antigen-specific CD8+ T cells producing IL-4 and GMCSF (Tc2 cytokines) have also been isolated from actively infected individuals (153;154). Interestingly, IL-4 production by CD8+ T cells seems to be associated with active disease in individuals and may be a surrogate marker for the infectious stage (194). M tuberculosis-specific CD8+ T cells in the lungs of latently infected people produce GM-CSF and IFNγ (178). Although GM-CSF is not thought to function as an effector molecule, it may have a role in immunoregulation. Similarly, transcription of IL-12p40, a Th1-promoting cytokine produced by infected DC and macrophages and a key cytokine in immunity to M. tuberculosis, is transcribed by human CD8+ T lymphocytes, in situ (195).
B. M. tuberculosis-specific CD8+ T cells are cytotoxic lymphocytes
A hallmark of CD8+ T cells in many immune responses is their ability mediate cellular cytotoxicity (15;88;196). Cellular lysis of M. tuberculosis-infected macrophages could be beneficial if the released bacteria, or bacteria sequestered in apoptotic bodies, are subsequently taken up by nearby macrophages that can mediate bacterial killing as hypothesized by Kaufmann (197). For example, when uninfected macrophages were added to M. avium infected macrophages dying by apoptosis, a decrease in bacterial survival was observed (198). Since apoptosis of target cells is the final common pathway following recognition by CTL, this mechanism may be particularly important following the killing of infected macrophages by CD8+ T cells (Figure 2). This may explain the inverse relationship between cytolytic activity of isolated CD8+ and CD4+ T lymphocytes against infected target cells and the severity of tuberculosis in people (199). CTL activity can be mediated by several molecular mechanisms that include 1) the release of cytotoxic granules containing perforin and granzymes; 2) CD95/CD95L-dependent (Fas/FasL) killing; and 3) the production of TNF that induces apoptosis in some cellular targets (see Figure 2).
FIGURE 2.
Molecular pathways mediate CD8+ T cell cytotoxic activity. Efficient CTL-mediated lysis of target cells requires cell-cell interaction and CTL activation mediated by signaling via the TCR triggered by recognition of the class I MHC/peptide complex (not shown). T cell activation leads to polarization of the T cell and development of an immunological synapse between the CTL and the target cell. CTL induction of target cell death can be mediated by three distinct molecular pathways. The cytotoxic-granule exocytosis pathway is dependent upon perforin, a pore-forming protein, which facilitates the entry of granzymes into the target cell cytosol, where they induce apoptosis. Although this is the principal mechanism by which CTL kill target cells, activated CTL also express CD95L (FasL) which can bind to CD95 (Fas) on the target cell surface and initiate Fas-induced apoptosis. Finally, expression of membrane TNF or secretion of TNF by CTL can promote TNF receptor induced death of the target cells.
1. Cytotoxic activity of M. tuberculosis-specific CD8+ T cells is detected in vitro and in vivo
M. tuberculosis-specific CD8+ cytolytic T cells isolated from both PPD+ actively M. tuberculosis-infected individuals have been used to establish cytolytic T cells lines that can recognize and kill infected APC in vitro (15;22;147;196). In vitro cytolytic activity towards M. tuberculosis-antigen presenting target cells has been used to define that antigen specificity of M. tuberculosis-specific CD8+ T cells (157). M. tuberculosis-specific CD8+ cytolytic T cells lines have also been generated from mice infected with BCG and M. tuberculosis (122;200).
While both human and murine M. tuberculosis-specific CD8+ cytolytic T cell lines have been established in vitro, it has been difficult to demonstrate cytolytic activity of freshly isolated CD8+ T cells against infected macrophages (18;19;157;197;201;202). One possible explanation for is that freshly isolated CD8+ T cells obtained from infected people and experimental animals has a low frequency of antigen-specific CD8+ T cells, which leads to a lower effector-to-target cell ratio than is typically used in standard cytotoxicity assays. Alternately, it has been argued that the repeated stimulation and propagation of CD8+ T cells in vitro induces CTL activity. More recently, in vivo CTL activity of M. tuberculosis-specific CD8+ T cells has been identified for class I MHC-restricted peptide epitopes in the murine infection model (126;203). This is vivo CTL activity was detected using an in vivo cytolytic assay first used in a murine viral infection model (204). In this assay, uninfected splenic target cells are fluorescently labeled and surface loaded with a class I MHC-restricted peptide from a defined M. tuberculosis antigen. The target cells are then mixed with a differentially labeled, non-peptide pulsed control target cell population and injected into uninfected and M. tuberculosis-infected mice. The cells are recovered 20 hours after transfer and the specific killing of the peptide-loaded target cells is determined by flow cytometry (see Figure 3). This assay can be used to detect M. tuberculosis-specific CD8+ T cell cytolytic activity during an ongoing immune response in vivo without the need to restimulate T cells in vitro. In 2004, M. tuberculosis-specific CD8+ T cell cytolytic activity using this method was reported by two groups. Using a class I MHC-restricted peptide from the putative mycobacterial phosphate receptor, PstS-3, Romano et al report that ~20–30% of the peptide-pulsed splenic target cells transferred in vivo are specifically killed in C57Bl/6 mice within 18 hours assay (203). In our own studies, in vivo cytolytic activity towards CFP1032–39-loaded targets is detected as early as 3 weeks post aerosol infection in the lung, spleen, and and spleen tissue of C3H/HeJ mice (126). The total cytolytic activity increase up to week 6-post infection, reaching >75% specific target cells killing of splenic and lung target cell re-isolated in an 18 hour assay. Furthermore, using the TB10.3/10.420–28 epitope, which is recognized by H-2 Kd-restricted CD8+ T cells following infection of BALB/c mice, we found that M. tuberculosis-specific CD8+ T cell cytolytic activity could be detected as late as 37 weeks-post infection, showing that CD8+ T cell cytolytic activity is long lived following M. tuberculosis infection.
FIGURE 3.
In vivo CTL assay. To monitor in vivo CTL activity, splenocytes from uninfected mice are used as target cells. One population of target cells is labeled with low concentrations of the fluorescent dye CFSE and pulsed with the CFP1032–39 synthetic peptide. A second population of cells is labeled with a higher concentration of CFSE in the absence of peptide. After extensive washing, the two cell populations are mixed at a 1:1 ratio. Flow cytometric analysis of these cells (in vitro), identifies two distinct peaks of CFSElo and CFSEHi cells, which correspond to peptide-pulsed and unpulsed target cells. These target cells are injected intravenously into uninfected or M. tuberculosis infected recipients. After 20 hrs, the recipient mice are sacrificed and the target cells in the different tissues are analyzed by flow cytometry. The two target cell populations in the tissue of the uninfected recipients maintain their original 1:1 ratio. In contrast, the ratio is dramatically altered in the tissues of the M. tuberculosis infected mice. In this analysis, the peptide pulsed CFSElo cells are markedly reduced representing their selective killing by CD8+ T cells in vivo. The “percent lysis” of the peptide pulsed cells can be calculated from the ratio of CFSElo/CFSEHi target cells from infected and uninfected recipients (see references for details). The specificity of this assay for CD8+ T cells is based on the use of a minimal epitope from a well-characterized class I MHC-presented peptide antigen.
2. CD8+ T cells kill M. tuberculosis infected macrophages using multiple mechanisms
The establishment of both long-term and short-term T cell lines from M. tuberculosis-infected people and experimental animals allows investigators to determine how M. tuberculosis-specific CD8+ T cells mediate cytolysis. These experiments typically use a chromium release assay to measure the ability of M. tuberculosis-specific CD8+ cytolytic T cells to lyse target cells that are either infected or antigen-loaded. While certain antigen-specific T cell clones exclusively use a single cytotoxic mechanism, more heterogeneous M. tuberculosis-specific CD8+ cytolytic T cells use multiple cytotoxic pathways, which suggest an overlap or redundancy in the molecular requirements for their cytolytic activity.
Serbina et al stimulated pulmonary CD8+ T cells from M. tuberculosis infected mice using infected class II MHC −/− DC to establish short-term T cell lines. These T cell lines acquired the ability to lyse infected macrophages in vitro and the lysis is dependent upon granule exocytosis (200). Similarly, Stenger et al analyzed mycobacterial lipid specific group 1 CD1-restricted human T cell clones and found that the CD8+ T cell clones exclusively used granule exocytosis for target cell lysis, whereas the CD8−CD4− T cell clones relied on the CD95/CD95L pathway (15). Additionally, Silva and Lowrie cloned hsp65-specific CD8+ T cells from M. tuberculosis infected mice that were dependent upon each the granule release and the CD95/CD95L pathways to kill infected macrophages (205). In contrast, the cytolytic activity of short term CD8+ T cell lines established from PPD+ patients was inhibited by blocking of both CD95/CD95L ligation and granule release/perforin polymerization, suggesting that these pathways are simultaneously activated during target cell recognition (206). A second study similarly found that CD8+ T cells from PPD+ and PPD− individuals used both pathways; however, the lysis of in vitro infected target cells by CD8+ T cells obtained from patients with active tuberculosis was dependent on perforin (199). Redundancies in the molecular mechanism used by CTL could operate at the level of individual clones (clonal cells can use more than one mechanism to kill target cells) or a population level (antigen-specific T cells are a mixture of clones, each one using one of three possible mechanisms to kill target cells).
Evidence in tumor-models highlight the potential disconnect between dependence on specific cytolytic mechanisms in vitro and in vivo (207). Hence, while these studies clearly illustrate the cytolytic mechanisms that are employed by M. tuberculosis-specific CD8+ in vitro, they inherently fall short of illuminating the importance of these effects in vivo.
3. Analysis of cytotoxic pathways using in vivo experimental animal models
Through the use of genetic tools, the mouse model initially appeared to offer a way to assess which of the different cytotoxic pathways was required for mycobacterial immunity in vivo (Figure 2). However, conflicting studies have made it difficult to conclude whether mice lacking critical mediators of cytolysis, such as perforin, granzyme, or CD95L, are more susceptible to M. tuberculosis infection (208;209). The interpretation of these studies is complicated since none of these molecules are specific for CD8+ T cells, and most are expressed by NK cells and some CD4+ T cells. Underlying this complexity is the observation that both perforin and CD95 can have a role in lymphocyte homeostasis (210). One manifestation of this function is the finding that CD95 and perforin −/− mice have increased type 1 cytokine production. For example, perforin −/− mice have more IFNγ-producing CD8+ T cells in the lung compared to WT mice following M. tuberculosis infection (200). These opposing effects (i.e., decreased cytotoxicity vs. increased type 1 cytokine production), could potentially obscure the effector role of these molecules in vivo. Alternately, a role in mediating immunopathology and controlling bacterial replication would also confuse the interpretation of in vivo experiments. One interpretation of the published studies is the idea that the requirement for cytotoxic CD8+ T cells may be more critical late during the chronic or recrudescent phase of infection, which is why only experiments measuring CFU at late time points or survival measurements, detected a difference between knockout and WT mice. Nevertheless, reliance on these genetic murine models is unlikely to add any additional insight into the role of CD8+ T cells in tuberculosis.
a. Granule-exocytosis pathway
While some reports did not detect an impairment of immunity to M. tuberculosis immunity in granzyme or perforin −/− mice (42;208;209), other data suggest that perforin −/− mice do not control bacterial replication as efficiently as WT mice, and have decreased survival following M. tuberculosis infection (42;44).
b. CD95/CD95L (Fas/FasL) pathway
Studies involving mice deficient in the CD95/CD95L pathway are also contradictory. An initial study that examined early time points after infection generally did not observe significant differences in CFU between CD95 −/− (lpr) and WT mice after up to 5 weeks post-mycobacterial IV infection (209). Granuloma formation in CD95 −/− mice appeared normal, and the authors suggest that measured compensatory cytokine production may have compensated for a lack of CD95 function. In contrast, in study in 2001, Turner et al monitored later time points post-low dose aerosol infection (up to 140 days), and reported a requirement for CD95/CD95L cytotoxic molecules (44). Interestingly, in these studies perforin −/− showed only a relatively very small increase in CFU.
c. The possibility of redundant pathways or previously undescribed cytotoxic pathways
Interestingly, mice lacking CD8+ T cells (i.e., β2m, TAP1, and CD8 knockout mice) die more rapidly than mice lacking either CD95 or perforin (42;44;211;212). While some immune responses appear wholly dependent on a single cytolytic mechanism, these results suggest that these two major cytotoxic pathways may be functionally redundant in tuberculosis, as for certain viruses (212–214), or may be compensated by another (undefined) mechanism.
4. CD8+ T cells kill M. tuberculosis
In vitro studies demonstrate that apoptosis of infected cells may have a bactericidal effect, which benefits host immunity by destroying a niche where the bacteria grow or by a more direct bactericidal effect of the apoptotic process itself. TNF-dependent apoptosis of both primary murine and human in vitro M. tuberculosis-infected macrophages leads to reduced bacterial viability in culture (192;215). Similarly, apoptosis induced by recombinant CD95L binding to CD95 on infected human macrophages also leads to reduced viability of M. tuberculosis in vitro and supports the possibility that CD4+ and CD8+ T cells could use the CD95/CD95L pathway to control M. tuberculosis infection (216). Conversely, virulent M. tuberculosis inhibit apoptosis of infected macrophages while simultaneous promoting a more necrotic cell death that does not reduce bacterial viability and may lead to increased bacterial dissemination in vivo (217;218).
In addition to cytolytic activity CD8+ T cells have unique effector mechanisms that can directly kill intracellular bacteria (219). For example, human CD8+ T cells (as well as NK cells, γδ T cells and CD4+ T cells) express granulysin in their cytotoxic granules that can directly kill M. tuberculosis by disruption of the bacterial cell wall and cell membrane, and can kill intracellular bacteria in a perforin-dependent manner (88;220). Indeed, CD1b-restricted CD8+ T cell clones dependent upon granule exocytosis for cytolytic activity, still retained their bactericidal effectiveness even when target cell apoptosis was blocked by caspase inhibitors (221). In addition, an analysis of hsp65-specific CD8+ T cell clones grown from infected mice correlated bactericidal activity with granule exocytosis-dependent cytolysis of infected macrophages (205). Thus, the granule contents have a direct bactericidal effect independent of the induction of apoptosis. Finally, the possibility exists that CD8+ T cells can use other mechanisms to prevent bacterial growth. Class I MHC-restricted CD8+ T cells lines derived from PPD+ individuals had bactericidal effects on intracellular M. tuberculosis via a perforin and CD95/CD95L-independent mechanism (206). Taken together, these studies show that M. tuberculosis-specific CD8+ T cells have both apoptosis-dependent and independent mechanisms of bactericidal activity.
While M. tuberculosis-specific CD8+ CTL can be isolated and grown in vitro, and their activity detected in vivo, importance of their cytolytic activity in mycobacterial immunity in vivo remains unknown. As discussed above, the studies performed by Tascon et al cannot properly take into account the potential effect of CD8+ cytolytic activity during natural infection (27), and the use of knockout mice to investigate the importance of CD8+ T cell cytotoxic activity lacks a straight forward interpretation (42;44;206;208;209). In a complex immune response, such as that directed against M. tuberculosis, the protective capacity of CD8+ T cells may require a synergistic combination of both cytokine- and cytolytic-mediated effector functions that are interdependent. For example, IFNγ production (both by CD4+ and CD8+ T cells) can activate infected macrophages to 1) be bactericidal via LRG-47 and NOS2, and 2) upregulate MHC I molecules on their surface to make them better targets for T cell-mediated cytolysis leading to a decreased survival of released bacteria and/or direct killing of intracellular bacteria by T cell-produced factors. Unraveling these potentially interdependent effector functions remains a prerequisite to fully understanding the role of CD8+ T cells in immunity to tuberculosis.
V. Is there a unique role for CD8+ T cells in immunity to M. tuberculosis infection?
The idea that a specific function can be associated with a specific T cell subset can be seen in many examples of immune responses. Indeed, when Scanga et al induced reactivation of murine M. tuberculosis infection via antibody depletion of CD4+ T cells, CD8+ T cell numbers, and the relative production of IFNγ by lymphocytes in lung tissue increased, but did not prevent recrudescence (222). These data suggest that while CD8+ T cell are capable of providing potentially compensatory IFNγ in the absence of, and probably even in parallel to, CD4+ T cells, the specific source of immune mediator may be essential to a protective effect in this particular infection model. Based on the specialized function of CD8+ T cells, there are several unique roles that CD8+ T cells could serve during a protective immune response to M. tuberculosis. For example, recognition of other cells by class I MHC-restricted CD8+ T cells requires presentation of antigens via the “endogenous” antigen processing pathway. Therefore M. tuberculosis-specific CD8+ T cells preferentially recognize infected cells, while CD4+ T cells can recognize infected cells or cells that have phagocytosed dead bacteria and their antigens. In fact, human CD8+ T cells clones preferentially recognize and lyse heavily infected cells, in vitro, suggesting a specific role for M. tuberculosis-specific CD8+ T cells in the surveillance of cells in which the containment of the bacterium has been compromised (86).
The protective effect of specific T cell populations may be time-dependent. For example, CD4+ T cell-deficient mice have a transient deficiency of IFNγ in the lung after aerosol infection (77). This deficiency is overcome by a CD8+ T cell response within four weeks; however, this is not sufficient to restore control of the infection and host resistance in CD4+ T cell-deficient mice is severely impaired. This example highlights the idea that the relative importance of T cell subsets following M. tuberculosis infection may vary with the stage of the infection.
While CD4+ T cell may be important during the early response to M. tuberculosis, CD8+ T cells may be more critical during other stages of the infection. This interpretation arises in part from the finding that perforin, CD95, and CD95L −/− mice succumb to infection late during disease. As discussed above, van Pinxteren et al used a mouse model of latency and found that CD8+ T cells are most important during latency (85). Since 90% of infected individuals successfully contain primary M. tuberculosis infection, a better understanding the role of T cells during latency is an important goal because of its relevance to human disease. Why would CD8+ T cells be uniquely important during latency? CD8+ T cells are particularly well-suited for the recognition of class II MHC− infected cells. A speculative possibility is that latent M. tuberculosis evades the immune system by infecting epithelial cells. While there is little evidence for this in vivo, in vitro study clearly demonstrates that M. tuberculosis has the potential to infect a variety of epithelial cells. The data that CD8+ T cells are more critical than CD4+ T cells in the control of M. tuberculosis during the latent phase of infection would support the conjecture that latent bacteria reside in a cell that can not be recognized by CD4+ T cells (85). Although the relevance of class II MHC− epithelial cells is only hypothetical, there is increasing evidence that infected macrophages down regulate class II MHC in the presence of IFNγ under some circumstances (223;224). This phenomenon may allow the bacteria to “hide” from class II MHC-restricted CD4+ T cells. In contrast, class I MHC does not appear to be altered under these conditions. Finally, the presence of class I MHC tetramer+ CD8+ T cells specific for M. tuberculosis are present in granulomatous lesions of latently infected people lends critical support to the hypothesis that CD8+ T cells are important in surveillance during this stage of infection (178).
VI. Conclusions
There is now a large body of evidence that reveals the protective role that CD8+ T cells play in immunity to M. tuberculosis. However, there are major gaps in our understanding of why CD8+ T cells are important, and which of their critical effector functions are required for host immunity. CD8+ T cells specific for mycobacterial antigens can recognize infected cells, produce cytokines such as TNF and IFNγ, lyse infected cells and kill M. tuberculosis. Understanding the protective and potentially pleiotropic effects of class I MHC-restricted CD8+ T cells, both mechanistically and functionally, during the course of M. tuberculosis infection is a prerequisite to the development of new and more effective vaccines. CD8+ T cells appear to play a critical role in host defense late during infection and are be important in preventing reactivation of latent M. tuberculosis (85). These results may provide impetus to harness the potential of CD8+ T cells for immunotherapy (225;226). A major advance during the past 10 years has been the identification of the minimal peptide epitopes that are recognized by class I MHC-restricted CD8+ T cells. Combined with tetramer technology, these peptide epitopes make it possible to identify and track antigen-specific CD8+ T cells, which will make possible a more precise and detailed assessment of their function. By combining these newly defined class I MHC-restricted antigens with new emerging concepts about the role that CD8+ T cells play in host defense, specific systems can be designed to reveal the effector mechanisms used by CD8+ T cells to protect the host during the immune response to M. tuberculosis. These recent advances should accelerate the development of new insights into the biology of CD8+ T cells during M. tuberculosis infection.
Acknowledgments
This work was supported by National Institutes of Health grants AI49093, AI067731, and an American Lung Association Career Investigator Award to SMB.
Footnotes
Abbreviations: −/−, genetically deficient (i.e., “knockout”); AIDS, acquired immunodeficiency syndrome; APC, antigen presenting cell; β2m, β2 microglobulin; BCG, M. bovis Bacille Calmette-Geurin; CFU, colony forming unit; CFP, culture filtrate protein; CTL, cytotoxic lymphocyte; DC, dendritic cell; DTH, delayed type hypersensitivity; ER, endoplasmic reticulum; HIV, human immunodeficiency virus; IFNγ, γ-interferon; IL, interleukin; IV, intravenous; LN, lymph node; NK, natural killer; MHC, major histocompatibility complex; MW, molecular weight; PBMC, peripheral blood mononuclear cells; PPD, purified protein derivative; TAP, transporter associated with antigen processing; TCR, T cell receptor; TNF, tumor necrosis factor; TST, tuberculin skin test; WT, wild-type.
Reference List
- 1.Sterne JA, Rodrigues LC, Guedes IN. Does the efficacy of BCG decline with time since vaccination? Int J Tuberc Lung Dis. 1998;2(3):200–207. [PubMed] [Google Scholar]
- 2.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 3.Orme IM. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138(1):293–298. [PubMed] [Google Scholar]
- 4.Orme IM, Collins FM. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 1984;84(1):113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- 5.Mustafa AS, Kvalheim G, Degre M, Godal T. Mycobacterium bovis BCG-induced human T-cell clones from BCG-vaccinated healthy subjects: antigen specificity and lymphokine production. Infect Immun. 1986;53(3):491–497. doi: 10.1128/iai.53.3.491-497.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boom WH, Wallis RS, Chervenak KA. Human Mycobacterium tuberculosis-reactive CD4+ T-cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phagocytes. Infect Immun. 1991;59(8):2737–2743. doi: 10.1128/iai.59.8.2737-2743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pithie AD, Rahelu M, Kumararatne DS, Drysdale P, Gaston JS, Iles PB, Innes JA, Ellis CJ. Generation of cytolytic T cells in individuals infected by Mycobacterium tuberculosis and vaccinated with BCG. Thorax. 1992;47(9):695–701. doi: 10.1136/thx.47.9.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewinsohn DM, Bement TT, Xu J, Lynch DH, Grabstein KH, Reed SG, Alderson MR. Human purified protein derivative-specific CD4+ T cells use both CD95-dependent and CD95-independent cytolytic mechanisms. J Immunol. 1998;160(5):2374–2379. [PubMed] [Google Scholar]
- 9.Randhawa PS. Lymphocyte subsets in granulomas of human tuberculosis: an in situ immunofluorescence study using monoclonal antibodies. Pathology. 1990;22(3):153–155. doi: 10.3109/00313029009063555. [DOI] [PubMed] [Google Scholar]
- 10.Guzman J, Bross KJ, Wurtemberger G, Freudenberg N, Costabel U. Tuberculous pleural effusions: lymphocyte phenotypes in comparison with other lymphocyte-rich effusions. Diagn Cytopathol. 1989;5(2):139–144. doi: 10.1002/dc.2840050206. [DOI] [PubMed] [Google Scholar]
- 11.Manca F, Rossi G, Valle MT, Lantero S, Li PG, Fenoglio D, De Bruin J, Costantini M, Damiani G, Balbi B. Limited clonal heterogeneity of antigen-specific T cells localizing in the pleural space during mycobacterial infection. Infect Immun. 1991;59(2):503–513. doi: 10.1128/iai.59.2.503-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rees A, Scoging A, Mehlert A, Young DB, Ivanyi J. Specificity of proliferative response of human CD8 clones to mycobacterial antigens. Eur J Immunol. 1988;18(12):1881–1887. doi: 10.1002/eji.1830181203. [DOI] [PubMed] [Google Scholar]
- 13.Bothamley GH, Festenstein F, Newland A. Protective role for CD8 cells in tuberculosis. Lancet. 1992;339(8788):315–316. doi: 10.1016/0140-6736(92)91397-q. [DOI] [PubMed] [Google Scholar]
- 14.Smith SM, Malin AS, Pauline T, Lukey, Atkinson SE, Content J, Huygen K, Dockrell HM. Characterization of Human Mycobacterium bovis Bacille Calmette-Guerin-Reactive CD8+ T Cells. Infect Immun. 1999;67(10):5223–5230. doi: 10.1128/iai.67.10.5223-5230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stenger S, Mazzaccaro RJ, Uyemura K, Cho S, Barnes PF, Rosat JP, Sette A, Brenner MB, Porcelli SA, Bloom BR, Modlin RL. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276(5319):1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 16.Lewinsohn DM, Alderson MR, Briden AL, Riddell SR, Reed SG, Grabstein KH. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. J Exp Med. 1998;187(10):1633–1640. doi: 10.1084/jem.187.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canaday DH, Ziebold C, Noss EH, Chervenak KA, Harding CV, Boom WH. Activation of Human CD8+ {alpha}{beta} TCR+ Cells by Mycobacterium tuberculosis Via an Alternate Class I MHC Antigen-Processing Pathway. J Immunol. 1999;162(1):372–379. [PubMed] [Google Scholar]
- 18.Mohagheghpour N, Gammon D, Kawamura LM, van Vollenhoven A, Benike CJ, Engleman EG. CTL response to Mycobacterium tuberculosis: identification of an immunogenic epitope in the 19-kDa lipoprotein. J Immunol. 1998;161(5):2400–2406. [PubMed] [Google Scholar]
- 19.Lalvani A, Brookes R, Wilkinson RJ, Malin AS, Pathan AA, Andersen P, Dockrell H, Pasvol G, Hill AV. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1998;95(1):270–275. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosat JP, Grant EP, Beckman EM, Dascher CC, Sieling PA, Frederique D, Modlin RL, Porcelli SA, Furlong ST, Brenner MB. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ alphabeta T cell pool [In Process Citation] J Immunol. 1999;162(1):366–371. [PubMed] [Google Scholar]
- 21.Heinzel AS, Grotzke JE, Lines RA, Lewinsohn DA, McNabb AL, Streblow DN, Braud VM, Grieser HJ, Belisle JT, Lewinsohn DM. HLA-E-dependent Presentation of Mtb-derived Antigen to Human CD8+ T Cells. J Exp Med. 2002;196(11):1473–1481. doi: 10.1084/jem.20020609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewinsohn DM, Zhu L, Madison VJ, Dillon DC, Fling SP, Reed SG, Grabstein KH, Alderson MR. Classically restricted human CD8+ T lymphocytes derived from Mycobacterium tuberculosis-infected cells: definition of antigenic specificity. J Immunol. 2001;166(1):439–446. doi: 10.4049/jimmunol.166.1.439. [DOI] [PubMed] [Google Scholar]
- 23.Kawashima T, Norose Y, Watanabe Y, Enomoto Y, Narazaki H, Watari E, Tanaka S, Takahashi H, Yano I, Brenner MB, Sugita M. Cutting edge: major CD8 T cell response to live bacillus Calmette-Guerin is mediated by CD1 molecules. J Immunol. 2003;170(11):5345–5348. doi: 10.4049/jimmunol.170.11.5345. [DOI] [PubMed] [Google Scholar]
- 24.Muller I, Cobbold SP, Waldmann H, Kaufmann SH. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987;55(9):2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedrazzini T, Hug K, Louis JA. Importance of L3T4+ and Lyt-2+ cells in the immunologic control of infection with Mycobacterium bovis strain bacillus Calmette-Guerin in mice. Assessment by elimination of T cell subsets in vivo. J Immunol. 1987;139(6):2032–2037. [PubMed] [Google Scholar]
- 26.Cox JH, Knight BC, Ivanyi J. Mechanisms of recrudescence of Mycobacterium bovis BCG infection in mice. Infect Immun. 1989;57(6):1719–1724. doi: 10.1128/iai.57.6.1719-1724.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tascon RE, Stavropoulos E, Lukacs KV, Colston MJ. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun. 1998;66(2):830–834. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng CG, Britton WJ. CD4+ and CD8+ T cells mediate adoptive immunity to aerosol infection of Mycobacterium bovis bacillus Calmette-Guerin. J Infect Dis. 2000;181(5):1846–1849. doi: 10.1086/315466. [DOI] [PubMed] [Google Scholar]
- 29.Orme IM, Collins FM. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983;158(1):74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonato VL, Lima VM, Tascon RE, Lowrie DB, Silva CL. Identification and Characterization of Protective T Cells in hsp65 DNA-Vaccinated and Mycobacterium tuberculosis-Infected Mice. Infect Immun. 1998;66(1):169–175. doi: 10.1128/iai.66.1.169-175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva CL, Silva MF, Pietro RC, Lowrie DB. Characterization of T cells that confer a high degree of protective immunity against tuberculosis in mice after vaccination with tumor cells expressing mycobacterial hsp65. Infect Immun. 1996;64(7):2400–2407. doi: 10.1128/iai.64.7.2400-2407.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva CL, Silva MF, Pietro RC, Lowrie DB. Protection against tuberculosis by passive transfer with T-cell clones recognizing mycobacterial heat-shock protein 65. Immunology. 1994;83(3):341–346. [PMC free article] [PubMed] [Google Scholar]
- 33.Hussein S, Curtis J, Akuffo H, Turk JL. Dissociation between delayed-type hypersensitivity and resistance to pathogenic mycobacteria demonstrated by T-cell clones. Infect Immun. 1987;55(3):564–567. doi: 10.1128/iai.55.3.564-567.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A. 1992;89(24):12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moody DB, Porcelli SA. Intracellular pathways of CD1 antigen presentation. Nat Rev Immunol. 2003;3(1):11–22. doi: 10.1038/nri979. [DOI] [PubMed] [Google Scholar]
- 36.Lindahl KF, Byers DE, Dabhi VM, Hovik R, Jones EP, Smith GP, Wang CR, Xiao H, Yoshino M. H2-M3, a full-service class Ib histocompatibility antigen. Annu Rev Immunol. 1997;15:851–879. doi: 10.1146/annurev.immunol.15.1.851. [DOI] [PubMed] [Google Scholar]
- 37.Bouwer HG, Seaman MS, Forman J, Hinrichs DJ. MHC class Ib-restricted cells contribute to antilisterial immunity: evidence for Qa-1b as a key restricting element for Listeria-specific CTLs. J Immunol. 1997;159(6):2795–2801. [PubMed] [Google Scholar]
- 38.Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999;189(12):1973–1980. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Kaer L, Ashton-Rickardt PG, Ploegh HL, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4–8+ T cells. Cell. 1992;71(7):1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 40.Ashton-Rickardt PG, Van Kaer L, Schumacher TN, Ploegh HL, Tonegawa S. Peptide contributes to the specificity of positive selection of CD8+ T cells in the thymus. Cell. 1993;73(5):1041–1049. doi: 10.1016/0092-8674(93)90281-t. [DOI] [PubMed] [Google Scholar]
- 41.D’Souza CD, Cooper AM, Frank AA, Ehlers S, Turner J, Bendelac A, Orme IM. A novel nonclassic beta2-microglobulin-restricted mechanism influencing early lymphocyte accumulation and subsequent resistance to tuberculosis in the lung. Am J Respir Cell Mol Biol. 2000;23(2):188–193. doi: 10.1165/ajrcmb.23.2.4063. [DOI] [PubMed] [Google Scholar]
- 42.Sousa AO, Mazzaccaro RJ, Russell RG, Lee FK, Turner OC, Hong S, Van Kaer L, Bloom BR. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc Natl Acad Sci U S A. 2000;97(8):4204–4208. doi: 10.1073/pnas.97.8.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rolph MS, Raupach B, Kobernick HH, Collins HL, Perarnau B, Lemonnier FA, Kaufmann SH. MHC class Ia-restricted T cells partially account for beta2-microglobulin-dependent resistance to Mycobacterium tuberculosis. Eur J Immunol. 2001;31(6):1944–1949. doi: 10.1002/1521-4141(200106)31:6<1944::aid-immu1944>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 44.Turner J, D’Souza CD, Pearl JE, Marietta P, Noel M, Frank AA, Appelberg R, Orme IM, Cooper AM. CD8− and CD95/95L-dependent mechanisms of resistance in mice with chronic pulmonary tuberculosis. Am J Respir Cell Mol Biol. 2001;24(2):203–209. doi: 10.1165/ajrcmb.24.2.4370. [DOI] [PubMed] [Google Scholar]
- 45.Urdahl KB, Liggitt D, Bevan MJ. CD8+ T cells accumulate in the lungs of Mycobacterium tuberculosis-infected Kb−/− Db−/− mice, but provide minimal protection. J Immunol. 2003;170(4):1987–1994. doi: 10.4049/jimmunol.170.4.1987. [DOI] [PubMed] [Google Scholar]
- 46.Rolph MS, Raupach B, Kobernick HH, Collins HL, Perarnau B, Lemonnier FA, Kaufmann SH. MHC class Ia-restricted T cells partially account for beta2-microglobulin-dependent resistance to Mycobacterium tuberculosis. Eur J Immunol. 2001;31(6):1944–1949. doi: 10.1002/1521-4141(200106)31:6<1944::aid-immu1944>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 47.Mogues T, Goodrich M, Ryan L, LaCourse R, North R. The Relative Importance of T Cell Subsets in Immunity and Immunopathology of Airborne Mycobacterium tuberculosis Infection in Mice. J Exp Med. 2001;193(3):271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabaczewski P, Stroynowski I. Expression of secreted and glycosylphosphatidylinositol-bound Qa-2 molecules is dependent on functional TAP-2 peptide transporter. J Immunol. 1994;152(11):5268–5274. [PubMed] [Google Scholar]
- 49.Chiang EY, Stroynowski I. A nonclassical MHC class I molecule restricts CTL-mediated rejection of a syngeneic melanoma tumor. J Immunol. 2004;173(7):4394–4401. doi: 10.4049/jimmunol.173.7.4394. [DOI] [PubMed] [Google Scholar]
- 50.He X, Tabaczewski P, Ho J, Stroynowski I, Garcia KC. Promiscuous antigen presentation by the nonclassical MHC Ib Qa-2 is enabled by a shallow, hydrophobic groove and self-stabilized peptide conformation. Structure. 2001;9(12):1213–1224. doi: 10.1016/s0969-2126(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 51.Tabaczewski P, Chiang E, Henson M, Stroynowski I. Alternative peptide binding motifs of Qa-2 class Ib molecules define rules for binding of self and nonself peptides. J Immunol. 1997;159(6):2771–2781. [PubMed] [Google Scholar]
- 52.Tsujimura K, Obata Y, Iwase S, Matsudaira Y, Ozeki S, Takahashi T. The epitope detected by cytotoxic T lymphocytes against thymus leukemia (TL) antigen is TAP independent. Int Immunol. 2000;12(9):1217–1225. doi: 10.1093/intimm/12.9.1217. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Xiong Y, Naidenko OV, Liu JH, Zhang R, Joachimiak A, Kronenberg M, Cheroutre H, Reinherz EL, Wang JH. The crystal structure of a TL/CD8alphaalpha complex at 2. 1 A resolution: implications for modulation of T cell activation and memory. Immunity. 2003;18(2):205–215. doi: 10.1016/s1074-7613(03)00027-x. [DOI] [PubMed] [Google Scholar]
- 54.Leishman AJ, Naidenko OV, Attinger A, Koning F, Lena CJ, Xiong Y, Chang HC, Reinherz E, Kronenberg M, Cheroutre H. T cell responses modulated through interaction between CD8alphaalpha and the nonclassical MHC class I molecule, TL. Science. 2001;294(5548):1936–1939. doi: 10.1126/science.1063564. [DOI] [PubMed] [Google Scholar]
- 55.Rodgers JR, Mehta V, Cook RG. Surface expression of beta 2-microglobulin-associated thymus-leukemia antigen is independent of TAP2. Eur J Immunol. 1995;25(4):1001–1007. doi: 10.1002/eji.1830250421. [DOI] [PubMed] [Google Scholar]
- 56.Chun T, Grandea AG, III, Lybarger L, Forman J, Van Kaer L, Wang CR. Functional roles of TAP and tapasin in the assembly of M3-N-formylated peptide complexes. J Immunol. 2001;167(3):1507–1514. doi: 10.4049/jimmunol.167.3.1507. [DOI] [PubMed] [Google Scholar]
- 57.Rolph MS, Kaufmann SH. Partially TAP-independent protection against Listeria monocytogenes by H2-M3-restricted CD8+ T cells. J Immunol. 2000;165(8):4575–4580. doi: 10.4049/jimmunol.165.8.4575. [DOI] [PubMed] [Google Scholar]
- 58.Chun T, Serbina NV, Nolt D, Wang B, Chiu NM, Flynn JL, Wang CR. Induction of M3-restricted cytotoxic T lymphocyte responses by N-formylated peptides derived from Mycobacterium tuberculosis. J Exp Med. 2001;193(10):1213–1220. doi: 10.1084/jem.193.10.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Orazio SE, Shaw CA, Starnbach MN. H2-M3-restricted CD8+ T cells are not required for MHC class Ib-restricted immunity against Listeria monocytogenes. J Exp Med. 2006;203(2):383–391. doi: 10.1084/jem.20052256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu H, Chun T, Choi HJ, Wang B, Wang CR. Impaired response to Listeria in H2-M3-deficient mice reveals a nonredundant role of MHC class Ib-specific T cells in host defense. J Exp Med. 2006;203(2):449–459. doi: 10.1084/jem.20051866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seaman MS, Wang CR, Forman J. MHC class Ib-restricted CTL provide protection against primary and secondary Listeria monocytogenes infection. J Immunol. 2000;165(9):5192–5201. doi: 10.4049/jimmunol.165.9.5192. [DOI] [PubMed] [Google Scholar]
- 62.Jiang H, Chess L. The Specific Regulation Of Immune Responses By CD8+ T Cells Restricted By The MHC Class Ib Molecule, Qa-1. Annual Review of Immunology. 2000;18(1):185–216. doi: 10.1146/annurev.immunol.18.1.185. [DOI] [PubMed] [Google Scholar]
- 63.Sarantopoulos S, Lu L, Cantor H. Qa-1 restriction of CD8+ suppressor T cells. J Clin Invest. 2004;114(9):1218–1221. doi: 10.1172/JCI23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li L, Sullivan BA, Aldrich CJ, Soloski MJ, Forman J, Grandea AG, III, Jensen PE, Van Kaer L. Differential Requirement for Tapasin in the Presentation of Leader- and Insulin-Derived Peptide Antigens to Qa-1b-Restricted CTLs. J Immunol. 2004;173(6):3707–3715. doi: 10.4049/jimmunol.173.6.3707. [DOI] [PubMed] [Google Scholar]
- 65.Wilson IA, Bjorkman PJ. Unusual MHC-like molecules; CD1, Fc receptor, the hemochromatosis gene product, and viral homologs. Current Opinion in Immunology. 1998;10(1):67–73. doi: 10.1016/s0952-7915(98)80034-4. [DOI] [PubMed] [Google Scholar]
- 66.Muckenthaler MU, Rodrigues P, Macedo MG, Minana B, Brennan K, Cardoso EM, Hentze MW, de Sousa M. Molecular analysis of iron overload in [beta]2-microglobulin-deficient mice. Blood Cells, Molecules, and Diseases. 2004;33(2):125–131. doi: 10.1016/j.bcmd.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 67.Schaible UE, Collins HL, Priem F, Kaufmann SHE. Correction of the Iron Overload Defect in {beta}-2-Microglobulin Knockout Mice by Lactoferrin Abolishes Their Increased Susceptibility to Tuberculosis. J Exp Med. 2002;196(11):1507–1513. doi: 10.1084/jem.20020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miranda CJ, Makui H, Andrews NC, Santos MM. Contributions of {beta}2-microglobulin-dependent molecules and lymphocytes to iron regulation: insights from HfeRag1−/− and {beta}2mRag1−/− double knock-out mice. Blood. 2004;103(7):2847–2849. doi: 10.1182/blood-2003-09-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Natarajan K, Dimasi N, Wang J, Mariuzza RA, Margulies DH. Structure and function of natural killer cell receptors: multiple molecular solutions to self, nonself discrimination. Annu Rev Immunol. 2002;20:853–85. 853–885. doi: 10.1146/annurev.immunol.20.100301.064812. [DOI] [PubMed] [Google Scholar]
- 70.Dorfman JR, Zerrahn J, Coles MC, Raulet DH. The basis for self-tolerance of natural killer cells in beta2- microglobulin- and TAP-1- mice. J Immunol. 1997;159(11):5219–5225. [PubMed] [Google Scholar]
- 71.Ljunggren HG, Van Kaer L, Ploegh HL, Tonegawa S. Altered natural killer cell repertoire in Tap-1 mutant mice. Proc Natl Acad Sci U S A. 1994;91(14):6520–6524. doi: 10.1073/pnas.91.14.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253(5016):199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- 73.Fung-Leung WP, Schilham MW, Rahemtulla A, Kundig TM, Vollenweider M, Potter J, van Ewijk W, Mak TW. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65(3):443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 74.Urdahl KB, Liggitt D, Bevan MJ. CD8+ T cells accumulate in the lungs of Mycobacterium tuberculosis-infected Kb−/− Db−/− mice, but provide minimal protection. J Immunol. 2003;170(4):1987–1994. doi: 10.4049/jimmunol.170.4.1987. [DOI] [PubMed] [Google Scholar]
- 75.Dalloul AH, Chmouzis E, Ngo K, Fung-Leung WP. Adoptively transferred CD4+ lymphocytes from CD8 −/− mice are sufficient to mediate the rejection of MHC class II or class I disparate skin grafts. J Immunol. 1996;156(11):4114–4119. [PubMed] [Google Scholar]
- 76.Dalloul AH, Ngo K, Fung-Leung WP. CD4-negative cytotoxic T cells with a T cell receptor alpha/beta intermediate expression in CD8-deficient mice. Eur J Immunol. 1996;26(1):213–218. doi: 10.1002/eji.1830260133. [DOI] [PubMed] [Google Scholar]
- 77.Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162(9):5407–5416. [PubMed] [Google Scholar]
- 78.Derrick SC, Repique C, Snoy P, Yang AL, Morris S. Immunization with a DNA vaccine cocktail protects mice lacking CD4 cells against an aerogenic infection with Mycobacterium tuberculosis. Infect Immun. 2004;72(3):1685–1692. doi: 10.1128/IAI.72.3.1685-1692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rahemtulla A, Kundig TM, Narendran A, Bachmann MF, Julius M, Paige CJ, Ohashi PS, Zinkernagel RM, Mak TW. Class II major histocompatibility complex-restricted T cell function in CD4-deficient mice. Eur J Immunol. 1994;24(9):2213–2218. doi: 10.1002/eji.1830240942. [DOI] [PubMed] [Google Scholar]
- 80.Locksley RM, Reiner SL, Hatam F, Littman DR, Killeen N. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science. 1993;261(5127):1448–1451. doi: 10.1126/science.8367726. [DOI] [PubMed] [Google Scholar]
- 81.Tyznik AJ, Sun JC, Bevan MJ. The CD8 population in CD4-deficient mice is heavily contaminated with MHC class II-restricted T cells. J Exp Med. 2004;199(4):559–565. doi: 10.1084/jem.20031961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pearce EL, Shedlock DJ, Shen H. Functional characterization of MHC class II-restricted CD8+CD4− and CD8-CD4− T cell responses to infection in CD4−/− mice. J Immunol. 2004;173(4):2494–2499. doi: 10.4049/jimmunol.173.4.2494. [DOI] [PubMed] [Google Scholar]
- 83.Serbina NV, Lazarevic V, Flynn JL. CD4(+) T cells are required for the development of cytotoxic CD8(+) T cells during Mycobacterium tuberculosis infection. J Immunol. 2001;167(12):6991–7000. doi: 10.4049/jimmunol.167.12.6991. [DOI] [PubMed] [Google Scholar]
- 84.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5(9):927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Pinxteren LA, Cassidy JP, Smedegaard BH, Agger EM, Andersen P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur J Immunol. 2000;30(12):3689–3698. doi: 10.1002/1521-4141(200012)30:12<3689::AID-IMMU3689>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 86.Lewinsohn DA, Heinzel AS, Gardner JM, Zhu L, Alderson MR, Lewinsohn DM. Mycobacterium tuberculosis-specific CD8+ T Cells Preferentially Recognize Heavily Infected Cells. Am J Respir Crit Care Med. 2003;168(11):1346–1352. doi: 10.1164/rccm.200306-837OC. [DOI] [PubMed] [Google Scholar]
- 87.Serbina NV, Flynn JL. CD8(+) T cells participate in the memory immune response to Mycobacterium tuberculosis. Infect Immun. 2001;69(7):4320–4328. doi: 10.1128/IAI.69.7.4320-4328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, Melian A, Bogdan C, Porcelli SA, Bloom BR, Krensky AM, Modlin RL. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282(5386):121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 89.Porcelli SA, Modlin RL. THE CD1 SYSTEM: Antigen-Presenting Molecules for T Cell Recognition of Lipids and Glycolipids. Annual Review of Immunology. 1999;17(1):297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 90.Brigl M, Brenner MB. CD1: Antigen Presentation and T Cell Function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 91.Skold M, Behar SM. The role of group 1 and group 2 CD1-restricted T cells in microbial immunity. Microbes Infect. 2005;7(3):544–551. doi: 10.1016/j.micinf.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 92.Ulrichs T, Moody DB, Grant E, Kaufmann SH, Porcelli SA. T-cell responses to CD1-presented lipid antigens in humans with Mycobacterium tuberculosis infection. Infect Immun. 2003;71(6):3076–3087. doi: 10.1128/IAI.71.6.3076-3087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dascher CC, Hiromatsu K, Xiong X, Morehouse C, Watts G, Liu G, McMurray DN, LeClair KP, Porcelli SA, Brenner MB. Immunization with a mycobacterial lipid vaccine improves pulmonary pathology in the guinea pig model of tuberculosis. Int Immunol. 2003;15(8):915–925. doi: 10.1093/intimm/dxg091. [DOI] [PubMed] [Google Scholar]
- 94.Dascher CC, Brenner MB. Evolutionary constraints on CD1 structure: insights from comparative genomic analysis. Trends Immunol. 2003;24(8):412–418. doi: 10.1016/s1471-4906(03)00179-0. [DOI] [PubMed] [Google Scholar]
- 95.Dascher CC, Hiromatsu K, Xiong X, Sugita M, Buhlmann JE, Dodge IL, Lee SY, Roura-Mir C, Watts GF, Roy CJ, Behar SM, Clemens DL, Porcelli SA, Brenner MB. Conservation of CD1 intracellular trafficking patterns between mammalian species. J Immunol. 2002;169(12):6951–6958. doi: 10.4049/jimmunol.169.12.6951. [DOI] [PubMed] [Google Scholar]
- 96.Hiromatsu K, Dascher CC, Sugita M, Gingrich-Baker C, Behar SM, LeClair KP, Brenner MB, Porcelli SA. Characterization of guinea-pig group 1 CD1 proteins. Immunology. 2002;106(2):159–172. doi: 10.1046/j.1365-2567.2002.01422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dascher CC, Hiromatsu K, Naylor JW, Brauer PP, Brown KA, Storey JR, Behar SM, Kawasaki ES, Porcelli SA, Brenner MB, LeClair Conservation of a CD1 Multigene Family in the Guinea Pig. J Immunol. 1999;163(10):5478–5488. [PubMed] [Google Scholar]
- 98.Robinson JH, Delvig AA. Diversity in MHC class II antigen presentation. Immunology. 2002;105(3):252–262. doi: 10.1046/j.0019-2805.2001.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yewdell JW, Reits E, Neefjes J. Making sense of mass destruction: quantitating MHC class I antigen presentation. Nat Rev Immunol. 2003;3(12):952–961. doi: 10.1038/nri1250. [DOI] [PubMed] [Google Scholar]
- 100.Antoniou AN, Powis SJ, Elliott T. Assembly and export of MHC class I peptide ligands. Curr Opin Immunol. 2003;15(1):75–81. doi: 10.1016/s0952-7915(02)00010-9. [DOI] [PubMed] [Google Scholar]
- 101.Wong P, Pamer EG. CD8 T cell responses to infectious pathogens. Annu Rev Immunol. 2003;21:29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- 102.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 103.Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol Rev. 2005;207:145–157. doi: 10.1111/j.0105-2896.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 104.Denis O, Tanghe A, Palfliet K, Jurion F, van den Berg TP, Vanonckelen A, Ooms J, Saman E, Ulmer JB, Content J, Huygen K. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect Immun. 1998;66(4):1527–1533. doi: 10.1128/iai.66.4.1527-1533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vogel TU, Horton H, Fuller DH, Carter DK, Vielhuber K, O’Connor DH, Shipley T, Fuller J, Sutter G, Erfle V, Wilson N, Picker LJ, Watkins DI. Differences Between T Cell Epitopes Recognized After Immunization and After Infection. J Immunol. 2002;169(8):4511–4521. doi: 10.4049/jimmunol.169.8.4511. [DOI] [PubMed] [Google Scholar]
- 106.Zhu X, Venkataprasad N, Thangaraj HS, Hill M, Singh M, Ivanyi J, Vordermeier HM. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158(12):5921–5926. [PubMed] [Google Scholar]
- 107.Vordermeier HM, Harris DP, Lathigra R, Roman E, Moreno C, Ivanyi J. Recognition of peptide epitopes of the 16,000 MW antigen of Mycobacterium tuberculosis by murine T cells. Immunology. 1993;80(1):6–12. [PMC free article] [PubMed] [Google Scholar]
- 108.Smith SM, Brookes R, Klein MR, Malin AS, Lukey PT, King AS, Ogg GS, Hill AV, Dockrell HM. Human CD8+ CTL specific for the mycobacterial major secreted antigen 85A. J Immunol. 2000;165(12):7088–7095. doi: 10.4049/jimmunol.165.12.7088. [DOI] [PubMed] [Google Scholar]
- 109.Geluk A, van Meijgaarden KE, Franken KL, Drijfhout JW, D’Souza S, Necker A, Huygen K, Ottenhoff TH. Identification of major epitopes of Mycobacterium tuberculosis AG85B that are recognized by HLA-A*0201-restricted CD8+ T cells in HLA-transgenic mice and humans. J Immunol. 2000;165(11):6463–6471. doi: 10.4049/jimmunol.165.11.6463. [DOI] [PubMed] [Google Scholar]
- 110.Klein MR, Smith SM, Hammond AS, Ogg GS, King AS, Vekemans J, Jaye A, Lukey PT, McAdam KP. HLA-B*35-restricted CD8 T cell epitopes in the antigen 85 complex of Mycobacterium tuberculosis. J Infect Dis. 2001;183(6):928–934. doi: 10.1086/319267. [DOI] [PubMed] [Google Scholar]
- 111.Zhu X, Stauss HJ, Ivanyi J, Vordermeier HM. Specificity of CD8+ T cells from subunit-vaccinated and infected H-2b mice recognizing the 38 kDa antigen of Mycobacterium tuberculosis. Int Immunol. 1997;9(11):1669–1676. doi: 10.1093/intimm/9.11.1669. [DOI] [PubMed] [Google Scholar]
- 112.Vordermeier HM, Zhu X, Harris DP. Induction of CD8+ CTL recognizing mycobacterial peptides. Scand J Immunol. 1997;45(5):521–526. doi: 10.1046/j.1365-3083.1997.d01-432.x. [DOI] [PubMed] [Google Scholar]
- 113.Friscia G, Vordermeier HM, Pasvol G, Harris DP, Moreno C, Ivanyi J. Human T cell responses to peptide epitopes of the 16-kD antigen in tuberculosis. Clin Exp Immunol. 1995;102(1):53–57. doi: 10.1111/j.1365-2249.1995.tb06635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen W, Carbone FR, McCluskey J. Electroporation and commercial liposomes efficiently deliver soluble protein into the MHC class I presentation pathway. Priming in vitro and in vivo for class I-restricted recognition of soluble antigen. J Immunol Methods. 1993;160(1):49–57. doi: 10.1016/0022-1759(93)90007-t. [DOI] [PubMed] [Google Scholar]
- 115.Reddy R, Zhou F, Huang L, Carbone F, Bevan M, Rouse BT. pH sensitive liposomes provide an efficient means of sensitizing target cells to class I restricted CTL recognition of a soluble protein. J Immunol Methods. 1991;141(2):157–163. doi: 10.1016/0022-1759(91)90142-3. [DOI] [PubMed] [Google Scholar]
- 116.Lewinsohn DA, Lines RA, Lewinsohn DM. Human dendritic cells presenting adenovirally expressed antigen elicit Mycobacterium tuberculosis--specific CD8+ T cells. Am J Respir Crit Care Med. 2002;166(6):843–848. doi: 10.1164/rccm.2110094. [DOI] [PubMed] [Google Scholar]
- 117.Nussbaum AK, Kuttler C, Tenzer S, Schild H. Using the World Wide Web for predicting CTL epitopes. Curr Opin Immunol. 2003;15(1):69–74. doi: 10.1016/s0952791502000043. [DOI] [PubMed] [Google Scholar]
- 118.Lewinsohn DM, Zhu L, Madison VJ, Dillon DC, Fling SP, Reed SG, Grabstein KH, Alderson MR. Classically Restricted Human CD8+ T Lymphocytes Derived from Mycobacterium tuberculosis-Infected Cells: Definition of Antigenic Specificity. J Immunol. 2001;166(1):439–446. doi: 10.4049/jimmunol.166.1.439. [DOI] [PubMed] [Google Scholar]
- 119.Sospedra M, Pinilla C, Martin R. Use of combinatorial peptide libraries for T-cell epitope mapping. Methods. 2003;29(3):236–247. doi: 10.1016/s1046-2023(02)00346-8. [DOI] [PubMed] [Google Scholar]
- 120.Lewinsohn DM, Briden AL, Reed SG, Grabstein KH, Alderson MR. Mycobacterium tuberculosis-reactive CD8+ T lymphocytes: the relative contribution of classical versus nonclassical HLA restriction. J Immunol. 2000;165(2):925–930. doi: 10.4049/jimmunol.165.2.925. [DOI] [PubMed] [Google Scholar]
- 121.Dong Y, Demaria S, Sun X, Santori FR, Jesdale BM, De Groot AS, Rom WN, Bushkin Y. HLA-A2-Restricted CD8+-Cytotoxic-T-Cell Responses to Novel Epitopes in Mycobacterium tuberculosis Superoxide Dismutase, Alanine Dehydrogenase, and Glutamine Synthetase. Infect Immun. 2004;72(4):2412–2415. doi: 10.1128/IAI.72.4.2412-2415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Majlessi L, Rojas MJ, Brodin P, Leclerc C. CD8+-T-cell responses of Mycobacterium-infected mice to a newly identified major histocompatibility complex class I-restricted epitope shared by proteins of the ESAT-6 family. Infect Immun. 2003;71(12):7173–7177. doi: 10.1128/IAI.71.12.7173-7177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hammond AS, Klein MR, Corrah T, Fox A, Jaye A, McAdam KP, Brookes RH. Mycobacterium tuberculosis genome-wide screen exposes multiple CD8+ T cell epitopes. Clinical and Experimental Immunology. 2005;140(1):109–116. doi: 10.1111/j.1365-2249.2005.02751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gomez-Nunez M, Pinilla-Ibarz J, Dao T, May RJ, Pao M, Jaggi JS, Scheinberg DA. Peptide binding motif predictive algorithms correspond with experimental binding of leukemia vaccine candidate peptides to HLA-A*0201 molecules. Leuk Res. 2006 doi: 10.1016/j.leukres.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 125.Andersen MH, Tan L, Sondergaard I, Zeuthen J, Elliott T, Haurum JS. Poor correspondence between predicted and experimental binding of peptides to class I MHC molecules. Tissue Antigens. 2000;55(6):519–531. doi: 10.1034/j.1399-0039.2000.550603.x. [DOI] [PubMed] [Google Scholar]
- 126.Kamath AB, Woodworth J, Xiong X, Taylor C, Weng Y, Behar SM. Cytolytic CD8+ T Cells Recognizing CFP10 Are Recruited to the Lung after Mycobacterium tuberculosis Infection. J Exp Med. 2004;200:1479–1489. doi: 10.1084/jem.20041690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Regner M, Mullbacher A, Blanden RV, Lobigs M. Immunogenicity of two peptide determinants in the cytolytic T-cell response to flavivirus infection: inverse correlation between peptide affinity for MHC class I and T-cell precursor frequency. Viral Immunol. 2001;14(2):135–149. doi: 10.1089/088282401750234510. [DOI] [PubMed] [Google Scholar]
- 128.Gray PM, Parks GD, Alexander-Miller MA. High avidity CD8+ T cells are the initial population elicited following viral infection of the respiratory tract. J Immunol. 2003;170(1):174–181. doi: 10.4049/jimmunol.170.1.174. [DOI] [PubMed] [Google Scholar]
- 129.Moreira AL, Wang J, Tsenova-Berkova L, Hellmann W, Freedman VH, Kaplan G. Sequestration of Mycobacterium tuberculosis in tight vacuoles in vivo in lung macrophages of mice infected by the respiratory route. Infect Immun. 1997;65(1):305–308. doi: 10.1128/iai.65.1.305-308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Clemens DL, Horwitz MA. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181(1):257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dillon DC, Alderson MR, Day CH, Bement T, Campos-Neto A, Skeiky YA, Vedvick T, Badaro R, Reed SG, Houghton R. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J Clin Microbiol. 2000;38(9):3285–3290. doi: 10.1128/jcm.38.9.3285-3290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arend SM, Andersen P, van Meijgaarden KE, Skjot RL, Subronto YW, van Dissel JT, Ottenhoff TH. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J Infect Dis. 2000;181(5):1850–1854. doi: 10.1086/315448. [DOI] [PubMed] [Google Scholar]
- 133.Tsai B, Ye Y, Rapoport TA. Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat Rev Mol Cell Biol. 2002;3(4):246–255. doi: 10.1038/nrm780. [DOI] [PubMed] [Google Scholar]
- 134.Schaible UE, Winau F, Sieling PA, Fischer K, Collins HL, Hagens K, Modlin RL, Brinkmann V, Kaufmann SH. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med. 2003;9(8):1039–1046. doi: 10.1038/nm906. [DOI] [PubMed] [Google Scholar]
- 135.Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, Sandhoff K, Brinkmann V, Kaufmann SH, Schaible UE. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24(1):105–117. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 136.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Barrell BG. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 137.Brosch R, Gordon SV, Marmiesse M, Brodin P, Buchrieser C, Eiglmeier K, Garnier T, Gutierrez C, Hewinson G, Kremer K, Parsons LM, Pym AS, Samper S, van Soolingen D, Cole ST. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci U S A. 2002;99(6):3684–3689. doi: 10.1073/pnas.052548299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol. 2002;46(3):709–717. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- 139.Harboe M, Oettinger T, Wiker HG, Rosenkrands I, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64(1):16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sorensen AL, Nagai S, Houen G, Andersen P, Andersen AB. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63(5):1710–1717. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, Griffiths KE, Marchal G, Leclerc C, Cole ST. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med. 2003;9(5):533–539. doi: 10.1038/nm859. [DOI] [PubMed] [Google Scholar]
- 142.Renshaw PS, Panagiotidou P, Whelan A, Gordon SV, Hewinson RG, Williamson RA, Carr MD. Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6*CFP-10 complex. Implications for pathogenesis and virulence. J Biol Chem. 2002;277(24):21598–21603. doi: 10.1074/jbc.M201625200. [DOI] [PubMed] [Google Scholar]
- 143.Skjot RL, Brock I, Arend SM, Munk ME, Theisen M, Ottenhoff TH, Andersen P. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12. 9, which constitute a subfamily of the esat-6 gene family. Infect Immun. 2002;70(10):5446–5453. doi: 10.1128/IAI.70.10.5446-5453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Andersen P, Andersen AB, Sorensen AL, Nagai S, Srensen AL. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154(7):3359–3372. [PubMed] [Google Scholar]
- 145.Berthet FX, Rasmussen PB, Rosenkrands I, Andersen P, Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10) Microbiology. 1998;144(Pt 11):3195–3203. doi: 10.1099/00221287-144-11-3195. [DOI] [PubMed] [Google Scholar]
- 146.Hsu T, Hingley-Wilson SM, Chen B, Chen M, Dai AZ, Morin PM, Marks CB, Padiyar J, Goulding C, Gingery M, Eisenberg D, Russell RG, Derrick SC, Collins FM, Morris SL, King CH, Jacobs WR., Jr The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc Natl Acad Sci U S A. 2003;100(21):12420–12425. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shams H, Klucar P, Weis SE, Lalvani A, Moonan PK, Safi H, Wizel B, Ewer K, Nepom GT, Lewinsohn DM, Andersen P, Barnes PF. Characterization of a Mycobacterium tuberculosis peptide that is recognized by human CD4+ and CD8+ T cells in the context of multiple HLA alleles. J Immunol. 2004;173(3):1966–1977. doi: 10.4049/jimmunol.173.3.1966. [DOI] [PubMed] [Google Scholar]
- 148.Pathan AA, Wilkinson KA, Wilkinson RJ, Latif M, McShane H, Pasvol G, Hill AV, Lalvani A. High frequencies of circulating IFN-gamma-secreting CD8 cytotoxic T cells specific for a novel MHC class I-restricted Mycobacterium tuberculosis epitope in M. tuberculosis-infected subjects without disease. Eur J Immunol. 2000;30(9):2713–2721. doi: 10.1002/1521-4141(200009)30:9<2713::AID-IMMU2713>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 149.Suzuki M, Aoshi T, Nagata T, Koide Y. Identification of murine H2-Dd- and H2-Ab-restricted T-cell epitopes on a novel protective antigen, MPT51, of Mycobacterium tuberculosis. Infect Immun. 2004;72(7):3829–3837. doi: 10.1128/IAI.72.7.3829-3837.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Young DB, Garbe TR. Lipoprotein antigens of Mycobacterium tuberculosis. Res Microbiol. 1991;142(1):55–65. doi: 10.1016/0923-2508(91)90097-t. [DOI] [PubMed] [Google Scholar]
- 151.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285(5428):732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 152.Neyrolles O, Gould K, Gares MP, Brett S, Janssen R, O’Gaora P, Herrmann JL, Prevost MC, Perret E, Thole JE, Young D. Lipoprotein access to MHC class I presentation during infection of murine macrophages with live mycobacteria. J Immunol. 2001;166(1):447–457. doi: 10.4049/jimmunol.166.1.447. [DOI] [PubMed] [Google Scholar]
- 153.Hohn H, Kortsik C, Nilges K, Necker A, Freitag K, Tully G, Neukirch C, Maeurer MJ. Human leucocyte antigen-A2 restricted and Mycobacterium tuberculosis 19-kDa antigen-specific CD8+ T-cell responses are oligoclonal and exhibit a T-cell cytotoxic type 2 response cytokine-secretion pattern. Immunology. 2001;104(3):278–288. doi: 10.1046/j.1365-2567.2001.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.HOHN H, KORTSIK C, TULLY G, Nilges K, Necker A, FREITAG K, NEUKIRCH C, Galle P, Lohr H, Maeurer MJ. Longitudinal analysis of Mycobacterium tuberculosis 19-kDa antigen-specific T cells in patients with pulmonary tuberculosis: association with disease activity and cross-reactivity to a peptide from HIVenv gp120. Eur J Immunol. 2003;33(6):1613–1623. doi: 10.1002/eji.200323480. [DOI] [PubMed] [Google Scholar]
- 155.Chang Z, Primm TP, Jakana J, Lee IH, Serysheva I, Chiu W, Gilbert HF, Quiocho FA. Mycobacterium tuberculosis 16-kDa Antigen (Hsp16. 3) Functions as an Oligomeric Structure in Vitro to Suppress Thermal Aggregation. J Biol Chem. 1996;271(12):7218–7223. [PubMed] [Google Scholar]
- 156.Caccamo N, Milano S, Di Sano C, Cigna D, Ivanyi J, Krensky AM, Dieli F, Salerno A. Identification of epitopes of Mycobacterium tuberculosis 16-kDa protein recognized by human leukocyte antigen-A*0201 CD8(+) T lymphocytes. J Infect Dis. 2002;186(7):991–998. doi: 10.1086/344174. [DOI] [PubMed] [Google Scholar]
- 157.Feng CG, Demangel C, Kamath AT, Macdonald M, Britton WJ. Dendritic cells infected with Mycobacterium bovis bacillus Calmette Guerin activate CD8(+) T cells with specificity for a novel mycobacterial epitope. Int Immunol. 2001;13(4):451–458. doi: 10.1093/intimm/13.4.451. [DOI] [PubMed] [Google Scholar]
- 158.Irwin SM, Izzo AA, Dow SW, Skeiky YA, Reed SG, Alderson MR, Orme IM. Tracking antigen-specific CD8 T lymphocytes in the lungs of mice vaccinated with the Mtb72F polyprotein. Infect Immun. 2005;73(9):5809–5816. doi: 10.1128/IAI.73.9.5809-5816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Skeiky YA, Alderson MR, Ovendale PJ, Guderian JA, Brandt L, Dillon DC, Campos-Neto A, Lobet Y, Dalemans W, Orme IM, Reed SG. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J Immunol. 2004;172(12):7618–7628. doi: 10.4049/jimmunol.172.12.7618. [DOI] [PubMed] [Google Scholar]
- 160.Leslie DS, Vincent MS, Spada FM, Das H, Sugita M, Morita CT, Brenner MB. CD1-mediated gamma/delta T cell maturation of dendritic cells. J Exp Med. 2002;196(12):1575–1584. doi: 10.1084/jem.20021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Spada FM, Grant EP, Peters PJ, Sugita M, Melian A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, Majdic O, Porcelli SA, Morita CT, Brenner MB. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity [see comments] J Exp Med. 2000;191(6):937–948. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Taylor JL, Turner OC, Basaraba RJ, Belisle JT, Huygen K, Orme IM. Pulmonary necrosis resulting from DNA vaccination against tuberculosis. Infect Immun. 2003;71(4):2192–2198. doi: 10.1128/IAI.71.4.2192-2198.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Taylor JL, Ordway DJ, Troudt J, Gonzalez-Juarrero M, Basaraba RJ, Orme IM. Factors Associated with Severe Granulomatous Pneumonia in Mycobacterium tuberculosis-Infected Mice Vaccinated Therapeutically with hsp65 DNA. Infect Immun. 2005;73(8):5189–5193. doi: 10.1128/IAI.73.8.5189-5193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Skold M, Xiong X, Illarionov PA, Besra GS, Behar SM. Interplay of Cytokines and Microbial Signals in Regulation of CD1d Expression and NKT Cell Activation. J Immunol. 2005;175(6):3584–3593. doi: 10.4049/jimmunol.175.6.3584. [DOI] [PubMed] [Google Scholar]
- 165.Ehrt S, Schnappinger D, Bekiranov S, Drenkow J, Shi S, Gingeras TR, Gaasterland T, Schoolnik G, Nathan C. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J Exp Med. 2001;194(8):1123–1140. doi: 10.1084/jem.194.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science. 2003;302(5645):654–659. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- 167.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178(6):2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178(6):2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jouanguy E, Doffinger R, Dupuis S, Pallier A, Altare F, Casanova JL. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol. 1999;11(3):346–351. doi: 10.1016/s0952-7915(99)80055-7. [DOI] [PubMed] [Google Scholar]
- 170.Madariaga L, Amurrio C, Martin G, Garcia-Cebrian F, Bicandi J, Lardelli P, Suarez MD, Cisterna R. Detection of anti-interferon-gamma autoantibodies in subjects infected by Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 1998;2(1):62–68. [PubMed] [Google Scholar]
- 171.Doffinger R, Helbert MR, Barcenas-Morales G, Yang K, Dupuis S, Ceron-Gutierrez L, Espitia-Pinzon C, Barnes N, Bothamley G, Casanova JL, Longhurst HJ, Kumararatne DS. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis. 2004;38(1):e10–e14. doi: 10.1086/380453. [DOI] [PubMed] [Google Scholar]
- 172.Shams H, Wizel B, Weis SE, Samten B, Barnes PF. Contribution of CD8(+) T cells to gamma interferon production in human tuberculosis. Infect Immun. 2001;69(5):3497–3501. doi: 10.1128/IAI.69.5.3497-3501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Feng CG, Bean AG, Hooi H, Briscoe H, Britton WJ. Increase in gamma interferon-secreting CD8(+), as well as CD4(+), T cells in lungs following aerosol infection with Mycobacterium tuberculosis. Infect Immun. 1999;67(7):3242–3247. doi: 10.1128/iai.67.7.3242-3247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Feng CG, Collazo-Custodio CM, Eckhaus M, Hieny S, Belkaid Y, Elkins K, Jankovic D, Taylor GA, Sher A. Mice deficient in LRG-47 display increased susceptibility to mycobacterial infection associated with the induction of lymphopenia. J Immunol. 2004;172(2):1163–1168. doi: 10.4049/jimmunol.172.2.1163. [DOI] [PubMed] [Google Scholar]
- 175.Serbina NV, Flynn JL. Early emergence of CD8(+) T cells primed for production of type 1 cytokines in the lungs of Mycobacterium tuberculosis-infected mice. Infect Immun. 1999;67(8):3980–3988. doi: 10.1128/iai.67.8.3980-3988.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119(6):753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 177.North RJ, Jung YJ. Immunity to Tuberculosis. Annual Review of Immunology. 2004;22(1):599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 178.TULLY G, KORTSIK C, HOHN H, Zehbe I, Hitzler WE, NEUKIRCH C, FREITAG K, Kayser K, Maeurer MJ. Highly focused T cell responses in latent human pulmonary Mycobacterium tuberculosis infection. J Immunol. 2005;174(4):2174–2184. doi: 10.4049/jimmunol.174.4.2174. [DOI] [PubMed] [Google Scholar]
- 179.Goter-Robinson C, Derrick SC, Yang AL, Jeon BY, Morris SL. Protection against an aerogenic Mycobacterium tuberculosis infection in BCG-immunized and DNA-vaccinated mice is associated with early type I cytokine responses. Vaccine. 2006;24(17):3522–3529. doi: 10.1016/j.vaccine.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 180.McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, Fletcher HA, Hill AV. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10(11):1240–1244. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 181.Kamath AB, Woodworth J, Xiong X, Taylor C, Weng Y, Behar SM. Cytolytic CD8+ T Cells Recognizing CFP10 Are Recruited to the Lung after Mycobacterium tuberculosis Infection. J Exp Med. 2004;200(11):1479–1489. doi: 10.1084/jem.20041690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Temmerman ST, Place S, Debrie AS, Locht C, Mascart F. Effector functions of heparin-binding hemagglutinin-specific CD8+ T lymphocytes in latent human tuberculosis. J Infect Dis. 2005;192(2):226–232. doi: 10.1086/430930. [DOI] [PubMed] [Google Scholar]
- 183.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2(6):561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 184.Garcia I, Miyazaki Y, Marchal G, Lesslauer W, Vassalli P. High sensitivity of transgenic mice expressing soluble TNFR1 fusion protein to mycobacterial infections: synergistic action of TNF and IFN-gamma in the differentiation of protective granulomas. Eur J Immunol. 1997;27(12):3182–3190. doi: 10.1002/eji.1830271215. [DOI] [PubMed] [Google Scholar]
- 185.Bean AG, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, Britton WJ. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999;162(6):3504–3511. [PubMed] [Google Scholar]
- 186.Botha T, Ryffel B. Reactivation of latent tuberculosis infection in TNF-deficient mice. J Immunol. 2003;171(6):3110–3118. doi: 10.4049/jimmunol.171.6.3110. [DOI] [PubMed] [Google Scholar]
- 187.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis Associated with Infliximab, a Tumor Necrosis Factor {alpha}-Neutralizing Agent. N Engl J Med. 2001;345(15):1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 188.Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38(9):1261–1265. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 189.Wallis RS, Broder M, Wong J, Beenhouwer D. Granulomatous infections due to tumor necrosis factor blockade: correction. Clin Infect Dis. 2004;39(8):1254–1255. doi: 10.1086/424455. [DOI] [PubMed] [Google Scholar]
- 190.Scott Algood HM, Flynn JL. CCR5-Deficient Mice Control Mycobacterium tuberculosis Infection despite Increased Pulmonary Lymphocytic Infiltration. J Immunol. 2004;173(5):3287–3296. doi: 10.4049/jimmunol.173.5.3287. [DOI] [PubMed] [Google Scholar]
- 191.Barry SM, Lipman MC, Bannister B, Johnson MA, Janossy G. Purified protein derivative-activated type 1 cytokine-producing CD4+ T lymphocytes in the lung: a characteristic feature of active pulmonary and nonpulmonary tuberculosis. J Infect Dis. 2003;187(2):243–250. doi: 10.1086/346112. [DOI] [PubMed] [Google Scholar]
- 192.Keane J, Shurtleff B, Kornfeld H. TNF-dependent BALB/c murine macrophage apoptosis following Mycobacterium tuberculosis infection inhibits bacillary growth in an IFN-gamma independent manner. Tuberculosis (Edinb ) 2002;82(2–3):55–61. doi: 10.1054/tube.2002.0322. [DOI] [PubMed] [Google Scholar]
- 193.Flynn JL. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinb ) 2004;84(1–2):93–101. doi: 10.1016/j.tube.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 194.Smith SM, Klein MR, Malin AS, Sillah J, McAdam KP, Dockrell HM. Decreased IFN-gamma and increased IL-4 production by human CD8(+) T cells in response to Mycobacterium tuberculosis in tuberculosis patients. Tuberculosis (Edinb ) 2002;82(1):7–13. doi: 10.1054/tube.2001.0317. [DOI] [PubMed] [Google Scholar]
- 195.Fenhalls G, Stevens L, Bezuidenhout J, Amphlett GE, Duncan K, Bardin P, Lukey PT. Distribution of IFN-gamma, IL-4 and TNF-alpha protein and CD8 T cells producing IL-12p40 mRNA in human lung tuberculous granulomas. Immunology. 2002;105(3):325–335. doi: 10.1046/j.1365-2567.2002.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cho S, Mehra V, Thoma-Uszynski S, Stenger S, Serbina N, Mazzaccaro RJ, Flynn JL, Barnes PF, Southwood S, Celis E, Bloom BR, Modlin RL, Sette A. Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc Natl Acad Sci U S A. 2000;97(22):12210–12215. doi: 10.1073/pnas.210391497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.De Libero G, Flesch I, Kaufmann SH. Mycobacteria-reactive Lyt-2+ T cell lines. Eur J Immunol. 1988;18(1):59–66. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- 198.Fratazzi C, Arbeit RD, Carini C, Remold HG. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J Immunol. 1997;158(9):4320–4327. [PubMed] [Google Scholar]
- 199.De La Barrera SS, Finiasz M, Frias A, Aleman M, Barrionuevo P, Fink S, Franco MC, Abbate E, del CS. Specific lytic activity against mycobacterial antigens is inversely correlated with the severity of tuberculosis. Clin Exp Immunol. 2003;132(3):450–461. doi: 10.1046/j.1365-2249.2003.02176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Serbina NV, Liu CC, Scanga CA, Flynn JL. CD8+ CTL from lungs of Mycobacterium tuberculosis-infected mice express perforin in vivo and lyse infected macrophages. J Immunol. 2000;165(1):353–363. doi: 10.4049/jimmunol.165.1.353. [DOI] [PubMed] [Google Scholar]
- 201.Kaufmann SH, Vath U, Thole JE, Van Embden JD, Emmrich F. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur J Immunol. 1987;17(3):351–357. doi: 10.1002/eji.1830170308. [DOI] [PubMed] [Google Scholar]
- 202.Orme IM. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988;56(12):3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Romano M, Denis O, D’Souza S, Wang XM, Ottenhoff TH, Brulet JM, Huygen K. Induction of in vivo functional Db-restricted cytolytic T cell activity against a putative phosphate transport receptor of Mycobacterium tuberculosis. J Immunol. 2004;172(11):6913–6921. doi: 10.4049/jimmunol.172.11.6913. [DOI] [PubMed] [Google Scholar]
- 204.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171(1):27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 205.Silva CL, Lowrie DB. Identification and characterization of murine cytotoxic T cells that kill Mycobacterium tuberculosis. Infect Immun. 2000;68(6):3269–3274. doi: 10.1128/iai.68.6.3269-3274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Canaday DH, Wilkinson RJ, Li Q, Harding CV, Silver RF, Boom WH. CD4(+) and CD8(+) T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism. J Immunol. 2001;167(5):2734–2742. doi: 10.4049/jimmunol.167.5.2734. [DOI] [PubMed] [Google Scholar]
- 207.Seki N, Brooks AD, Carter CR, Back TC, Parsoneault EM, Smyth MJ, Wiltrout RH, Sayers TJ. Tumor-specific CTL kill murine renal cancer cells using both perforin and Fas ligand-mediated lysis in vitro, but cause tumor regression in vivo in the absence of perforin. J Immunol. 2002;168(7):3484–3492. doi: 10.4049/jimmunol.168.7.3484. [DOI] [PubMed] [Google Scholar]
- 208.Cooper AM, D’Souza C, Frank AA, Orme IM. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect Immun. 1997;65(4):1317–1320. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Laochumroonvorapong P, Wang J, Liu CC, Ye W, Moreira AL, Elkon KB, Freedman VH, Kaplan G. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect Immun. 1997;65(1):127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.de Saint BG, Fischer A. The role of cytotoxicity in lymphocyte homeostasis. Curr Opin Immunol. 2001;13(5):549–554. doi: 10.1016/s0952-7915(00)00257-0. [DOI] [PubMed] [Google Scholar]
- 211.Topham DJ, Cardin RC, Christensen JP, Brooks JW, Belz GT, Doherty PC. Perforin and Fas in murine gammaherpesvirus-specific CD8(+) T cell control and morbidity. J Gen Virol. 2001;82(Pt 8):1971–1981. doi: 10.1099/0022-1317-82-8-1971. [DOI] [PubMed] [Google Scholar]
- 212.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159(11):5197–5200. [PubMed] [Google Scholar]
- 213.Gupta M, Greer P, Mahanty S, Shieh WJ, Zaki SR, Ahmed R, Rollin PE. CD8-mediated protection against Ebola virus infection is perforin dependent. J Immunol. 2005;174(7):4198–4202. doi: 10.4049/jimmunol.174.7.4198. [DOI] [PubMed] [Google Scholar]
- 214.Wang Y, Lobigs M, Lee E, Mullbacher A. Exocytosis and Fas mediated cytolytic mechanisms exert protection from West Nile virus induced encephalitis in mice. Immunol Cell Biol. 2004;82(2):170–173. doi: 10.1046/j.0818-9641.2004.01227.x. [DOI] [PubMed] [Google Scholar]
- 215.Keane J, Balcewicz-Sablinska MK, Remold HG, Chupp GL, Meek BB, Fenton MJ, Kornfeld H. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997;65(1):298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Oddo M, Renno T, Attinger A, Bakker T, MacDonald HR, Meylan PR. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J Immunol. 1998;160(11):5448–5454. [PubMed] [Google Scholar]
- 217.Keane J, Remold HG, Kornfeld H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol. 2000;164(4):2016–2020. doi: 10.4049/jimmunol.164.4.2016. [DOI] [PubMed] [Google Scholar]
- 218.Lee J, Remold HG, Ieong MH, Kornfeld H. Macrophage Apoptosis in Response to High Intracellular Burden of Mycobacterium tuberculosis Is Mediated by a Novel Caspase-Independent Pathway. J Immunol. 2006;176(7):4267–4274. doi: 10.4049/jimmunol.176.7.4267. [DOI] [PubMed] [Google Scholar]
- 219.Brookes RH, Pathan AA, McShane H, Hensmann M, Price DA, Hill AV. CD8+ T cell-mediated suppression of intracellular Mycobacterium tuberculosis growth in activated human macrophages. Eur J Immunol. 2003;33(12):3293–3302. doi: 10.1002/eji.200324109. [DOI] [PubMed] [Google Scholar]
- 220.Ernst WA, Thoma-Uszynski S, Teitelbaum R, Ko C, Hanson DA, Clayberger C, Krensky AM, Leippe M, Bloom BR, Ganz T, Modlin RL. Granulysin, a T cell product, kills bacteria by altering membrane permeability. J Immunol. 2000;165(12):7102–7108. doi: 10.4049/jimmunol.165.12.7102. [DOI] [PubMed] [Google Scholar]
- 221.Thoma-Uszynski S, Stenger S, Modlin RL. CTL-mediated killing of intracellular Mycobacterium tuberculosis is independent of target cell nuclear apoptosis. J Immunol. 2000;165(10):5773–5779. doi: 10.4049/jimmunol.165.10.5773. [DOI] [PubMed] [Google Scholar]
- 222.Scanga CA, Mohan VP, Yu K, Joseph H, Tanaka K, Chan J, Flynn JL. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J Exp Med. 2000;192(3):347–358. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Noss EH, Harding CV, Boom WH. Mycobacterium tuberculosis inhibits MHC class II antigen processing in murine bone marrow macrophages. Cell Immunol. 2000;201(1):63–74. doi: 10.1006/cimm.2000.1633. [DOI] [PubMed] [Google Scholar]
- 224.Noss EH, Pai RK, Sellati TJ, Radolf JD, Belisle J, Golenbock DT, Boom WH, Harding CV. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J Immunol. 2001;167(2):910–918. doi: 10.4049/jimmunol.167.2.910. [DOI] [PubMed] [Google Scholar]
- 225.Lowrie DB, Tascon RE, Bonato VLD, Lima VMF, Faccioli LH, Stavropoulos E, Colston MJ, Hewinson RG, Moelling K, Silva CL. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400(6741):269–271. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- 226.Ha SJ, Jeon BY, Kim SC, Kim DJ, Song MK, Sung YC, Cho SN. Therapeutic effect of DNA vaccines combined with chemotherapy in a latent infection model after aerosol infection of mice with Mycobacterium tuberculosis. Gene Ther. 2003;10(18):1592–1599. doi: 10.1038/sj.gt.3302057. [DOI] [PubMed] [Google Scholar]
- 227.Dong Y, Demaria S, Sun X, Santori FR, Jesdale BM, De Groot AS, Rom WN, Bushkin Y. HLA-A2-restricted CD8+-cytotoxic-T-cell responses to novel epitopes in Mycobacterium tuberculosis superoxide dismutase, alanine dehydrogenase, and glutamine synthetase. Infect Immun. 2004;72(4):2412–2415. doi: 10.1128/IAI.72.4.2412-2415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]