Abstract
Background
Epithelial-to-mesenchymal transition (EMT) is a series of molecular changes allowing epithelial cancer cells to acquire properties of mesenchymal cells: increased motility and invasion and protection from apoptosis. Transcriptional regulators such as Slug mediate EMT, working in part to repress E-cadherin transcription. We report a novel, non-invasive in vivo rectal cancer model to explore the role of Slug in colorectal cancer (CRC) tumor development.
Methods
For the generation of DLD-1 cells overexpressing Slug (Slug DLD-1), a Slug or empty (Empty DLD-1) pCMV-3Tag-1 (kanamycin resistant) vector was used for transfection. Cells were evaluated for Slug and E-cadherin expression, and cell migration and invasion. For the in vivo study, colon cancer cells (parental DLD-1, Slug DLD-1, empty DLD-1, and HCT-116) were submucosally injected into the posterior rectum of nude mice using endoscopic guidance. After 28 days, tumors were harvested and tissue was analyzed.
Results
Slug expression in our panel of colon cancer cell lines was inversely correlated with E-cadherin expression and enhanced migration/invasion. Slug DLD-1 cells demonstrated a 21-fold increased Slug and 19-fold decreased E-cadherin expression compared with empty DLD-1. Similarly, the Slug DLD-1 cells had significantly enhanced cellular migration and invasion. In the orthotopic rectal cancer model, Slug DLD-1 cells formed rectal tumors in 9/10 (90%) of the mice (mean volume = 458 mm3) compared with only 1/10 (10%) with empty DLD-1 cells.
Conclusion
Slug mediates EMT with enhanced in vivo rectal tumor formation. Our non-invasive in vivo model enables researchers to explore the molecular consequences of altered genes in a clinically relevant rectal cancer in an effort to develop novel therapeutic approaches for patients with rectal cancer.
Keywords: colorectal cancer, e-cadherin, slug, rectal tumor model
Introduction
With the goal of developing novel cancer therapeutic strategies, investigations exploring critical pathways driving cancer cell growth and invasion may highlight potential molecular-based targets. The epithelial-to-mesenchymal transition (EMT) is a unique process initially characterized in embryonic development in which cells lose epithelial features and gain mesenchymal properties with increased cellular motility and invasion (1, 2). More recently, molecular pathways associated with EMT have been identified in cancer cells with analogous roles as those observed in development (2, 3). The critical difference with EMT associated with the carcinogenic process is that tumor cells are unresponsive to normal growth-regulatory signals and thus continue to grow, detach from the host environment, invade other tissues, and metastasize (3).
Loss of E-cadherin is a critical step in EMT leading to the breakdown of cell-cell adhesions and morphologic alterations (4). The discovery of the zinc finger transcription factor SNAI1 (Snail), a direct repressor of E-cadherin, in Drosophila provided insight into one of the mechanisms driving EMT (5). This novel finding uncovered a critical link between intracellular signaling and transcriptional inhibition of E-cadherin. Subsequently, other critical E-cadherin transcriptional repressors have been discovered, including Slug (SNAI2), ZEB1, ZEB2, SMAD interacting protein 1 (SIP1), and the basic helix-loop-helix family member TWIST (2). The impact of E-cadherin transcriptional repressors on colorectal cancer development and growth is poorly understood. Besides enhancing motility, Slug, in particular, has been implicated as an anti-apoptotic factor (6, 7).
Evaluating in vivo tumor effects in colorectal cancer (CRC) is limited by the paucity of primary relevant CRC models. Ectopic subcutaneous tumor models have been extensively used in the past although the relevance to clinical application is debatable. Orthotopic models, on the other hand, offer the advantage of evaluating tumor growth in the tissue of origin. For CRC, direct cecal and hepatic tumor implantation models have been developed modeling primary colon cancer and hepatic metastasis, although both models require a surgical incision (8, 9). The physiologic impact of the surgical procedure on tumor growth is difficult to quantitate. Direct rectal submucosal injection has been previously reported although necessitates use of a murine colon cancer cell line (10). Our study demonstrates a novel non-invasive in vivo rectal cancer model using endoscopic visualization for reliable tumor implantation of human colon cancer cells to explore the role of Slug in CRC tumor development.
Materials and Methods
Cell lines and culture conditions
Human colon cancer cell lines (DLD1, HCT116, HT29, CaCo2, SW480, SW620, LS174, and LoVo) were cultured according to American Type Culture Collection recommendations and maintained at 37°C with 5% CO2. Mycoplasma-negative cultures were ensured by PCR testing prior to the investigations. Cells were monitored throughout with consistent morphology and doubling-time.
Reagents
Antibodies used for immunofluorescence (IF) staining and western blot analyses were as follows: mouse anti-E-cadherin antibody (Zymed Laboratories, Carlsbad, CA), rabbit anti-actin (Sigma-Aldrich, St. Louis, MO). Goat anti-rabbit and horse anti-mouse horseradish peroxidase-conjugated antibodies were acquired from Cell Signaling Technology, Inc. (Danvers, MA). Alexa Fluor488-conjugated antibodies specific for rabbit and mouse IgG were from Molecular Probes, Inc. (Eugene, OR).
Reverse transcription-PCR
Total RNA from cultured cells was extracted using the RNeasy Plus Mini kit (Qiagen, Valencia, CA). 1 ug total RNA was reverse transcribed in a 20 μl reaction using iScript (Bio-Rad, Hercules, CA). Real-Time PCR was performed with 5 μl of a 1/20 dilution of reverse-transcribed cDNA for the cell line samples using the UPL mono-color probes in the Roche LightCycler 480 machine (Roche Diagnostics, Basel, Switzerland). The cycling conditions for all genes were preincubation at 95°C for 10 min followed by 55 cycles of denaturation at 95 °C for 15 sec and amplification/extension at 60 °C for 30 sec; after cycle completion cooling was held for 30 sec at 40 °C. Triplicate reactions were run for each cDNA sample. Data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and confirmed with biologic replicate samples. Sequences for gene-specific primers are provided in Table 1.
TABLE 1.
SLUG- AND GENE-SPECIFIC PRIMERS
| Primer name | Forward sequence | Reverse sequence | UPL probe # | Amplicon size (bp) |
|---|---|---|---|---|
| Slug cDNA | gcgcggatccccgcgctccttcctggtc | gcgcgaattctcagtgtgctacacagcagc | N/A | 815 |
| qPCR-Slug | tggttgcttcaaggacacat | gttgcagtgagggcaagaa | 7 | 66 |
| qPCR-E-Cadherin | cccgggacaacgtttattac | gctggctcaagtcaaagtcc | 35 | 72 |
| qPCR-Gapdh | agccacatcgctcagacac | gcccaatacgaccaaatcc | 60 | 66 |
Western Blot Analysis
Cells were suspended in radioimmunoprecipitation assay (RIPA) protein lysis buffer (pH 7.4), containing 20 mM sodium phosphate, 150 mM sodium chloride, 1% Triton X-100, 5 mM EDTA, 5 mM phenylmethylsulfonyl fluoride, 1% aprotinin, 1 μg/ml leupeptin, and 500 μM Na3VO4. Protein concentration was quantified using Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Twenty micrograms of total protein was resolved with SDS-PAGE (10% polyacrylamide gel), and transferred to a polyvinylidene difluoride membrane. Immunoblotting was performed with enhanced chemiluminescence (GE Healthcare, Piscataway, NJ). Blot was probed with commercially available antibodies as outlined above. All membranes were stripped and reprobed with actin antibody for loading control.
Transwell migration and invasion assay
Cells were seeded into the upper chamber of a Transwell insert precoated with 5 μg/ml fibronectin for migration or a BD Matrigel invasion chamber for invasion in serum-free medium at a density of 50,000 cells per well (24-well insert; 8 μm pore size, BD Biosciences, San Jose, CA). Medium containing 10% fetal bovine serum (FBS) was placed in the lower chamber to act as a chemoattractant, and cells were further incubated for the indicated time points. Non-migratory cells were removed from the upper chamber by scraping the upper surface of the transwell membrane. The migratory cells remaining on the lower surface of the insert were fixed and stained using Diff-Quick dye (Dade Behring, Inc, Newark, DE). Cells were quantified as the average number of cells found in five random microscope fields in three independent inserts.
Slug gene cloning
Slug was amplified from genomic DNA using Slug-specific primers (Table 1) and sequence validated. For the generation of DLD-1 cells overexpressing Slug (Slug DLD-1), a Slug or empty pCMV-3Tag-1 (kanamycin resistant) vector was transfected into DLD-1 cells using Lipofectamine 2000 according to the manufacturer’s recommendations (Invitrogen, Carlsbad, CA). Stable transfected pools of cells were selected in medium containing G418 (400 μg/ml; Invitrogen).
Morphologic and immunofluorescence analysis
Cells were seeded as monolayers onto sterile, confocal glass coverslips (35 mm; MatTek Corp., Ashland, MA), coated with 5 μg/ml fibronectin in culture dishes and allowed to attach overnight. Cells were then fixed for 10 min with 3.7% formaldehyde, washed three times with 1X phosphate-buffered saline (PBS), permeabilized with 0.1% Triton X-100, and blocked in 2% bovine serum albumin (BSA) in 1X PBS for 1 h at room temperature. E-cadherin primary antibody (Zymed Laboratories, Carlsbad, CA) was used overnight at 4 °C at a 1:100 dilution in 1X PBS containing 2% BSA. Overnight incubation was followed by 3 × 10 min washes in 1X PBS, followed by incubation in Alexa Fluor 488 secondary antibody for 2 h in the dark at room temperature. DRAQ5 (Alexis Corporation, San Diego, CA)) was used for nuclear counterstaining. Slides were examined under a confocal laser-scanning microscope (Zeiss LSM 510 META) with a Plan Apo Chromat X63 oil objective (N.A. 1.4), and images were captured using LSM 510 META software version 3.2.
In vivo rectal cancer model
The experimental protocol was approved by the Institution’s Animal Care and Use Committee and conducted in compliance with the Principles of Laboratory Animal Care published by the US National Institute of Health. Nude athymic mice were anesthetized with isoflurane and subjected to gentle anal dilation using blunt-tipped forceps. Using the Stryker 1.9 mm × 30 degree rigid arthroscope (Stryker Endoscopy, San Jose, CA) for rectal visualization, 5 × 106 human colon cancer cells were submucosally injected with a 25 gauge hypodermic needle in 100 ul of HBSS. Four groups were used for comparison including: parental HCT116, DLD-1 human colon cancer cells and the stably transfected Slug DLD-1 and empty DLD-1 cells. Mice were monitored daily for 28 days. Tumors were excised, weighed, and measured with calipers. Volume was calculated as V= (A x B2)/0.5, where “A” is the larger and “B” is the smaller of the tumor dimensions as described previously (25). Thick (10 μm) sections were obtained from paraffin-embedded tumor sections for H&E staining. Slides were visualized with the Olympus BH.2 optical microscope and images were taken with the Olympus DP70 camera.
Statistical Analysis
Statistical analyses were performed using the paired Student’s t-test. P<0.05 was considered significant.
Results
Variable Slug expression in human colon cancer cell lines
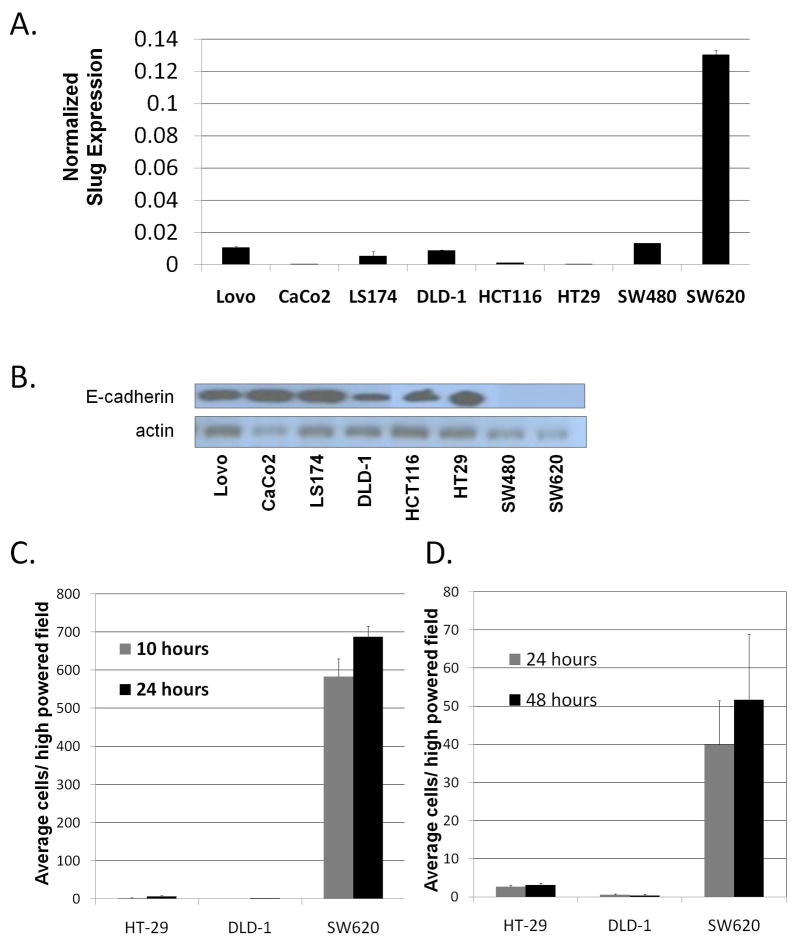
We surveyed a panel of eight human colon cancer cell lines for Slug expression by real-time RT-PCR. Slug expression had variable intensity across the cell lines sampled (Figure 1A). The well-characterized pair of cell lines SW480 (primary tumor) and SW620 (metastatic tumor) cell lines had significantly greater Slug expression than the rest of the human cell lines. Furthermore, SW620, the cell line derived from the metastatic lesion, had the highest level of Slug expression (11, 12).
Figure 1. Slug expression in human colon cancer cell lines is correlated with properties consistent with EMT.
A) Real time RT-PCR analysis of Slug expression normalized to GAPDH in a panel of human colon cancer cell lines. B) Western Blot Analysis demonstrating E-cadherin expression in a panel of human colon cancer cell lines. Actin is shown as a loading control. C) Cellular migration quantified as mean number of cells migrating through the transwell inserts for a Boyden chamber assay in three colon cancer cell lines with variable Slug expression: HT-29 (low), DLD-1(medium), SW620 (high). D) Cellular invasion quantified as mean number of cells invading with Matrigel-coated transwell inserts in the three cell lines (HT-29, DLD-1, and SW620). Columns, average of cells invading per high-powered field. Measured differences of migration at 10 and 24 hours was statistically significant for SW620 cells compared with HT-29 and DLD-1 cells, p < 0.0001. Measured differences of invasion at 24 and 48 h were statistically significant for SW620 cells, p<0.01.
The correlation between Slug expression, E-cadherin and motility in human colon cancer cell lines
We explored the relative significance of Slug expression in our panel of human colon cancer cell lines for critical properties of EMT including E-cadherin expression, invasion and migration. Based on western blot, E-cadherin protein levels inversely correlated with Slug expression with both SW480 and SW620 demonstrating no detectable E-cadherin protein relative to the other human colon cancer cell lines (Figure 1B). We further assessed three cell lines with low (HT29), intermediate (DLD-1), and high (SW620) Slug expression for migration (Figure 1C) and invasion (Figure 1D). Chemokinetic migration and invasion assays were performed using 10% FBS as a chemoattractant. Compared to HT29 and DLD-1 colon cancer cell lines (low and intermediate Slug expression), the SW620 cells demonstrated significantly enhanced migration (p<0.0001) and invasion (p<0.01) at both time points (Figure 1C and D). We selected the DLD-1 colon cancer cells for further investigations.
Slug overexpression results in EMT phenotypic and molecular changes
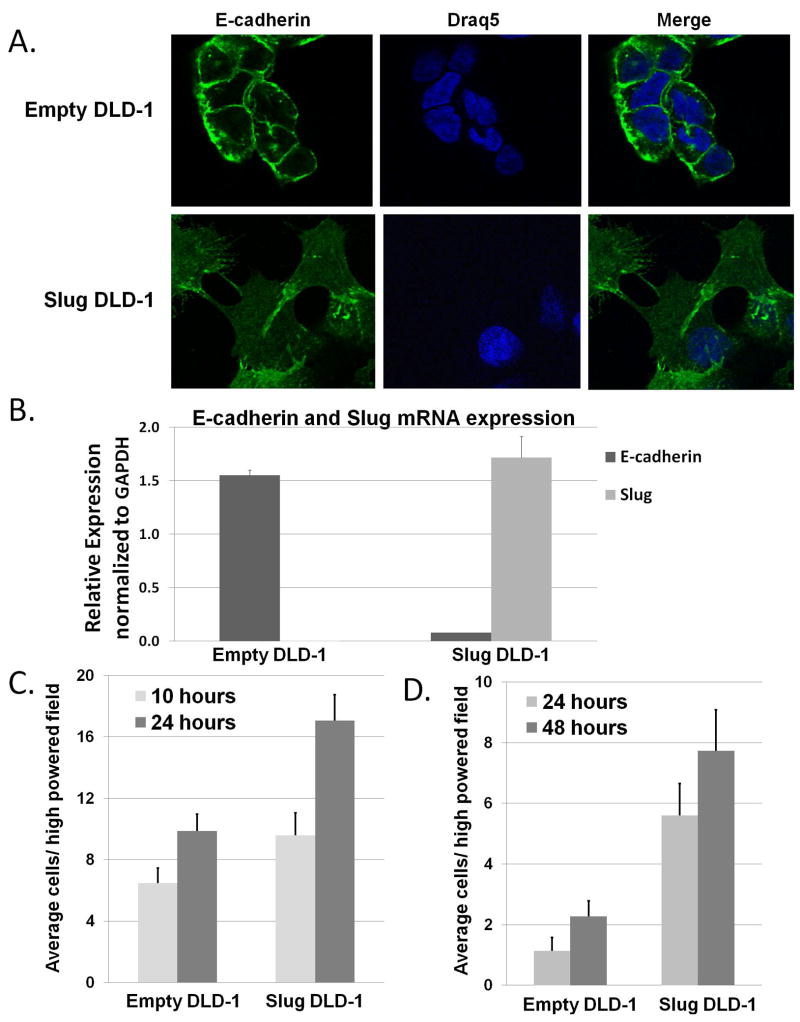
Slug-expressing DLD-1 (Slug-DLD-1) cells had morphologic changes consistent with EMT compared with control DLD-1 (empty DLD1) cells including: increased spindle shape, pseudopodia, and inter-cellular space as indicated by immunofluorescent staining (Figure 2A). Slug-DLD-1 cells also had altered E-cadherin localization as observed with immunofluorescent staining (Figure 2A) and decreased E-cadherin expression according to real-time PCR (Figure 2B, p<0.001). Slug DLD-1 cells demonstrated 21-fold increased Slug expression compared with the empty DLD-1 by qRT-PCR (Figure 2B, p<0.001).
Figure 2. Expression of Slug in the DLD-1 cells leads to loss of E cadherin and enhanced cellular migration and invasion.
A) Representative images of immunofluorescence staining for E-cadherin staining at x100 objective power. DRAQ5 was used as a nuclear stain. B) Real time RT-PCR analysis of E-cadherin and Slug mRNA expression in the Slug DLD-1 cells compared to empty DLD-1 cells (p <0.001). C) Cellular migration quantified as mean number of cells migrating through the transwell inserts for a Boyden chamber assay. D) Cellular invasion quantified as mean number of cells invading with Matrigel-coated transwell inserts. Columns, average of cells invading per high-powered field. Measured differences of migration at 24 hours was statistically significant, p < 0.002. Measured differences of invasion at 24 and 48 h were statistically significant, p<0.001.
Slug overexpression increased cellular migration and invasion
Slug-DLD-1 cells had enhanced migration at 24 h (1.7-fold p=0.0013) relative to the empty DLD-1 control cells (Figure 2C). Slug expression further impacted cellular invasion through Matrigel at 24 h (5.0-fold) and 48 h (3.5-fold) relative to the empty DLD-1 cells (Figure 2D, p<0.001).
Slug overexpression enhanced in vivo tumor development
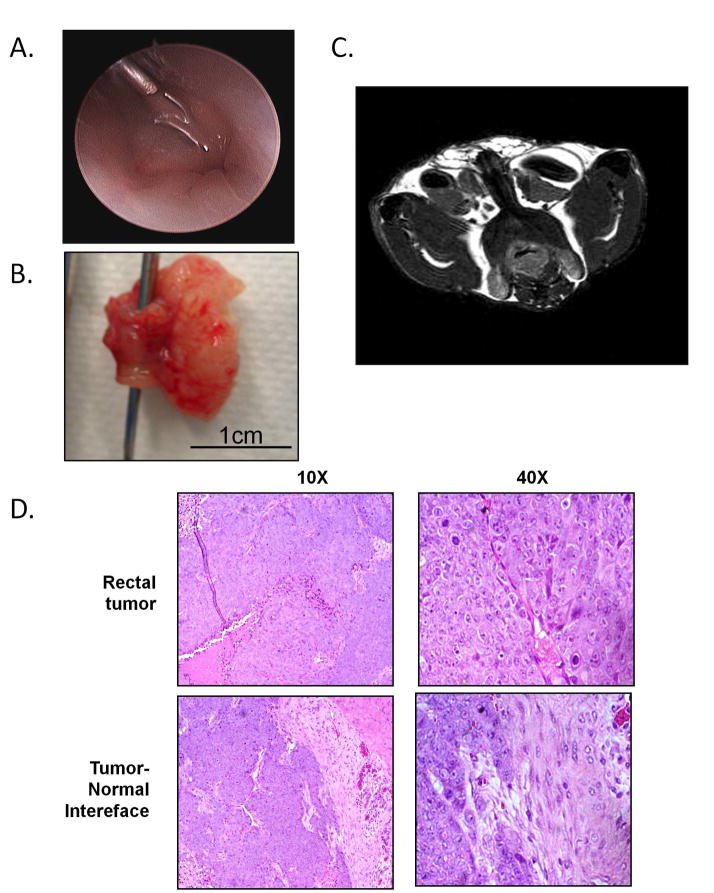
Using our novel orthotopic rectal model, we assessed the impact of Slug expression on tumor growth compared with empty DLD-1 cells. Parental DLD-1 and HCT116 cells were also used for comparison as well as secondary controls for the experiment. 5 X 106 human colon cancer cells were submucosally injected with a 25 gauge hypodermic needle in 100 ul of HBSS with the Stryker 1.9 mm × 30 degree rigid arthroscope for rectal visualization (Figure 3A). No procedure-related mortalities were observed. The experiment ended at 4 weeks following submucosal implantation of tumor cells as per protocol.
Figure 3. Slug expressing DLD-1 cells form tumors in vivo with morphology consistent with human CRC.
A) Endoscopic image using the Stryker 1.9 mm × 30 degree rigid arthroscope demonstrates submucosal injection procedure. B) Gross image of tumor dissection en bloc with rectum (forceps in place displaying rectal lumen). C) Magnetic resonance image taken prior to tumor dissection reveals near-obstructing rectal tumor. D) H&E staining reveals morphology of rectal tumors consistent with a poorly differentiated adenocarcinoma formed in vivo from Slug expressing DLD-1 cells at 10 × and 40 × magnifications.
Tumor growth was significantly enhanced in the Slug DLD-1 group with tumor formation in 9/10 mice (90%) compared with the empty DLD-1 group that developed tumors in only 1/9 (11%) mice. Mean tumor volume in the Slug DLD-1 group was 458mm3 compared with 352 mm3 in the one mouse that developed a tumor with the empty DLD-1 cells (Figure 3B). The parental DLD-1 injected mice failed to generate tumors in 10 mice injected. As a second control, the HCT116 human colon cancer cells developed tumors in 6/6 mice injected. No lung, liver or intra-peritoneal metastases were observed. In two mice injected with the HCT116 cells, magnetic resonance imaging was performed at the termination of the experiment to assess for tumor growth using a 9.4 T horizontal INOVA Varian system (Varian, Palo Alto, CA) (13) (Figure 3C).
H&E stained sections were obtained from tumors generated from the Slug DLD-1 human colon cancer cells for further characterization. Microscopic images were taken from the normal rectal wall and from the tumor sections including the tumor-normal interface at 10 and 40X magnification (Figure 3D). Images were reviewed by our gastrointestinal pathologist who characterized sections from the Slug DLD-1 tumors as representative of a poorly differentiated adenocarcinoma.
Discussion
Our understanding of tumor biology and therapies for cancer patients continues to expand. However, in the case of rectal cancer, treatment response to neoadjuvant chemoradiation for locally advanced disease has plateaued with only 60% of patients demonstrating tumor downstaging at surgery (14, 15). Understanding gene expression alterations associated with aggressive tumor biology will provide insight into tumor survival mechanisms and highlight novel CRC therapeutic strategies.
Preclinical investigations serve as the foundation for exploring of future potential novel molecular-targeted therapies. In the case of CRC, relevant pre-clinical models have previously been limited and have either required a surgical incision or use of murine cancer cell lines (8–10). Our report describes a non-invasive orthotopic rectal cancer model using endoscopic visualization for tumor implantation of human colon cancer cells to characterize the effect of Slug overexpression.
Our investigations highlight the function of Slug as a mediator of EMT in human colon cancer cells. We demonstrated that the relative level of Slug expression in a panel of human colon cancer cell lines is correlated with critical EMT properties including loss of E-cadherin as well as increased migration and invasion. To examine this correlation experimentally, we examined the impact of Slug expression in DLD-1 cells., Slug expression in the DLD-1 colon cancer cells leads to morphologic and phenotypic changes consistent with EMT, including increased spindle shape and pseudopodia formation and decreased E-cadherin expression. We further demonstrated that Slug overexpression resulted in enhanced cellular migration, invasion, and in vivo tumor development. In our orthotopic model, Slug expressing DLD-1 cells developed significantly more tumor growth than both the parental DLD-1 cell line as well as the empty vector (control) DLD-1 cells. Essentially, Slug expression converted a well-differentiated minimally invasive colon cancer cell line into an invasive cell line (16). Unfortunately, with the lack of growth in the control cell lines, further tumor analysis was not performed and limited immunohistochemical and molecular characterization of tumor growth in our model. Technical reproducibility was achieved with 100% tumor growth with use of the HCT116 cell line. In our study, MRI was used for descriptive purposes only but demonstrates the capability of MRI to characterize and possibly quantitate tumor burden in future investigations. Our results confirm observations in a cecal orthotopic model demonstrating significantly increased tumor growth and development in HCT 116 cells compared with DLD-1 cells (16).
Our findings are consistent with prior observations characterizing expression of EMT transcriptional mediators in cancer cells resulting in enhanced motility and invasion (17, 18). Recently, mediators of EMT have also been associated with enhanced cellular survival (6, 19). Snail expression in Madin-Darby canine kidney cells attenuated cell death in response to serum starvation and TNF-α treatment. The anti-apoptotic response as a result of Snail expression was associated with activation of both MAPK and PI3K pathways (19). Similarly, transfection of Slug into MCF7 breast cancer cells promoted resistance to DNA damage-mediated programmed cell death via inhibiting multiple pro-apoptotic factors including p53, DFF40, and BID (6). Colon cancer cells resistant to oxaliplatin chemotherapy-induced apoptosis had alterations consistent with EMT, including loss of cellular polarity, spindle shape, and increased motility as well as loss of membrane-bound E-cadherin expression (20). Consistent with previous investigations, our in vitro and in vivo experiments demonstrated that Slug expression generated more invasive and resilient cancer cells with increased capacity for tumor formation.
The pro-neoplastic qualities enhanced by Slug expression suggest that Slug may represent a novel molecular therapeutic target for CRC. Our pre-clinical rectal model can serve as a foundation for further investigations evaluating the therapeutic impact of EMT signaling and potential novel molecular therapeutic strategies targeting the transcriptional EMT mediators.
TABLE 2.
IN VIVO RECTAL TUMOR DEVELOPMENT
| Cell line | Tumor Growth (%) | Mean Tumor Volume (mm3) |
|---|---|---|
| DLD-1 | 0/10 (0) | N/A |
| Empty DLD-1 | 1/9 (11) | 352 |
| Slug DLD-1 | 9/10 (90) | 458 |
| HCT116 | 10/10 (100) | 750 |
Acknowledgments
The authors thank Dr. M. Rita Young and Kim Sutton for use of the arthroscope and assistance with the experimental procedures, Dr. Mehmet Bilgen and Peter Chou for performing the MRI procedures and Dr. Jennifer Schnellmann and Stacy Miers for assistance with manuscript preparation and submission.
Supported in part by: Hollings Cancer Center Translational Research Award 2008, American Cancer Society: Institutional Research Grant 2009 and the National Institutes of Health, 1K08CA142904 (ERC)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. The Journal of clinical investigation. 2009;119 :1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nature reviews. 2006;7 :131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139 :871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Olmeda D, Moreno-Bueno G, Flores JM, Fabra A, Portillo F, Cano A. SNAI1 is required for tumor growth and lymph node metastasis of human breast carcinoma MDA-MB-231 cells. Cancer research. 2007;67 :11721–11731. doi: 10.1158/0008-5472.CAN-07-2318. [DOI] [PubMed] [Google Scholar]
- 5.Leptin M. twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes & development. 1991;5 :1568–1576. doi: 10.1101/gad.5.9.1568. [DOI] [PubMed] [Google Scholar]
- 6.Kajita M, McClinic KN, Wade PA. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Molecular and cellular biology. 2004;24 :7559–7566. doi: 10.1128/MCB.24.17.7559-7566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tribulo C, Aybar MJ, Sanchez SS, Mayor R. A balance between the anti-apoptotic activity of Slug and the apoptotic activity of msx1 is required for the proper development of the neural crest. Developmental biology. 2004;275 :325–342. doi: 10.1016/j.ydbio.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 8.Cespedes MV, Espina C, Garcia-Cabezas MA, Trias M, Boluda A, Gomez del Pulgar MT, Sancho FJ, Nistal M, Lacal JC, Mangues R. Orthotopic microinjection of human colon cancer cells in nude mice induces tumor foci in all clinically relevant metastatic sites. Am J Pathol. 2007;170 :1077–1085. doi: 10.2353/ajpath.2007.060773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer TW, Fan F, Liu W, Camp ER, Yang A, Somcio RJ, Bucana CD, Singh R, Ellis LM. Targeting of insulin-like growth factor-I receptor with a monoclonal antibody inhibits growth of hepatic metastases from human colon carcinoma in mice. Annals of surgical oncology. 2007;14 :2838–2846. doi: 10.1245/s10434-007-9486-5. [DOI] [PubMed] [Google Scholar]
- 10.Donigan M, Norcross LS, Aversa J, Colon J, Smith J, Madero-Visbal R, Li S, McCollum N, Ferrara A, Gallagher JT, Baker CH. Novel murine model for colon cancer: non-operative trans-anal rectal injection. The Journal of surgical research. 2009;154 :299–303. doi: 10.1016/j.jss.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Gagos S, Hopwood VL, Iliopoulos D, Kostakis A, Karayannakos P, Yatzides H, Skalkeas GD, Pathak S. Chromosomal markers associated with metastasis in two colon cancer cell lines established from the same patient. Anticancer research. 1995;15 :369–378. [PubMed] [Google Scholar]
- 12.Maehle L, Eilertsen E, Mollerup S, Schonberg S, Krokan HE, Haugen A. Effects of n-3 fatty acids during neoplastic progression and comparison of in vitro and in vivo sensitivity of two human tumour cell lines. British journal of cancer. 1995;71 :691–696. doi: 10.1038/bjc.1995.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatar I, Chou PC, Desouki MM, El Sayed H, Bilgen M. Evaluating regional blood spinal cord barrier dysfunction following spinal cord injury using longitudinal dynamic contrast-enhanced MRI. BMC Med Imaging. 2009;9 :10. doi: 10.1186/1471-2342-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balch GC, De Meo A, Guillem JG. Modern management of rectal cancer: a 2006 update. World J Gastroenterol. 2006;12 :3186–3195. doi: 10.3748/wjg.v12.i20.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guillem JG, Chessin DB, Cohen AM, Shia J, Mazumdar M, Enker W, Paty PB, Weiser MR, Klimstra D, Saltz L, Minsky BD, Wong WD. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Annals of surgery. 2005;241:829–836. doi: 10.1097/01.sla.0000161980.46459.96. discussion 836–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki H, Miura K, Horii A, Kaneko N, Fujibuchi W, Kiseleva L, Gu Z, Murata Y, Karasawa H, Mizoi T, Kobayashi T, Kinouchi M, Ohnuma S, Yazaki N, Unno M, Sasaki I. Orthotopic implantation mouse model and cDNA microarray analysis indicates several genes potentially involved in lymph node metastasis of colorectal cancer. Cancer science. 2008;99:711–719. doi: 10.1111/j.1349-7006.2008.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nature cell biology. 2000;2 :84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 18.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nature cell biology. 2000;2 :76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 19.Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes & development. 2004;18 :1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang AD, Fan F, Camp ER, van Buren G, Liu W, Somcio R, Gray MJ, Cheng H, Hoff PM, Ellis LM. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 2006;12 :4147–4153. doi: 10.1158/1078-0432.CCR-06-0038. [DOI] [PubMed] [Google Scholar]