Abstract
Background
Cushing's syndrome (CS) secondary to ectopic ACTH secretion (EAS) has been described in association with a variety of tumors. The current experience with this syndrome is based on a few case series and individual case reports. Limited data are available about the tumors associated with CS-EAS in cancer center setting. This report describes CS-EAS at MD Anderson Cancer Center to further enhance our understanding and management of this syndrome.
Methods
This is a retrospective review for 43 patients with CS-EAS who were diagnosed between 1979 and 2009 at our institution.
Results
Different neuroendocrine tumors were associated with CS-EAS. Twenty one patients (48.9%) had tumors located in the chest cavity with bronchial carcinoid and small cell lung cancer representing the two most common causes. The ACTH source remained occult in 4 patients (9.3 %) despite extensive work-up. Clinical presentation was variable and the classical features of CS were not evident in some patients. Death occurred in 27 patients (62.8%) and the median overall survival was 32.2 months. Major morbidities included new onset or worsening hyperglycemia (77%), symptomatic venous thromboembolism (14%) and infections (23%).
Conclusions
In CS-EAS cases seen at a comprehensive cancer center, tumors originating in the chest cavity were the leading tumors associated with this syndrome. We suspect that CS-EAS is underreported because of the atypical presentation in some cases. Thus, we suggest careful evaluation of patients with neuroendocrine tumors to avoid missing co-existing CS-EAS.
Keywords: Paraneoplastic syndrome, Adrenocorticotropic hormone, Cushing's syndrome, neuroendocrine tumors, localization studies
Cushing's syndrome (CS) in association with non-pituitary tumors was initially reported in 1928, shortly before Harvey Cushing reported his eponymous clinical syndrome associated with basophilic pituitary tumors in 1932 1, 2. In the following 3 decades, multiple cases were reported in which adrenal hyperplasia was associated with various tumors, but the link between CS and ectopic adrenocorticotropic hormone (ACTH) secretion was not established until 1962 3.
Our current understanding of CS associated with ectopic ACTH production is mainly derived from the published series from large institutions 4-7 as well as literature reviews and individual case reports 8-12.
Based on the available literature, it is estimated that CS secondary to ectopic ACTH secretion (CS-EAS) constitutes 8 – 18% of all causes of CS 8, 13, 14. A wide variety of tumors, most neuroendocrine in origin, are reported to be associated with this syndrome 11. These tumors are readily apparent in many cases, although some require a great deal of time and effort to localize 10, 12. There is some evidence that mortality is increased in patients with CS-EAS compared to controls without hypercortisolemia 15, 16. This excess mortality and morbidity could be in part because of susceptibility to infection; however, prospective validation of these findings is still lacking.
The purpose of the present review was to study our institutional experience with CS-EAS and add to further our understanding of this clinical entity in cancer center setting. To achieve this goal, we reviewed cases of CS to identify patients with CS-EAS managed at our institution. We summarized their clinical features, diagnostic studies performed, long-term outcomes, and selected complications.
Patients and Methods
A retrospective review of CS cases was undertaken at the University of Texas MD Anderson Cancer Center, after local Institutional Review Board approval. Cases were identified through an institutional tumor registry database in addition to departmental databases. We initially searched for all cases of Cushing's syndrome and then individually reviewed all cases to identify cases of CS-EAS based on clinical documentation and diagnostic studies.
Clinical data was obtained through a review of the medical records.
For the purpose of this review, CS-EAS was defined as:
ACTH-dependent CS ( plasma ACTH > 15 pg/ml) in patients with tumors known to be associated with ectopic ACTH secretion, or
ACTH-dependent CS with positive ACTH immunostaining of non-pituitary tumors, or
ACTH-dependent CS with inferior petrosal sinus sampling (IPSS) that suggested an ectopic source, determined by a central: peripheral ACTH ratio of less than 2 at baseline or less than 3 after CRH stimulation.
We also reviewed records to summarize important clinical and laboratory parameters associated with CS, including hypertension, hyperglycemia, and hematological and electrolyte abnormalities.
New-onset or worsening hypertension was defined as an elevated systolic blood pressure at presentation (>140 mmHg) in patients without a prior history of hypertension or clinical documentation of uncontrolled hypertension in patients with a pre-existing diagnosis of hypertension who had been well-controlled on anti-hypertensive medications. New-onset or worsening hyperglycemia was defined as fasting blood glucose of ≥126 mg/dL in patients without a prior history of diabetes mellitus or clinical documentation of worsening glycemic control in the 3 months prior to the diagnosis of CS. White blood cell counts (WBC) and differentials at presentation were studied and classified as follows: leukocytosis if WBC was >11,000/mm3, neutrophilia if neutrophil count was >7,300/mm3, lymphopenia if lymphocyte count was <1,000/mm3 and eosinopenia if eosinophil count was <40/mm3.
Hypokalemia was defined as a serum potassium <3.5 meq/liter at presentation or the need for potassium supplementation or potassium sparing agents to normalize potassium. Alkalosis was defined as serum bicarbonate >30 meq/liter at presentation.
Statistical Analysis
The primary analysis was to determine the overall survival (OS) for patients with CS-EAS. For OS, the time to death or censoring was calculated in months after the date of CS-EAS diagnosis. In the absence of death, survival was censored at the date of last known follow-up. Univariate Cox proportional hazards regression was used to model the association between gender and duration of OS. The Kaplan-Meier product limit method was used to estimate median OS. We performed the statistical analyses using STATA/SE version 11 statistical software (Stata Corp, College Station, TX).
Results
Patient characteristics
A total of 300 patients with CS were identified, including 43 patients with ACTH-dependent ectopic CS who had been diagnosed between 1979 and 2009. Patients' clinical features at presentation are summarized in Table 1.
Table.1.
Clinical features at diagnosis of 43 patients with CS-EAS
| Patient Characteristics | N=43 |
|---|---|
| Gender | Men 17 (39.5%) |
| Women 26 (60.5%) | |
| Ethnicity | White 33 |
| Black 6 | |
| Hispanic 4 | |
| Median age at diagnosis (range) | 48 years (19 – 75) |
| Weight change | Weight gain, 26/33 (79%) |
| Weight loss, 7/33 (21%) | |
| Median body mass index (BMI) | 27.7 kg/m2 (range, 19.1 – 54.9) |
| New onset or worsening hypertension | 35/41 (85%) |
| New onset or worsening hyperglycemia |
30/39 (77%) |
| Leukocytosis | 15/38 (39%) |
| Neutrophilia | 21/36 (58%) |
| Lymphopenia | 20/36 (56%) |
| Eosinopenia | 18/33 (55%) |
| Hypokalemia | 28/39 (72%) |
| Alkalosis | 23/35 (66%) |
Diagnostic tests for CS
24-hour urine free cortisol (UFC) test results were available and elevated in 30 patients. The median UFC level was 498 micrograms/ 24 hours, with a range of 71.7 –12515.0 micrograms/ 24 hours (Upper reference value, 50 micrograms/24 hours). Random plasma ACTH levels were available in 42 patients. Median ACTH level at presentation was 182.5 pg/mL, with a range of 43 – 5900 (Upper reference value varied from 46 to 52 pg/mL on different assays used during the review period). The ACTH value was not available at presentation in one patient who had a metastatic bronchial carcinoid tumor associated with a classic clinical presentation of CS and elevated cortisol.
Results of high-dose dexamethasone suppression tests (HDDSTs) were available in 16 patients, of whom 14 had 8 am serum cortisol values >5 μg/dL and 2 had serum cortisol values <5 μg/dL after completing either 2-day or overnight HDDSTs. Cortisol levels in 8 patients after HDDST showed median decrease of 16.6% (range 2.8-54.8%) from baseline values.
The median change in peripheral plasma ACTH during IPSS study (ACTH at 10 minutes after CRH injection compared with baseline value) was 4.9% with a range of −50% - 81.5%). The change was less than 50% in 7/8 studies. In one patient, the increase was 81.5% but the patient had a peak peripheral plasma ACTH value of 69 pg/ml and previously had no change in peripheral plasma ACTH after CRH injection during earlier IPSS study.
Tumor distribution and ACTH source
Tumors associated with EAS were distributed as follows: 9 (21%) patients had bronchial carcinoid tumors, 9 (21%) had SCLC, 5 (11.6%) had MTC, 3 (6.9%) had thymic carcinoid tumors, 6 (14%) had gastroenteropancreatic neuroendocrine tumors, 4 (9.3%) had genitourinary tumors (2 prostatic neuroendocrine tumors, 1 bladder neuroendocrine tumor, 1 ovarian endometrioid carcinoma), 3 (6.9%) had widely metastatic neuroendocrine tumors of unknown primary origin, and 4 (9.3%) had occult sources of ACTH despite extensive testing. Table 2 summarizes the tumors associated with CS-EAS in our series compared to other series in English literature.
Table 2.
Causes of CS-EAS in the current manuscript and previously published case series
| Tumor | Ref#5 1969* (n = 104) |
Ref#4 2001 (n =106) |
Ref# 6 2005 (n = 90) |
Ref#7 2006 (n = 40) |
Our report 2010 (n = 43) |
Combined data (n = 383) |
|---|---|---|---|---|---|---|
| Bronchial Carcinoid | 5 (4.8%) | 26 (24.5%) |
35(38.9%) | 12 (30%) | 9 (21%) | 87 (22.7%) |
| SCLC | 52 (50%) | 12 (11.3%) |
3 (3.3%) | 7 (17.5%) |
9 (21%) | 83 (21.7%) |
| Thymic Carcinoid | 11(10.6%) | 5 (4.7%) | 5 (5.6%) | 2 (5%) | 3 (6.9%) | 26 (6.8%) |
| MTC | 2 (1.9%) | 9 (8.5%) | 2 (2.2%) | 3 (7.5%) | 5 (11.6%) |
21 (5.5%) |
| GEP NET | 14(13.5%) | 18 (17%) | 8 (8.9%) | 5 (12.5%) |
6 (14%) | 51 (13.3%) |
| Pheochromocytoma/Paraganglioma | 4 (3.8%) | 3 (2.8%) | 5 (5.6%) | 1 (2.5%) | 0 (0%) | 13 (3.4%) |
| NET of unknown primary | 0 (0%) | 7 (6.6%) | 13(14.4%) | 2 (5%) | 3 (6.9%) | 25 (6.5%) |
| Occult | 7 (6.7%) | 17 (16%) | 17(18.9%) | 5 (12.5) | 4 (9.3%) | 50 (13.1%) |
| Other | 9 (8.7%) | 9 (8.5%) | 2 (2.2%) | 3 (7.5%) | 4 (9.3%) | 27 (7%) |
Literature review
NET: neuroendocrine tumor
SCLC: Small cell lung carcinoma
MTC: Medullary thyroid carcinoma
GEP: Gastroenteropancreatic
Localization studies
Several different localization studies were used, particularly in cases where the source of ACTH was not apparent at presentation.
○ IPSS: 8 patients had IPSS that further supported a diagnosis of EAS because of the lack of central-to-peripheral ACTH gradients in all 8 cases.
○ 6-mCi indium [In-111] pentetreotide scan (octreoscan): Twenty patients had octreoscans (including 8 for whom this scan was repeated at least twice). Octreoscans identified the source of ACTH production in 12 patients (60% of those tested). This included 5 patients with bronchial carcinoid, 2 with medullary thyroid carcinoma, 3 with pancreatic NET, 1 urinary bladder NET, and 1 with small bowel carcinoid. Octreoscan was negative in the remaining 8 (40%) that included 4 patients who had occult source of ACTH while other imaging studies localized ACTH source in the remaining 4 patients( 1 with bronchial carcinoid, 2 with thymic carcinoid, 1 with widely metastatic NET of unknown primary).
○ 18-fluorodeoxyglucose positron emission tomography (FDG-PET) scans: 6 patients had FDG-PET scans. The underlying tumor was seen with FDG-PET scans in 4 patients (SCLC, thymic carcinoid, bronchial carcinoid, and MTC), whereas the scan was negative in 2 patients (1 with a bronchial carcinoid tumor and 1 with an occult ACTH source).
○ Pituitary imaging: 34 patients had pituitary imaging, which was normal in 31 patients (91%), whereas 3 patients (9%) were shown to have incidental pituitary abnormalities. IPSS failed to localize ACTH secretion to the pituitary in 2 of these 3 patients with occult ACTH sources. In the third patient, there was an incidental 0.3-cm pituitary microadenoma on MRI imaging but she was found to have an ACTH-producing bronchial carcinoid tumor.
- ○ Cross-sectional body imaging:
- ■ Chest imaging (CT and/ or MRI) localized the source of ACTH in 25 of 37 patients who had such imaging. These patients had their primary tumor located in the chest or lower neck and included patients with 9 bronchial carcinoid, 9 SCLC, 4 MTC, and 3 thymic carcinoid.
- ■ Abdominal imaging (CT and/ or MRI) localized the source of ACTH or distant metastases to the abdomen in 9 of 32 patients who had such imaging.
Duration to localization
The time to ACTH localization after CS diagnosis was variable and ranged from 0 – 118 months. The times to localization were as follows:
⦵ Localization at time of diagnosis (within 1 month): 32 patients (74.4%)
⦵ Delayed localization (more than 1 month): 7 patients (16.3%), whose tumors were localized after a median of 22 months (range, 6 – 110 months). The diagnoses of these patients were: 4 bronchial carcinoid tumors, 1 thymic carcinoid tumor, 1 bladder neuroendocrine tumor, and 1 metastatic neuroendocrine tumor of unknown primary origin.
- ⦵ Occult source: In 4 patients (9.3%), the sources of ACTH remained unknown despite extensive work-up and the patients were labeled as having occult ACTH sources. Follow-up for these 4 patients ranged from 6 –118 months, and they had the following localization studies performed:
- Pituitary imaging: 2 were normal and 2 suggested pituitary adenoma, but IPSS testing did not confirm adenomas as the source of ACTH
- IPSS tests were done in all 4 patients, including 1 patient who had IPSS twice. Table. 3 summarizes the biochemical profile in these 4 patients.
- Chest and abdominal cross-sectional imaging scans were negative in all 4 patients
- An FDG-PET scan performed for 1 patient was negative
- Octreoscans were negative in all 4 patients
Table.3.
Summary of the 4 patients with occult ACTH source.
| Patient number |
Age at diagnosis |
Sex | 24-UFC (microgram) |
8 am cortisol after HDSST (microgram/dl) |
MRI pituitary | Central to Peripheral ACTH ratio before CRH injection* |
Central to Peripheral ACTH ratio after CRH injection* |
Pituitary Surgery |
|---|---|---|---|---|---|---|---|---|
| 1 | 46 | M | 398 | N/A | No adenoma (questionable) |
1.1 | 1.2 | Yes, but no adenoma |
| 2† | 41 | F | 71.7 | 15.3 | Suspicious 0.6 cm adenoma |
1 1.1 |
1.5 1.1 |
Yes, but no ACTH staining |
| 3 | 27 | M | 1250 | 68 | No adenoma | 1.1 | 1.1 | Not done |
| 4 | 43 | F | 283 | 8.3 | Suspicious 0.5 cm adenoma |
1.2 | 1.6 | Not done |
CRH injection during IPSS study
IPSS done twice after initial pituitary surgery
Management
Surgical and medical options to control hypercortisolemia were individualized, considering the variability in ACTH sources. Surgery was performed in 28 patients, including 7 patients who had bilateral adrenalectomy, 14 who had resections of their primary tumors (ACTH sources), and 7 who had combined bilateral adrenalectomy along with primary tumor resections.
Medical therapy was offered to 40 patients, mainly using metyrapone and ketoconazole, with variable success to control cortisol overproduction. Available data did not allow meaningful assessment for duration of response or magnitude of response considering the variability of clinical course and frequent deaths seen in this cohort.
Medical complications
Infectious complications were documented in 10 patients (23.3%; 5 had pneumonia alone, 2 had pneumonia and cellulitis, 2 had pneumonia and sepsis, and 1 had septicemia). Symptomatic venous thromboembolism (VTE) was documented in 6 patients (14%) including 4 patients with pulmonary embolism, 1 with unprovoked deep vein thrombosis of axillary/ subclavian veins, and 1 with symptomatic retinal vein thrombosis. Two patients with bronchial carcinoid died secondary to pulmonary embolism.
Prognosis
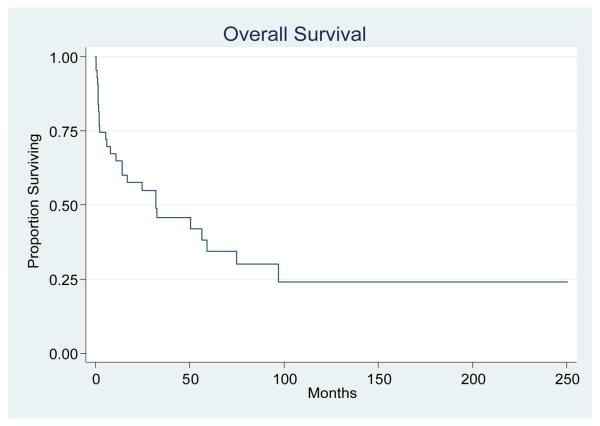
Death occurred in 27 patients and the median duration of OS was 32.2 months in all patients. There were no significant differences in median OS durations between men (32.2 months) and women (32.4 months; P = 0.714). The Kaplan-Meier curve for OS in all patients is illustrated in Figure 1. Progression of primary malignancy and systemic infections at the time of death were the leading causes of mortality and 2 patients who died from pulmonary embolism.
Figure. 1.
The Kaplan-Meier curve for overall survival in this series (n=43). Median OS was 32.2 months.
Discussion
Cushing's syndrome (CS) in association with non-pituitary tumors was described more than 8 decades ago but our current knowledge is limited to retrospective reviews in part because of the rarity of this syndrome , variability in clinical presentation, and heterogeneity of underlying tumors associated with this syndrome. Tumors associated with ectopic ACTH production have been well documented in medical literature mainly in case reports and case series from single institutions. It remains uncertain if patients seen in comprehensive cancer centers differ from patients reported from other tertiary referral centers. To clarify this uncertainty, we summarized our experience with CS-EAS patients seen and treated at a comprehensive cancer center and compared our findings with other case series from other major medical institutions. We identified a total of 300 with CS seen and treated in our institution out of which 43 patients (14.3%) had CS-EAS that is close to available reports in which CS-EAS constitutes 8 – 18% of all causes of CS 8, 13, 14. These estimates are derived mostly from major referral centers and likely subject to referral bias.
Current body of literature reported the prevalence of clinical CS-EAS in 1.6% – 4.5% of patients with SCLC 17-19 and biochemical abnormalities suggestive of CS or ectopic ACTH in as many as 30 – 50% of SCLC patients 20-22. Despite of extensive search of medical records, we found only 9 cases with SCLC associated EAS-CS and could not re-indentify previously reported cases of SCLC and CS published from our institution two decades ago 15 . Based on that, we feel that our report underestimates the true prevalence of CS-EAS in cancer patients.
Earlier reports have suggested that ectopic CS is more common in men, because initial cases of CS-EAS were often reported in patients with SCLC 17, 18; however, our report and some other more recent case series have estimated that men constitute between 40% – 50% of all patients with CS-EAS 6-9 and represent only 6% – 26% of patients with Cushing's disease 23-25.
The median age for patients at diagnosis was 48 years in our report, which falls within the range of mean ages at diagnosis (38 – 50 years) reported elsewhere 6-9.
Clinically, weight gain was not universal in patients with CS-EAS, and weight loss was reported in 21% of patients in our series. This differs from the 10% of patients reported to have weight loss in other reports 6, with the discrepancy possibly linked to the higher ratio of malignant, especially SCLC, tumors seen in our report. The majority of patients in our series had new onset or worsening hypertension or hyperglycemia, similar to that seen in other reported series 6, 7. On the other hand, leukocytosis was seen in only (39%) patients in our series, whereas neutrophilia, lymphopenia, and eosinopenia were found in 55% – 58% of patients at the time of initial diagnosis. Hypokalemia was noted in (72%) patients in our study, which is similar to the reported prevalence of 71% in literature 6. UFC and plasma ACTH were elevated in all tested patients, suggestive of ACTH-mediated CS, whereas serum cortisol remained elevated in patients (88%) after HDDST, suggestive of EAS and similar to the rates of (90%) patients and (91%) patients reported by Ilias et al 6 and Isidori et al 7, respectively.
The distribution of tumors associated with CS-EAS is summarized in Table 2. Looking at the combined data from multiple series in Table 2, it appears that bronchial carcinoid and SCLC tumors are the two most common causes of EAS and represent about 44.4% of all cases. Thus, chest imaging represents the most important diagnostic test in cases of ACTH-dependent CS with negative pituitary imaging or IPSS suggestive of EAS.
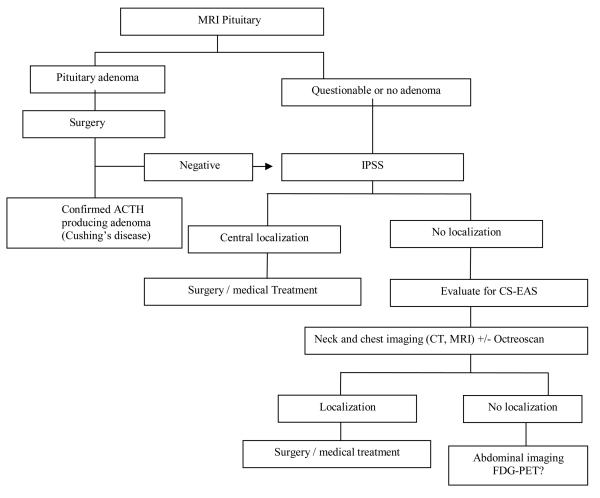
IPSS is a very helpful tool for ruling out pituitary sources of ACTH with very high sensitivity and specificity 13; however, the wider use of this procedure is still limited by the potential risk for serious complications and the availability of experienced neuroradiologists. After combining our data with 3 other reports 4, 6, 7, we found that 37/38 patients with occult CS-EAS who had IPSS studies showed no central/peripheral gradient suggestive of non-pituitary source for ACTH secretion. However, this assumption should be viewed in the light of the retrospective series where false negative IPSS results were seen in about 6.5% of patients with Cushing's disease 26, 27. On the other hand, octreoscans localized the sources of ACTH in only 12/20 (60%) patients in our series, close to other reports in the literature that showed limited sensitivity for octreoscans in patients with CS-EAS 6, 7. The use of FDG-PET is not established in the diagnosis and management of CS-EAS, although it is often used to stage and assess lung malignancies. Scattered reports have suggested the incidental findings of carcinoid tumors by using FDG-PET scans 28-30,with sensitivity of 75% to detect bronchial carcinoid tumors measuring 1– 8 cm 31. The combination of FDG-PET with CT imaging has been reported to enhance the localization of bronchial carcinoid tumors 32. In this series, FDG-PET localized ACTH sources in 4/6 patients (with SCLC, MTC, bronchial carcinoid, and thymic carcinoid tumors) in whom the primary tumor was also seen on the less-expensive cross-sectional imaging studies. In the fifth patient, FDG-PET was negative, as were all other imaging studies, at the time of diagnosis. With long-term follow-up, this patient was found by CT of the chest to have a 1-cm bronchial carcinoid tumor 28 months after her initial diagnosis. In the sixth patient with an occult ACTH source, FDG-PET was negative, similar to the other diagnostic studies. In 11 (25.6%) patients, the diagnosis of the source of ACTH production was either delayed more than 6 months or remained unknown after exhausting work-ups. This finding is compatible with the overall reported experience in the literature, especially with occult cases, as shown in Table 2. Considering the complex nature of this disease, we suggested an algorithm for ACTH source localization as shown in Figure. 2.
Figure. 2. Suggested algorithm for diagnostic work-up for ACTH-Dependent Cushing's Syndrome.
IPSS: Inferior Petrosal Sinus Sampling
Multiple questions remain unanswered regarding the association with infections and other medical complications previously reported in patients with CS. The association between CS and a predisposition to infection has been reported since the description of Cushing's disease in 1932 by Harvey Cushing, who noted that “The malady appears to leave the patients with a definite susceptibility to infections” 2. Ever since, retrospective evidence has accumulated to support the assumption of a higher risk of infection associated with hypercortisolemia 15, 16, 33. We also found an increased risk of infection in our series but the lack of a good control group and the nature of retrospective data reviews may affect the accurate assessment of the strength of this association between CS and infections.
The likelihood of VTE in association with CS has been debated and the true prevalence has not yet been well-established 34. It has been well-documented that CS patients have altered coagulation-factor profiles, including elevated levels of coagulation factors II, V, VIII, IX, XI, and XII, compatible with an increased risk for thrombosis 35. Despite the lack of quality evidence, a recent review suggested a risk of VTE in patients with CS of close to 2% in patients who did not have surgery and around 4% post surgery 36. It is unclear if patients with CS-EAS have higher risks of VTE compared to patients with CS secondary to other causes. The fact that ectopic CS is associated with underlying malignancy may theoretically put these patients at higher risk for VTE compared with other CS patients. Still, our finding of 6 cases of VTE (14%) is close to those of other reports who also combined prospective follow-up with retrospective analysis 34.
The median OS in our series was 32.2 months, with no effect of gender on survival. In our series, death occurred in 27/43 patients (62.8%) during the follow-up period, which is much higher than the reported death rate (19/90; 21%) of patients in the NIH series that had fewer patients with SCLC (3.3% of all cases) 6. Nevertheless, our findings are similar to those reported by Isidori et al 7, in which 25/40 (62.5%) patients died during follow-up; as they observed a percentage of patients with SCLC (17.5%) closer to ours (21%). While CS with an occult ACTH source can lead to significant morbidity, OS is better in such patients than in patients who have apparent malignant sources of ACTH, including SCLC and MTC 4, 8.
The heterogeneity of underlying tumors as well as the retrospective nature of our study limited our ability to assess the effect of cortisol lowering therapy on clinical outcomes especially infections and death. We speculate that lowering cortisol levels before attempting curative treatments (surgery or chemotherapy) may reduce the mortality and morbidity associated with CS and in particular reduce the rates of opportunistic infections. Prospective investigation of this syndrome is needed in order to find answers for the gaps in our knowledge related to CS-EAS and in its effect on cancer related therapy.
Conclusion
CS-EAS is a relatively uncommon clinical syndrome that accompanies a wide variety of tumors. Patients may not have obvious CS because their clinical presentation may be masked by the symptoms of the underlying tumor. Thus the diagnosis can be challenging, with the source of ACTH production difficult to identify. Additionally, the true prevalence of this syndrome has likely been underestimated in this retrospective review. This syndrome is associated with significant morbidity and mortality, which could be related in part to the expected increased incidence of infectious and thrombotic complications. Patients with neuroendocrine tumors need to be carefully assessed for CS-EAS. Prospective studies are needed to clarify the true prevalence of this syndrome in cancer patients and its clinical complications.
Acknowledgments
The authors thank Maude E. Veech from the Department of Scientific Publications, Sarah H. Taylor from Tumor Registry, and Graciela M. Nogueras González from the division of Quantitative Sciences for their assistance in this manuscript.
Research support: This paper is supported in part by the National Institutes of Health through The University of Texas MD Anderson Cancer Center's Cancer Center Support Grant CA16672.
Footnotes
Conflict of interest: None
References
- 1.Brown H. A CASE OF PLURIGLANDULAR SYNDROME: “DIABETES OF BEARDED WOMEN.”. The Lancet. 1928;212(5490):1022–23. [Google Scholar]
- 2.Cushing H. The basophilic adenomas of the pituitary body and their clinical manifestations(pituitary basophilism) Bull Johns Hopkins Hosp. 1932;50:138–95. [Google Scholar]
- 3.Meador CK, Liddle GW, Island DP, Nicholson WE, Lucas CP, Nuckton JG, et al. Cause of Cushing's syndrome in patients with tumors arising from “nonendocrine” tissue. J Clin Endocrinol Metab. 1962;22:693–703. doi: 10.1210/jcem-22-7-693. [DOI] [PubMed] [Google Scholar]
- 4.Aniszewski JP, Young WF, Jr., Thompson GB, Grant CS, van Heerden JA. Cushing syndrome due to ectopic adrenocorticotropic hormone secretion. World J Surg. 2001;25(7):934–40. doi: 10.1007/s00268-001-0032-5. [DOI] [PubMed] [Google Scholar]
- 5.Liddle GW, Nicholson WE, Island DP, Orth DN, Abe K, Lowder SC. Clinical and laboratory studies of ectopic humoral syndromes. Recent Prog Horm Res. 1969;25:283–314. doi: 10.1016/b978-0-12-571125-8.50009-0. [DOI] [PubMed] [Google Scholar]
- 6.Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing's syndrome due to ectopic corticotropin secretion: twenty years' experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90(8):4955–62. doi: 10.1210/jc.2004-2527. [DOI] [PubMed] [Google Scholar]
- 7.Isidori AM, Kaltsas GA, Pozza C, Frajese V, Newell-Price J, Reznek RH, et al. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J Clin Endocrinol Metab. 2006;91(2):371–7. doi: 10.1210/jc.2005-1542. [DOI] [PubMed] [Google Scholar]
- 8.Howlett TA, Drury PL, Perry L, Doniach I, Rees LH, Besser GM. Diagnosis and management of ACTH-dependent Cushing's syndrome: comparison of the features in ectopic and pituitary ACTH production. Clin Endocrinol (Oxf) 1986;24(6):699–713. doi: 10.1111/j.1365-2265.1986.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 9.Jex RK, van Heerden JA, Carpenter PC, Grant CS. Ectopic ACTH syndrome. Diagnostic and therapeutic aspects. Am J Surg. 1985;149(2):276–82. doi: 10.1016/s0002-9610(85)80085-4. [DOI] [PubMed] [Google Scholar]
- 10.Doppman JL, Nieman L, Miller DL, Pass HI, Chang R, Cutler GB, Jr., et al. Ectopic adrenocorticotropic hormone syndrome: localization studies in 28 patients. Radiology. 1989;172(1):115–24. doi: 10.1148/radiology.172.1.2544919. [DOI] [PubMed] [Google Scholar]
- 11.Wajchenberg BL, Mendonca BB, Liberman B, Pereira MA, Carneiro PC, Wakamatsu A, et al. Ectopic adrenocorticotropic hormone syndrome. Endocr Rev. 1994;15(6):752–87. doi: 10.1210/edrv-15-6-752. [DOI] [PubMed] [Google Scholar]
- 12.Findling JW, Tyrrell JB. Occult ectopic secretion of corticotropin. Arch Intern Med. 1986;146(5):929–33. [PubMed] [Google Scholar]
- 13.Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, et al. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing's syndrome. N Engl J Med. 1991;325(13):897–905. doi: 10.1056/NEJM199109263251301. [DOI] [PubMed] [Google Scholar]
- 14.Wajchenberg BL, Mendonca B, Liberman B, Adelaide M, Pereira A, Kirschner MA. Ectopic ACTH syndrome. J Steroid Biochem Mol Biol. 1995;53(1-6):139–51. doi: 10.1016/0960-0760(95)00044-z. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos MA, Fernandez JF, Samaan NA, Holoye PY, Vassilopoulou-Sellin R. Paraneoplastic Cushing's syndrome as an adverse prognostic factor in patients who die early with small cell lung cancer. Cancer. 1992;69(1):66–71. doi: 10.1002/1097-0142(19920101)69:1<66::aid-cncr2820690113>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Sarlis NJ, Chanock SJ, Nieman LK. Cortisolemic indices predict severe infections in Cushing syndrome due to ectopic production of adrenocorticotropin. J Clin Endocrinol Metab. 2000;85(1):42–7. doi: 10.1210/jcem.85.1.6294. [DOI] [PubMed] [Google Scholar]
- 17.Kato Y, Ferguson TB, Bennett DE, Burford TH. Oat cell carcinoma of the lung. A review of 138 cases. Cancer. 1969;23(3):517–24. doi: 10.1002/1097-0142(196903)23:3<517::aid-cncr2820230301>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd FA, Laskey J, Evans WK, Goss PE, Johansen E, Khamsi F. Cushing's syndrome associated with ectopic corticotropin production and small-cell lung cancer. J Clin Oncol. 1992;10(1):21–7. doi: 10.1200/JCO.1992.10.1.21. [DOI] [PubMed] [Google Scholar]
- 19.Delisle L, Boyer MJ, Warr D, Killinger D, Payne D, Yeoh JL, et al. Ectopic corticotropin syndrome and small-cell carcinoma of the lung. Clinical features, outcome, and complications. Arch Intern Med. 1993;153(6):746–52. [PubMed] [Google Scholar]
- 20.Gewirtz G, Yalow RS. Ectopic ACTH production in carcinoma of the lung. J Clin Invest. 1974;53(4):1022–32. doi: 10.1172/JCI107639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gropp C, Havemann K, Scheuer A. Ectopic hormones in lung cancer patients at diagnosis and during therapy. Cancer. 1980;46(2):347–54. doi: 10.1002/1097-0142(19800715)46:2<347::aid-cncr2820460223>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 22.Bondy PK, Gilby ED. Endocrine function in small cell undifferentiated carcinoma of the lung. Cancer. 1982;50(10):2147–53. doi: 10.1002/1097-0142(19821115)50:10<2147::aid-cncr2820501029>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 23.Etxabe J, Vazquez JA. Morbidity and mortality in Cushing's disease: an epidemiological approach. Clin Endocrinol (Oxf) 1994;40(4):479–84. doi: 10.1111/j.1365-2265.1994.tb02486.x. [DOI] [PubMed] [Google Scholar]
- 24.Lindholm J, Juul S, Jorgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, et al. Incidence and late prognosis of cushing's syndrome: a population-based study. J Clin Endocrinol Metab. 2001;86(1):117–23. doi: 10.1210/jcem.86.1.7093. [DOI] [PubMed] [Google Scholar]
- 25.Bochicchio D, Losa M, Buchfelder M. Factors influencing the immediate and late outcome of Cushing's disease treated by transsphenoidal surgery: a retrospective study by the European Cushing's Disease Survey Group. J Clin Endocrinol Metab. 1995;80(11):3114–20. doi: 10.1210/jcem.80.11.7593411. [DOI] [PubMed] [Google Scholar]
- 26.Bonelli FS, Huston J, 3rd, Carpenter PC, Erickson D, Young WF, Jr., Meyer FB. Adrenocorticotropic hormone-dependent Cushing's syndrome: sensitivity and specificity of inferior petrosal sinus sampling. AJNR Am J Neuroradiol. 2000;21(4):690–6. [PMC free article] [PubMed] [Google Scholar]
- 27.Swearingen B, Katznelson L, Miller K, Grinspoon S, Waltman A, Dorer DJ, et al. Diagnostic errors after inferior petrosal sinus sampling. J Clin Endocrinol Metab. 2004;89(8):3752–63. doi: 10.1210/jc.2003-032249. [DOI] [PubMed] [Google Scholar]
- 28.Gomard-Mennesson E, Seve P, De La Roche E, Collardeau-Frachon S, Lombard-Bohas C, Broussolle C. [Thymic carcinoid tumor revealed by a Cushing's syndrome: usefulness of positron emission tomography] Rev Med Interne. 2008;29(9):751–3. doi: 10.1016/j.revmed.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Kumar J, Spring M, Carroll PV, Barrington SF, Powrie JK. 18Flurodeoxyglucose positron emission tomography in the localization of ectopic ACTH-secreting neuroendocrine tumours. Clin Endocrinol (Oxf) 2006;64(4):371–4. doi: 10.1111/j.1365-2265.2006.02471.x. [DOI] [PubMed] [Google Scholar]
- 30.Markou A, Manning P, Kaya B, Datta SN, Bomanji JB, Conway GS. [18F]fluoro-2-deoxy-D-glucose ([18F]FDG) positron emission tomography imaging of thymic carcinoid tumor presenting with recurrent Cushing's syndrome. Eur J Endocrinol. 2005;152(4):521–5. doi: 10.1530/eje.1.01839. [DOI] [PubMed] [Google Scholar]
- 31.Daniels CE, Lowe VJ, Aubry MC, Allen MS, Jett JR. The utility of fluorodeoxyglucose positron emission tomography in the evaluation of carcinoid tumors presenting as pulmonary nodules. Chest. 2007;131(1):255–60. doi: 10.1378/chest.06-0711. [DOI] [PubMed] [Google Scholar]
- 32.Kruger S, Buck AK, Blumstein NM, Pauls S, Schelzig H, Kropf C, et al. Use of integrated FDG PET/CT imaging in pulmonary carcinoid tumours. J Intern Med. 2006;260(6):545–50. doi: 10.1111/j.1365-2796.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- 33.Plotz CM, Knowlton AI, Ragan C. The natural history of Cushing's syndrome. Am J Med. 1952;13(5):597–614. doi: 10.1016/0002-9343(52)90027-2. [DOI] [PubMed] [Google Scholar]
- 34.Small M, Lowe GD, Forbes CD, Thomson JA. Thromboembolic complications in Cushing's syndrome. Clin Endocrinol (Oxf) 1983;19(4):503–11. doi: 10.1111/j.1365-2265.1983.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 35.Kastelan D, Dusek T, Kraljevic I, Polasek O, Giljevic Z, Solak M, et al. Hypercoagulability in Cushing's syndrome: the role of specific haemostatic and fibrinolytic markers. Endocrine. 2009;36(1):70–4. doi: 10.1007/s12020-009-9186-y. [DOI] [PubMed] [Google Scholar]
- 36.Van Zaane B, Nur E, Squizzato A, Dekkers OM, Twickler MT, Fliers E, et al. Hypercoagulable state in Cushing's syndrome: a systematic review. J Clin Endocrinol Metab. 2009;94(8):2743–50. doi: 10.1210/jc.2009-0290. [DOI] [PubMed] [Google Scholar]