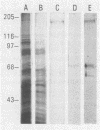
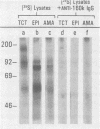
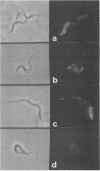
Abstract
Recent studies on the humoral immune response to Trypanosoma cruzi have shown that antibodies which are able to bind living parasites and lyse them in conjunction with complement are associated with host protection. Antibodies which support complement-mediated lysis (CML) of trypomastigotes are elicited as a result of an active infection and not after immunization with killed parasites. In spite of the requirement for immune antibodies, lysis proceeds mainly via the alternative complement pathway. We have purified a 160-kilodalton (kDa) glycoprotein from T. cruzi metacyclic trypomastigotes which appears to be a specific target for lytic antibodies. Rabbit antiserum to the purified 160-kDa protein was prepared, and we have determined that these antibodies will support CML of tissue-culture-derived trypomastigotes. The percentage of killing (65 to 70%) was consistent among three different T. cruzi strains tested. In order to examine the specificity of antibody-dependent CML, antibodies to T. cruzi neuraminidase, an unrelated trypomastigote membrane glycoprotein, were tested in the CML, assays and were not found lytic. Viable trypomastigotes bound anti-160-kDa antibodies uniformly as demonstrated by immunofluorescence, whereas antineuraminidase antibodies were extensively capped. The 160-kDa glycoprotein is specifically produced in infectious trypomastigotes (tissue culture derived and metacyclic) and was not detected in epimastigotes or amastigotes. The identification of the 160-kDa glycoprotein as a specific target for lytic antibodies, as well as its expression only in the infectious stage of the parasite, suggests an important role for this protein in eliciting host immunity.
Full text
PDF

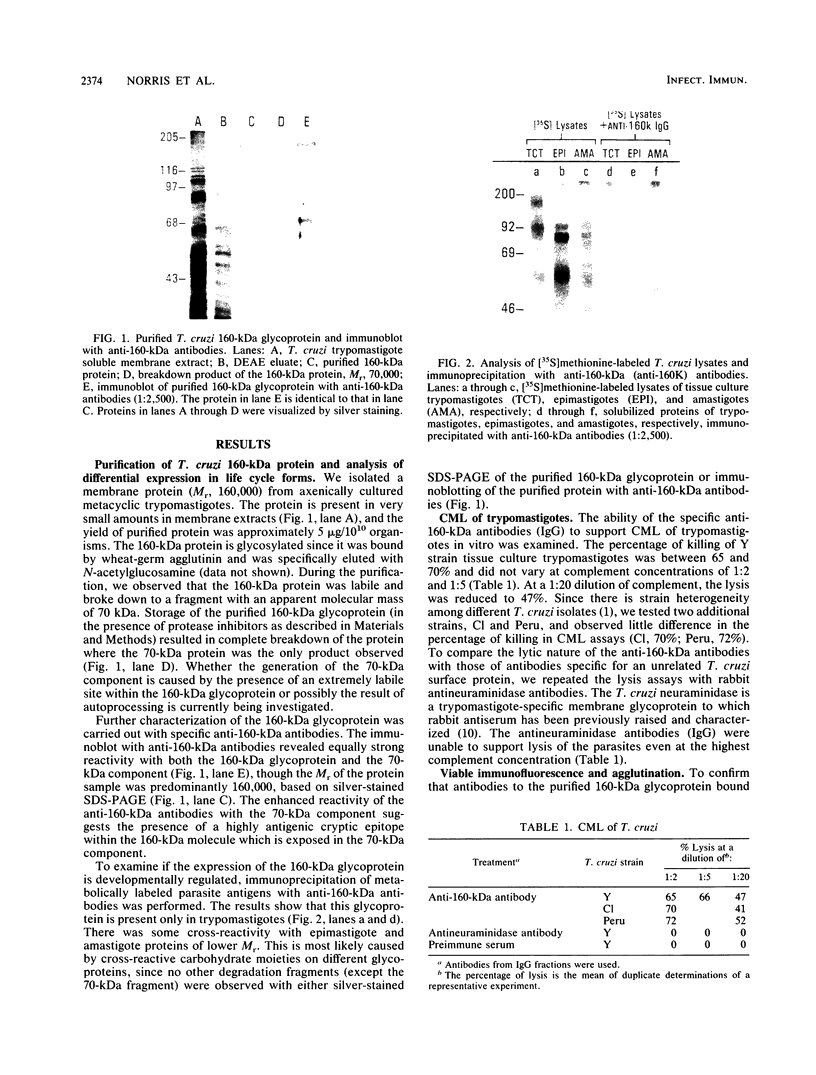


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brener Z. Why vaccines do not work in Chagas disease. Parasitol Today. 1986 Jul;2(7):196–197. doi: 10.1016/0169-4758(86)90193-6. [DOI] [PubMed] [Google Scholar]
- Budzko D. B., Pizzimenti M. C., Kierszenbaum F. Effects of complement depletion in experimental chagas disease: immune lysis of virulent blood forms of Trypanosoma cruzi. Infect Immun. 1975 Jan;11(1):86–91. doi: 10.1128/iai.11.1.86-91.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J. J., Behin R., Mauel J., Rowe D. S. Antibody-induced movement of membrane components of Leishmania enriettii. J Exp Med. 1974 May 1;139(5):1061–1069. doi: 10.1084/jem.139.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer D. M. Antibody-induced modulation of Leishmania donovani surface membrane antigens. J Immunol. 1976 Dec;117(6):2081–2091. [PubMed] [Google Scholar]
- Dzbeński T. H., Zielińska E. Antibody-induced formation of caps in Toxoplasma gondii. Experientia. 1976 Apr 15;32(4):454–456. doi: 10.1007/BF01920792. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Allison A. C. Alternative pathway activation of complement by African trypanosomes lacking a glycoprotein coat. Parasite Immunol. 1983 Sep;5(5):491–498. doi: 10.1111/j.1365-3024.1983.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Fischer E., Ouaissi M. A., Velge P., Cornette J., Kazatchkine M. D. gp 58/68, a parasite component that contributes to the escape of the trypomastigote form of T. cruzi from damage by the human alternative complement pathway. Immunology. 1988 Oct;65(2):299–303. [PMC free article] [PubMed] [Google Scholar]
- Franke E. D., McGreevy P. B., Katz S. P., Sacks D. L. Growth cycle-dependent generation of complement-resistant Leishmania promastigotes. J Immunol. 1985 Apr;134(4):2713–2718. [PubMed] [Google Scholar]
- Harth G., Haidaris C. G., So M. Neuraminidase from Trypanosoma cruzi: analysis of enhanced expression of the enzyme in infectious forms. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8320–8324. doi: 10.1073/pnas.84.23.8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., daSilva W. D., Rimoldi M. T., Hammer C. H., Sher A., Kipnis T. L. Biochemical characterization of a factor produced by trypomastigotes of Trypanosoma cruzi that accelerates the decay of complement C3 convertases. J Biol Chem. 1988 Aug 15;263(23):11327–11335. [PubMed] [Google Scholar]
- Kipnis T. L., Krettli A. U., Dias da Silva W. Transformation of trypomastigote forms of Trypanosoma cruzi into activators of alternative complement pathway by immune IgG fragments. Scand J Immunol. 1985 Aug;22(2):217–226. doi: 10.1111/j.1365-3083.1985.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Kipnis T. L., Tambourgi D. V., Sucupira M., Dias-da-Silva W. Effect of Trypanosoma cruzi membrane components on the formation of the classical pathway C3 convertase. Braz J Med Biol Res. 1986;19(2):271–278. [PubMed] [Google Scholar]
- Konigsberg W. H., Henderson L. Removal of sodium dodecyl sulfate from proteins by ion-pair extraction. Methods Enzymol. 1983;91:254–259. doi: 10.1016/s0076-6879(83)91022-4. [DOI] [PubMed] [Google Scholar]
- Krettli A. U., Brener Z. Resistance against Trypanosoma cruzi associated to anti-living trypomastigote antibodies. J Immunol. 1982 May;128(5):2009–2012. [PubMed] [Google Scholar]
- Krettli A. U., Cançado J. R., Brener Z. Effect of specific chemotherapy on the levels of lytic antibodies in Chagas's disease. Trans R Soc Trop Med Hyg. 1982;76(3):334–340. doi: 10.1016/0035-9203(82)90184-5. [DOI] [PubMed] [Google Scholar]
- Krettli A. U., Weisz-Carrington P., Nussenzweig R. S. Membrane-bound antibodies to bloodstream Trypanosoma cruzi in mice: strain differences in susceptibility to complement-mediated lysis. Clin Exp Immunol. 1979 Sep;37(3):416–423. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martins M. S., Hudson L., Krettli A. U., Cançado J. R., Brener Z. Human and mouse sera recognize the same polypeptide associated with immunological resistance to Trypanosoma cruzi infection. Clin Exp Immunol. 1985 Aug;61(2):343–350. [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Bianco C., Cohn Z. Studies on the selective lysis and purification of Trypanosoma cruzi. J Exp Med. 1975 Jul 1;142(1):224–229. doi: 10.1084/jem.142.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi M. T., Sher A., Heiny S., Lituchy A., Hammer C. H., Joiner K. Developmentally regulated expression by Trypanosoma cruzi of molecules that accelerate the decay of complement C3 convertases. Proc Natl Acad Sci U S A. 1988 Jan;85(1):193–197. doi: 10.1073/pnas.85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeiro S. A., Takehara H. A., Mota I. Isotype of lytic antibodies in serum of Chagas' disease patients. Clin Exp Immunol. 1984 Feb;55(2):413–418. [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., Thomas J. A., Twomey C. E. The growth of Trypanosoma cruzi in human diploid cells for the production of trypomastigotes. Parasitology. 1980 Feb;80(1):153–162. doi: 10.1017/s0031182000000615. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Güther M. L., Yoshida N. Mechanism of resistance to lysis by the alternative complement pathway in Trypanosoma cruzi trypomastigotes: effect of specific monoclonal antibody. J Immunol. 1986 Sep 1;137(5):1623–1628. [PubMed] [Google Scholar]
- Schmuñis G. A., Szarfman A., De Souza W., Langembach T. Trypanosoma cruzi: antibody-induced mobility of surface antigens. Exp Parasitol. 1980 Aug;50(1):90–102. doi: 10.1016/0014-4894(80)90011-9. [DOI] [PubMed] [Google Scholar]
- Schmuñis G. A., Szarfman A., Langembach T., de Souza W. Induction of capping in blood-stage trypomastigotes of Trypanosoma cruzi by human anti-Trypanosoma cruzi antibodies. Infect Immun. 1978 May;20(2):567–569. doi: 10.1128/iai.20.2.567-569.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. J. Metacyclogenesis of Trypanosoma cruzi in vitro: a simplified procedure. Trans R Soc Trop Med Hyg. 1982;76(3):300–303. doi: 10.1016/0035-9203(82)90173-0. [DOI] [PubMed] [Google Scholar]
- Teixeira A. R., Santos-Buch C. A. The immunology of experimental Chagas' disease. I. Preparation of Trypanosoma cruzi antigens and humoral antibody response to there antigens. J Immunol. 1974 Sep;113(3):859–869. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]