Abstract
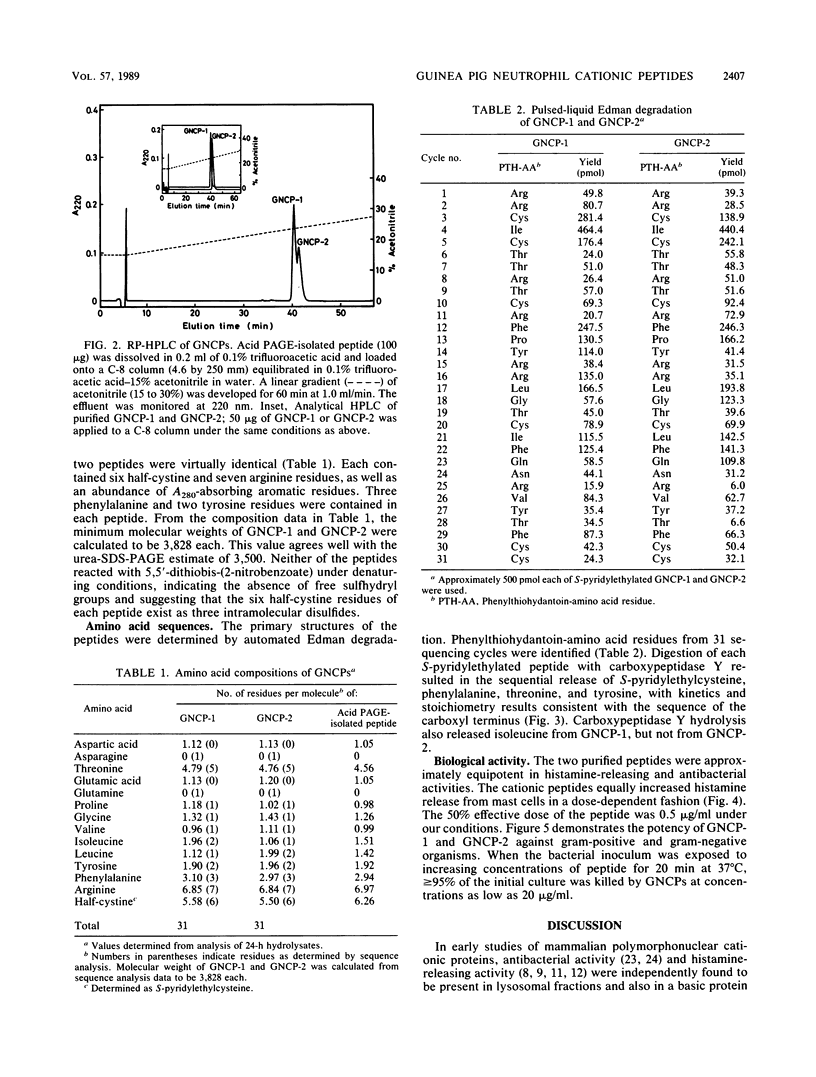
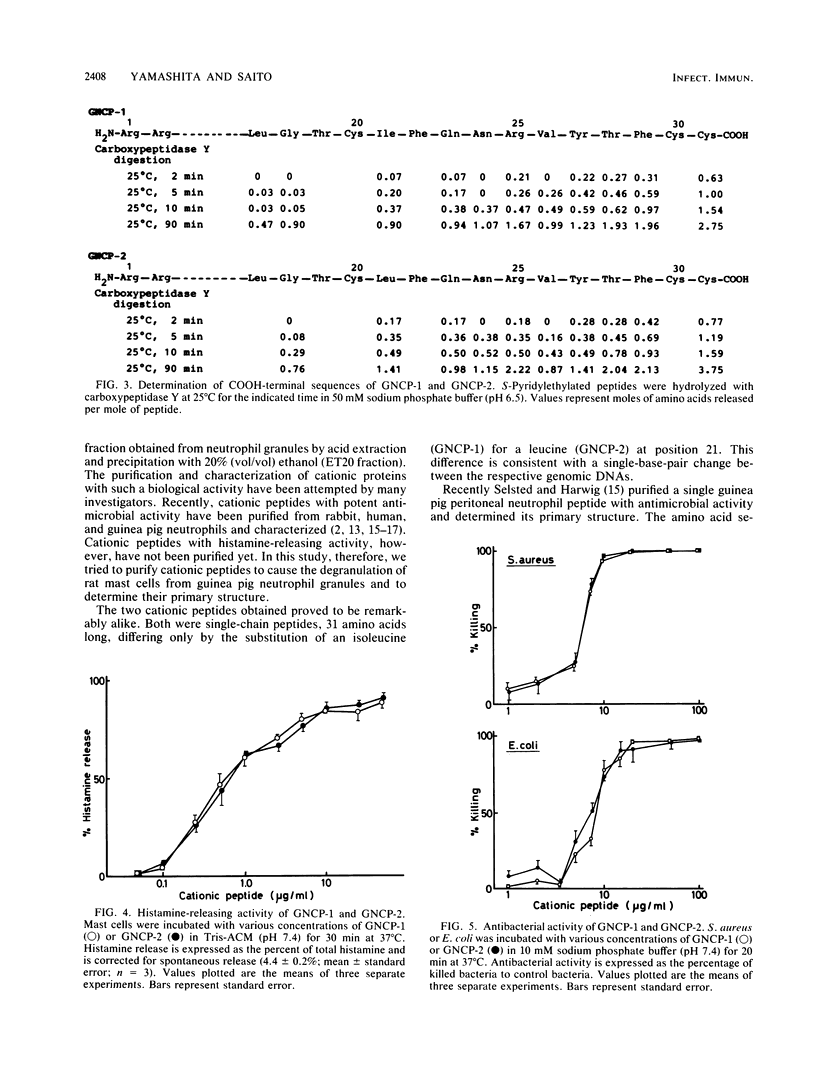
The guinea pig neutrophil cationic peptides GNCP-1 and GNCP-2 were purified from a granule-rich subcellular fraction of peritoneal exudate neutrophils by acid-gel electrophoresis and reversed-phase high-performance liquid chromatography. Both peptides not only released histamine from rat mast cells to the same extent but also were equally active against Staphylococcus aureus and Escherichia coli. The peptides were rich in arginine and cystine and lacked free sulfhydryl groups. Composition and sequence determinations revealed that GNCP-1 and GNCP-2 are each single polypeptides containing 31 amino acid residues and three intramolecular disulfide bonds. The complete amino acid sequence of GNCP-1 is Arg-Arg-Cys-Ile-Cys-Thr-Thr-Arg-Thr-Cys-Arg-Phe-Pro-Tyr-Arg-Arg-Leu-Gly- Thr-Cys - Ile-Phe-Gln-Asn-Arg-Val-Tyr-Thr-Phe-Cys-Cys. The sequence of GNCP-2 is identical except for the substitution of isoleucine for leucine at residue 21.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R. Carboxypeptidase Y in sequence determination of peptides. Methods Enzymol. 1977;47:84–93. doi: 10.1016/0076-6879(77)47010-1. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Neurath H., Walsh K. A. Determination of the amino acid sequence of porcine trypsin by sequenator aalysis. Biochemistry. 1973 Aug 14;12(17):3146–3153. doi: 10.1021/bi00741a002. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y., Yamashita T. Purification and characterization of a phagocytosis-stimulating factor from phagocytosing polymorphonuclear neutrophils: comparison with granule basic proteins. Infect Immun. 1985 Jun;48(3):799–805. doi: 10.1128/iai.48.3.799-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C. D., Lyman M., Alberto R., Cheng J. Procedures for immunochemical study of histamine release from leukocytes with small volume of blood. J Allergy. 1970 Jul;46(1):12–20. doi: 10.1016/0021-8707(70)90056-0. [DOI] [PubMed] [Google Scholar]
- Nagaoka I., Yamashita T. Possible involvement of aminopeptidase, an ecto-enzyme, in the inactivation of bradykinin by intact neutrophils. Biochim Biophys Acta. 1985 Oct 30;847(1):67–76. doi: 10.1016/0167-4889(85)90154-5. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Ranadive N. S., Cochrane C. G. Isolation and characterization of permeability factors from rabbit neutrophils. J Exp Med. 1968 Oct 1;128(4):605–622. doi: 10.1084/jem.128.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranadive N. S., Cochrane C. G. Mechanism of histamine release from mast cells by cationic protein (band 2) from neutrophil lysosomes. J Immunol. 1971 Feb;106(2):506–516. [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Scherer J., Janoff A. Mediators of inflammation in leukocyte lysosomes. VII. Observations on mast cell-rupturing agents in different species. Lab Invest. 1968 Feb;18(2):196–202. [PubMed] [Google Scholar]
- Seegers W., Janoff A. Mediators of inflammation in leukocyte lysosomes. VI. Partial purification and characterization of a mast cell-rupturing component. J Exp Med. 1966 Nov 1;124(5):833–849. doi: 10.1084/jem.124.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Brown D. M., DeLange R. J., Harwig S. S., Lehrer R. I. Primary structures of six antimicrobial peptides of rabbit peritoneal neutrophils. J Biol Chem. 1985 Apr 25;260(8):4579–4584. [PubMed] [Google Scholar]
- Selsted M. E., Brown D. M., DeLange R. J., Lehrer R. I. Primary structures of MCP-1 and MCP-2, natural peptide antibiotics of rabbit lung macrophages. J Biol Chem. 1983 Dec 10;258(23):14485–14489. [PubMed] [Google Scholar]
- Selsted M. E., Harwig S. S., Ganz T., Schilling J. W., Lehrer R. I. Primary structures of three human neutrophil defensins. J Clin Invest. 1985 Oct;76(4):1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Harwig S. S. Purification, primary structure, and antimicrobial activities of a guinea pig neutrophil defensin. Infect Immun. 1987 Sep;55(9):2281–2286. doi: 10.1128/iai.55.9.2281-2286.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Szklarek D., Lehrer R. I. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun. 1984 Jul;45(1):150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan T. J., Parker K. L., Stenson W., Parker C. W. Modulation of cyclic AMP in purified rat mast cells. I. Responses to pharmacologic, metabolic, and physical stimuli. J Immunol. 1975 May;114(5):1473–1479. [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Imaizumi N., Yuasa S. Effect of endocellular cryoprotectant upon polymorphonuclear neutrophil function during storage at low temperature. Cryobiology. 1979 Apr;16(2):112–117. doi: 10.1016/0011-2240(79)90020-8. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Saito K. No direct involvement of plasma membrane divalent cation-activated adenosine triphosphatase in histamine release from rat mast cells. Biochem Biophys Res Commun. 1987 Apr 29;144(2):951–956. doi: 10.1016/s0006-291x(87)80056-6. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. I. Resolution of antibacterial and enzymatic activities. J Bacteriol. 1966 Feb;91(2):750–754. doi: 10.1128/jb.91.2.750-754.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. II. Composition, properties, and mechanism of antibacterial action. J Bacteriol. 1966 Feb;91(2):755–762. doi: 10.1128/jb.91.2.755-762.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



