Abstract
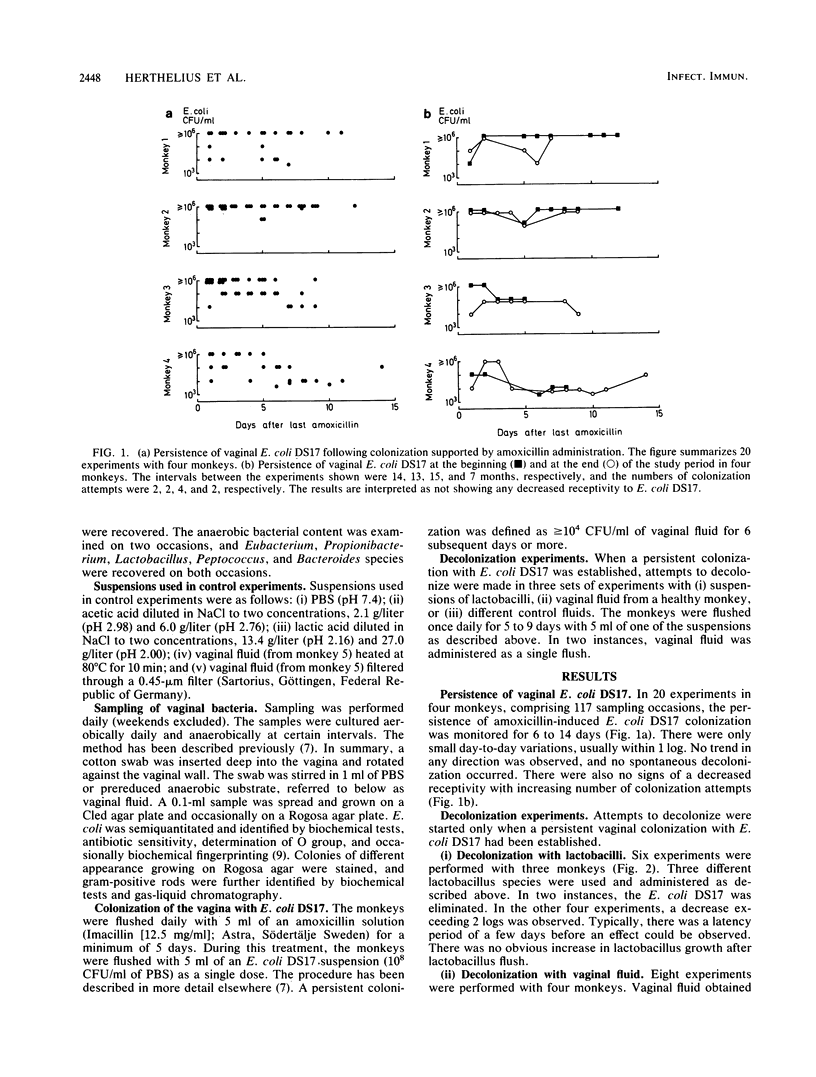
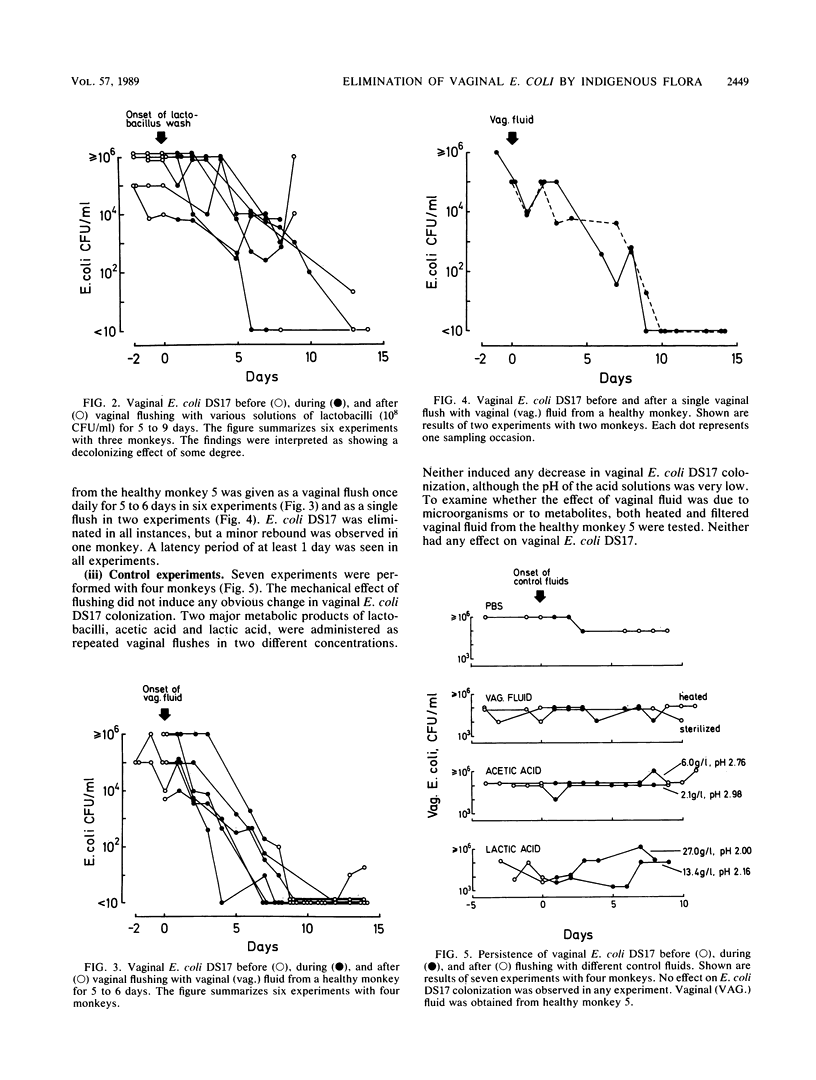
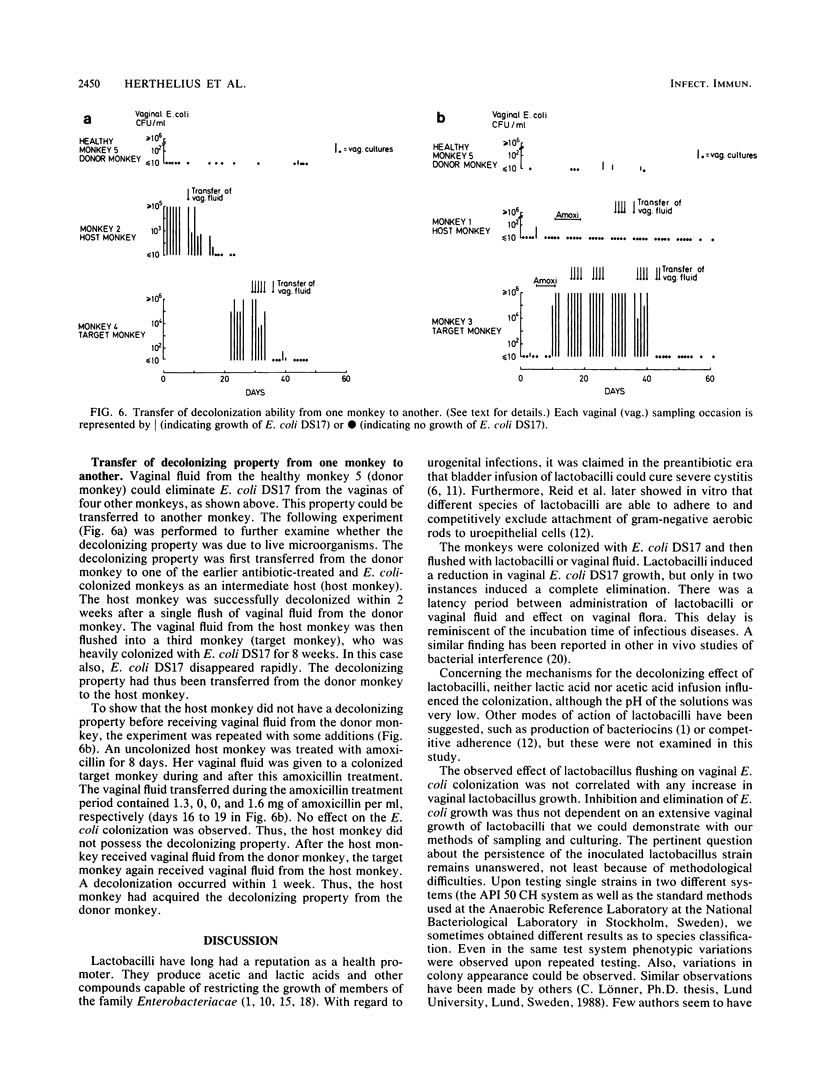
A persistent vaginal colonization with a pyelonephritogenic strain of Escherichia coli, induced by administration of amoxicillin, was established in four adult cynomolgus monkeys. This colonization mimicked the one seen in urinary tract infection-prone human females. Attempts to eliminate the E. coli colonization and restore normal conditions were made. Either suspensions of lactobacilli or vaginal fluid from a healthy unmanipulated monkey was administered as repeated vaginal flushes for 5 to 9 days. A total elimination of vaginal E. coli was observed in two of six experiments with lactobacilli, and a decrease was observed in the other four. A better result was obtained with flushes of vaginal fluid, which eliminated the E. coli colonization in eight of eight experiments. In two of these, a single flush was sufficient to obtain a decolonization. The ability of fresh vaginal fluid to eliminate E. coli from the vagina could be transferred from one monkey to another. This study demonstrates the role of the normal flora in the defense against genital colonization with potentially pathogenic adhering E. coli. The possible clinical relevance of these findings must be further examined.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barefoot S. F., Klaenhammer T. R. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl Environ Microbiol. 1983 Jun;45(6):1808–1815. doi: 10.1128/aem.45.6.1808-1815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollgren I., Winberg J. The periurethral aerobic bacterial flora in healthy boys and girls. Acta Paediatr Scand. 1976 Jan;65(1):74–80. doi: 10.1111/j.1651-2227.1976.tb04410.x. [DOI] [PubMed] [Google Scholar]
- Bollgren I., Winberg J. The periurethral aerobic flora in girls highly susceptible to urinary infections. Acta Paediatr Scand. 1976 Jan;65(1):81–87. doi: 10.1111/j.1651-2227.1976.tb04411.x. [DOI] [PubMed] [Google Scholar]
- Bruce A. W., Reid G. Intravaginal instillation of lactobacilli for prevention of recurrent urinary tract infections. Can J Microbiol. 1988 Mar;34(3):339–343. doi: 10.1139/m88-062. [DOI] [PubMed] [Google Scholar]
- Brumfitt W., Gargan R. A., Hamilton-Miller J. M. Periurethral enterobacterial carriage preceding urinary infection. Lancet. 1987 Apr 11;1(8537):824–826. doi: 10.1016/s0140-6736(87)91606-0. [DOI] [PubMed] [Google Scholar]
- Herthelius B. M., Hedström K. G., Möllby R., Nord C. E., Pettersson L., Winberg J. Pathogenesis of urinary tract infections--amoxicillin induces genital Escherichia coli colonization. Infection. 1988 Sep-Oct;16(5):263–266. doi: 10.1007/BF01645066. [DOI] [PubMed] [Google Scholar]
- Hofmann K., Marget W., Belohradsky B. Veränderte bakterielle Periurethralflora bei jungen Mädchen mit chronisch rekurrierenden Harnwegsinfektionen? Infection. 1981;9(5):252–254. doi: 10.1007/BF01640729. [DOI] [PubMed] [Google Scholar]
- Mårdh P. A., Soltész L. V. In vitro interactions between lactobacilli and other microorganisms occurring in the vaginal flora. Scand J Infect Dis Suppl. 1983;40:47–51. [PubMed] [Google Scholar]
- Reid G., Cook R. L., Bruce A. W. Examination of strains of lactobacilli for properties that may influence bacterial interference in the urinary tract. J Urol. 1987 Aug;138(2):330–335. doi: 10.1016/s0022-5347(17)43137-5. [DOI] [PubMed] [Google Scholar]
- Roberts J. A., Kaack B., Källenius G., Möllby R., Winberg J., Svenson S. B. Receptors for pyelonephritogenic Escherichia coli in primates. J Urol. 1984 Jan;131(1):163–168. doi: 10.1016/s0022-5347(17)50251-7. [DOI] [PubMed] [Google Scholar]
- Schwan A., Sjölin S., Trottestam U., Aronsson B. Relapsing Clostridium difficile enterocolitis cured by rectal infusion of normal faeces. Scand J Infect Dis. 1984;16(2):211–215. doi: 10.3109/00365548409087145. [DOI] [PubMed] [Google Scholar]
- Silva M., Jacobus N. V., Deneke C., Gorbach S. L. Antimicrobial substance from a human Lactobacillus strain. Antimicrob Agents Chemother. 1987 Aug;31(8):1231–1233. doi: 10.1128/aac.31.8.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamey T. A., Timothy M., Millar M., Mihara G. Recurrent urinary infections in adult women. The role of introital enterobacteria. Calif Med. 1971 Jul;115(1):1–19. [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Källenius G., Korhonen T. K., Möllby R., Roberts J. A., Tullus K., Winberg J. Initiation of clinical pyelonephritis--the role of P-fimbriae-mediated bacterial adhesion. Contrib Nephrol. 1984;39:252–272. doi: 10.1159/000409254. [DOI] [PubMed] [Google Scholar]
- Tramer J. Inhibitory effect of Lactobacillus acidophilus. Nature. 1966 Jul 9;211(5045):204–205. doi: 10.1038/211204a0. [DOI] [PubMed] [Google Scholar]
- Tullus K., Hörlin K., Svenson S. B., Källenius G. Epidemic outbreaks of acute pyelonephritis caused by nosocomial spread of P fimbriated Escherichia coli in children. J Infect Dis. 1984 Nov;150(5):728–736. doi: 10.1093/infdis/150.5.728. [DOI] [PubMed] [Google Scholar]


