Abstract
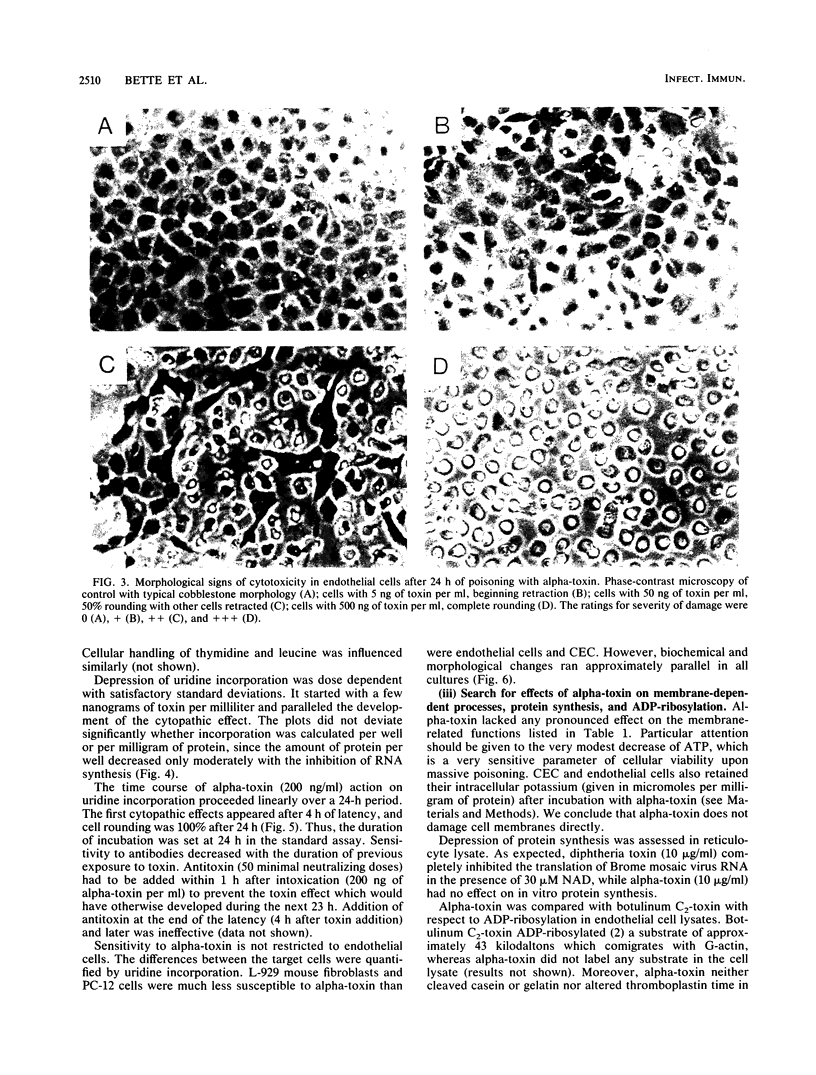
The actions of apparently homogeneous alpha-toxin from Clostridium novyi type A were studied in order to develop an in vitro system which closely mimics its in vivo effects and to search for the mode of poisoning. Time to death (by intravenous injection of mice) was inversely related to dose, with a detection limit of about 200 ng/kg of body weight at 100 h. Injections of 2.5 ng or more into the rat paw led to a slowly (maximum after about 30 h) developing, dose-dependent edema which was useful as a quantitative in vivo assay based on volumetry. Vascular leakage was due to gap formation between endothelial cells. Similarly, endothelial cells cultured from pig pulmonary artery lost their "cobblestone" arrangement after a dose-dependent lag period of some hours after poisoning. The morphological changes were accompanied by depression of uptake or incorporation of [3H]uridine. A quantitative in vitro assay was established on the inhibition of [3H]uridine incorporation. As in animals, the action of alpha-toxin started with a few nanograms per milliliter and proceeded slowly for at least 1 day but became resistant to antitoxin within 2 h of exposure. The toxin action is not limited to endothelial cells, since chicken embryonic cells, a mouse fibroblast line (L-929), and a rat phaeochromocytoma line (PC-12) behaved similarly. Alpha-toxin was found to differ from other bacterial toxins investigated whose modes of action are already known.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AIKAT B. K., DIBLE J. H. The local and general effects of cultures and culture-filtrates of Clostridium oedematiens, CL. septicum, CL. sporogenes and CL. histolyticum. J Pathol Bacteriol. 1960 Apr;79:227–241. doi: 10.1002/path.1700790203. [DOI] [PubMed] [Google Scholar]
- Aktories K., Bärmann M., Ohishi I., Tsuyama S., Jakobs K. H., Habermann E. Botulinum C2 toxin ADP-ribosylates actin. Nature. 1986 Jul 24;322(6077):390–392. doi: 10.1038/322390a0. [DOI] [PubMed] [Google Scholar]
- Albus U., Habermann E., Ferry D. R., Glossmann H. Novel 1,4-dihydropyridine (Bay K 8644) facilitates calcium-dependent [3H]noradrenaline release from PC 12 cells. J Neurochem. 1984 Apr;42(4):1186–1189. doi: 10.1111/j.1471-4159.1984.tb12729.x. [DOI] [PubMed] [Google Scholar]
- Bagadi H. O., Sewell M. M. Experimental studies on infectious necrotic hepatitis (Black Disease) of sheep. Res Vet Sci. 1973 Jul;15(1):53–61. [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. Cell. 1980 Sep;21(2):365–372. doi: 10.1016/0092-8674(80)90473-0. [DOI] [PubMed] [Google Scholar]
- Boroff D. A., Fleck U. Statistical analysis of a rapid in vivo method for the titration of the toxin of Clostridium botulinum. J Bacteriol. 1966 Nov;92(5):1580–1581. doi: 10.1128/jb.92.5.1580-1581.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal G. S., Ahnert-Hilger G., Beress L., Habermann E. Palytoxin both induces and inhibits the release of histamine from rat mast cells. Int Arch Allergy Appl Immunol. 1982;68(2):97–100. doi: 10.1159/000233075. [DOI] [PubMed] [Google Scholar]
- Cotran R. S. Studies on inflammation. Ultrastructure of the prolonged vascular response induced by Clostridium oedematiens toxin. Lab Invest. 1967 Jul;17(1):39–60. [PubMed] [Google Scholar]
- Detwiler T. C., Feinman R. D. Kinetics of the thrombin-induced release of adenosine triphosphate by platelets. Comparison with release of calcium. Biochemistry. 1973 Jun 19;12(13):2462–2468. doi: 10.1021/bi00737a015. [DOI] [PubMed] [Google Scholar]
- Habermann E., Ahnert-Hilger G., Chhatwal G. S., Beress L. Delayed haemolytic action of palytoxin. General characteristics. Biochim Biophys Acta. 1981 Dec 7;649(2):481–486. doi: 10.1016/0005-2736(81)90439-9. [DOI] [PubMed] [Google Scholar]
- Habermann E., Hudel M., Dauzenroth M. E. Palytoxin promotes potassium outflow from erythrocytes, HeLa and bovine adrenomedullary cells through its interaction with Na+, K+ -ATPase. Toxicon. 1989;27(4):419–430. doi: 10.1016/0041-0101(89)90204-3. [DOI] [PubMed] [Google Scholar]
- Habermann E. Inhibition by tetanus and botulinum A toxin of the release of [3H]noradrenaline and [3H]GABA from rat brain homogenate. Experientia. 1988 Mar 15;44(3):224–226. doi: 10.1007/BF01941714. [DOI] [PubMed] [Google Scholar]
- Izumi N., Kondo H., Ohishi I., Sakaguchi G. Purification and characterization of alpha-toxin of Clostridium oedematiens type A. Jpn J Med Sci Biol. 1983 Jun;36(3):135–146. [PubMed] [Google Scholar]
- Izumi N., Niiro M., Kondo H. Clostridium oedematiens type A toxin: the correlation between the lethal and edematizing activities. Jpn J Med Sci Biol. 1983 Apr;36(2):67–74. doi: 10.7883/yoken1952.36.67. [DOI] [PubMed] [Google Scholar]
- Joris I., Majno G., Corey E. J., Lewis R. A. The mechanism of vascular leakage induced by leukotriene E4. Endothelial contraction. Am J Pathol. 1987 Jan;126(1):19–24. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mitchell T. J., Ketley J. M., Burdon D. W., Candy D. C., Stephen J. The effects of Clostridium difficile toxins A and B on membrane integrity and protein synthesis in intestinal cells in vivo and in vitro and in McCoy cells in vitro. J Med Microbiol. 1987 May;23(3):205–210. doi: 10.1099/00222615-23-3-205. [DOI] [PubMed] [Google Scholar]
- OAKLEY C. L., WARRACK G. H. The soluble antigens of Clostridium oedematiens type D (CI. haemolyticum). J Pathol Bacteriol. 1959 Oct;78:543–551. doi: 10.1002/path.1700780221. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rutter J. M., Collee J. G. Studies on the soluble antigens of Clostridium oedematiens (Cl. novyi). J Med Microbiol. 1969 Nov 4;2(4):395–417. doi: 10.1099/00222615-2-4-395. [DOI] [PubMed] [Google Scholar]
- Schallehn G., Wolff M. H. Morphologische Veränderungen humaner embryonaler Lungenfibroblasten durch Cytotoxine verschiedener Clostridium-Spezies. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Jan;267(3):367–378. [PubMed] [Google Scholar]
- Selden S. C., 3rd, Schwartz S. M. Cytochalasin B inhibition of endothelial proliferation at wound edges in vitro. J Cell Biol. 1979 May;81(2):348–354. doi: 10.1083/jcb.81.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Sullivan J. M., Peach M. J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982 Nov;51(5):657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Galanos C., Neuhof H. Endotoxin alters arachidonate metabolism in pulmonary endothelial cells. Am J Physiol. 1987 Sep;253(3 Pt 1):C384–C390. doi: 10.1152/ajpcell.1987.253.3.C384. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Seeger W., Uhl J., Lutz F., Roka L. Pseudomonas aeruginosa cytotoxin stimulates prostacyclin production in cultured pulmonary artery endothelial cells: membrane attack and calcium influx. J Cell Physiol. 1985 Apr;123(1):64–72. doi: 10.1002/jcp.1041230111. [DOI] [PubMed] [Google Scholar]
- Urbanitz D., Wiegand H., Habermann E. Zur Frage der Bedeutung des Kininsystems beim thermischen Odem der Rattenpfote. Naunyn Schmiedebergs Arch Pharmakol. 1969;264(4):476–493. [PubMed] [Google Scholar]
- Wysolmerski R., Lagunoff D., Dahms T. Ethchlorvynol-induced pulmonary edema in rats. An ultrastructural study. Am J Pathol. 1984 Jun;115(3):447–457. [PMC free article] [PubMed] [Google Scholar]
- Wysolmerski R., Lagunoff D. The effect of ethchlorvynol on cultured endothelial cells. A model for the study of the mechanism of increased vascular permeability. Am J Pathol. 1985 Jun;119(3):505–512. [PMC free article] [PubMed] [Google Scholar]




