Abstract
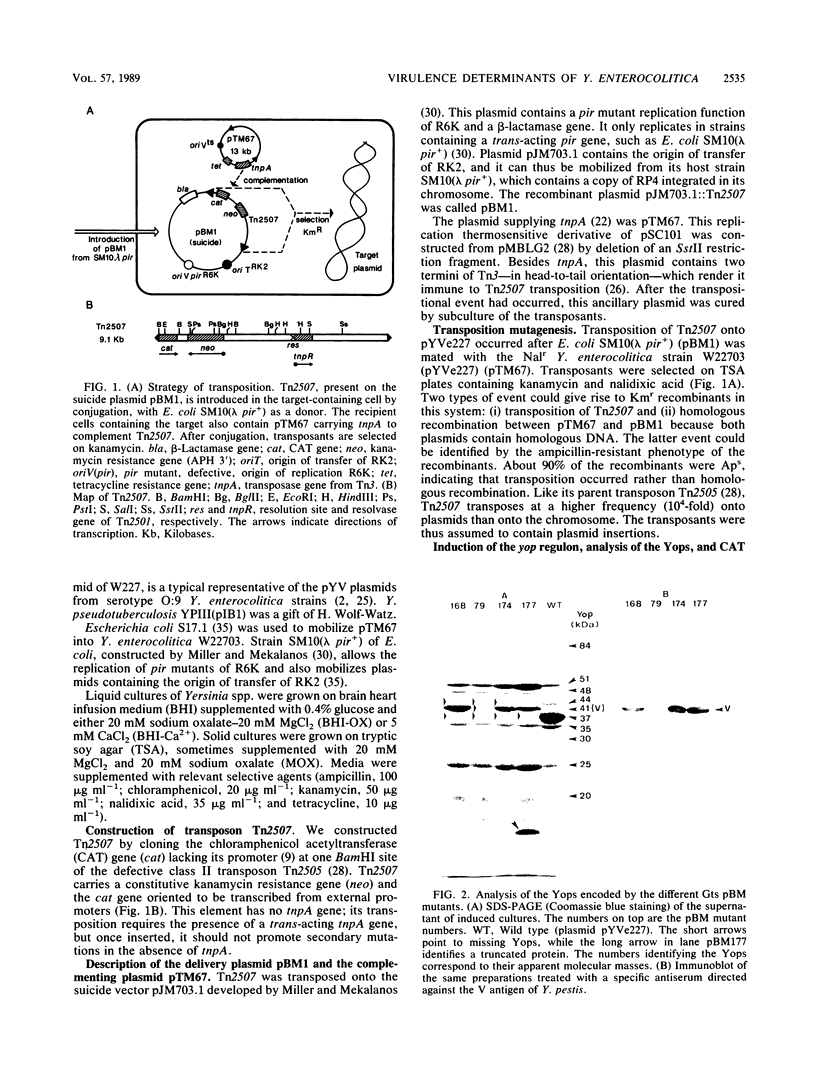
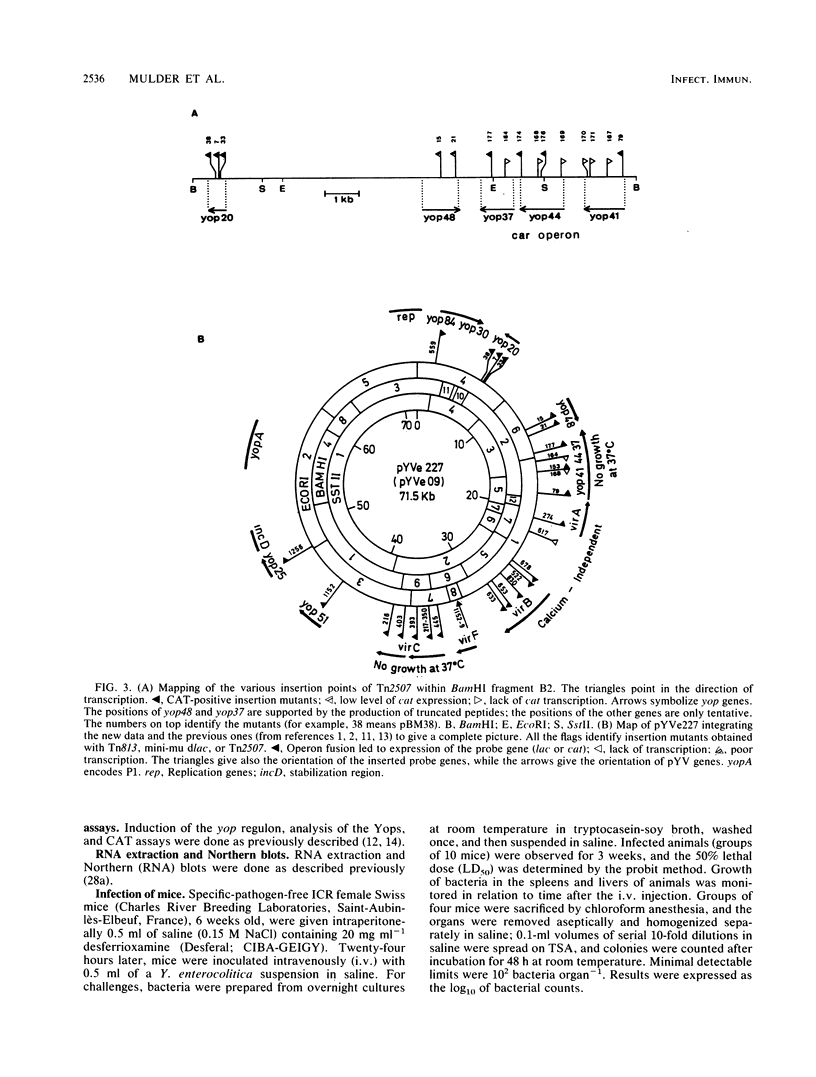
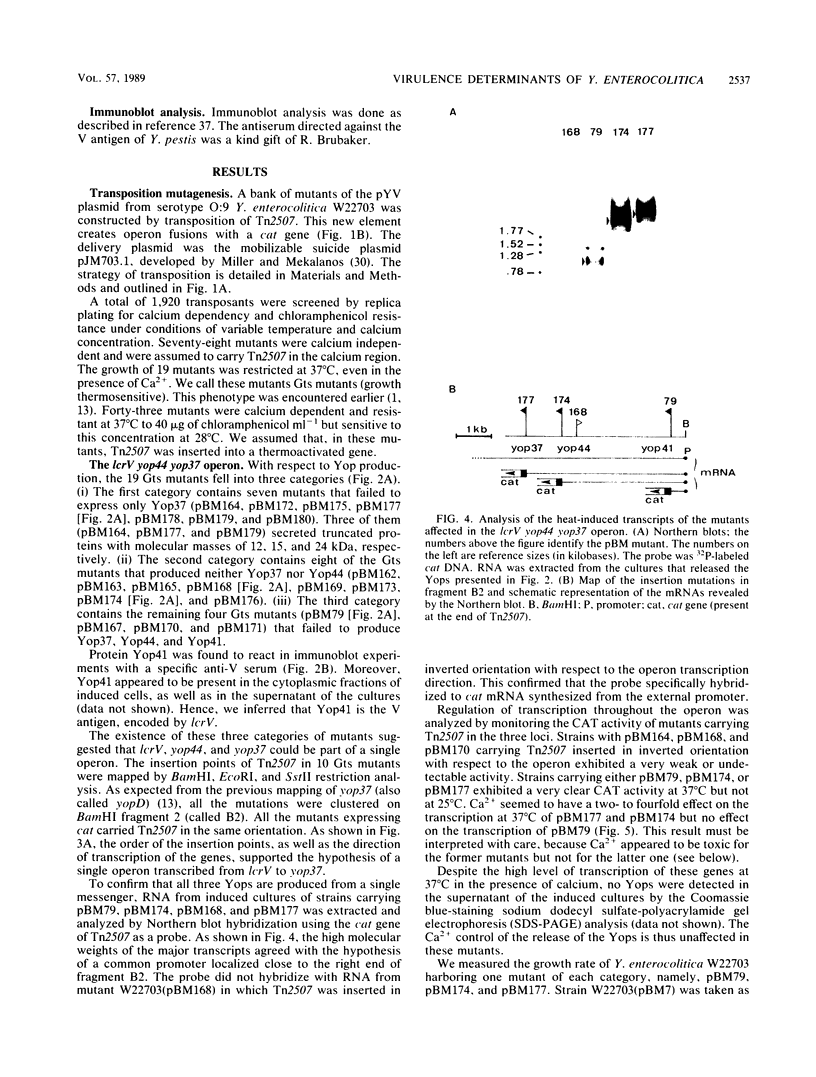
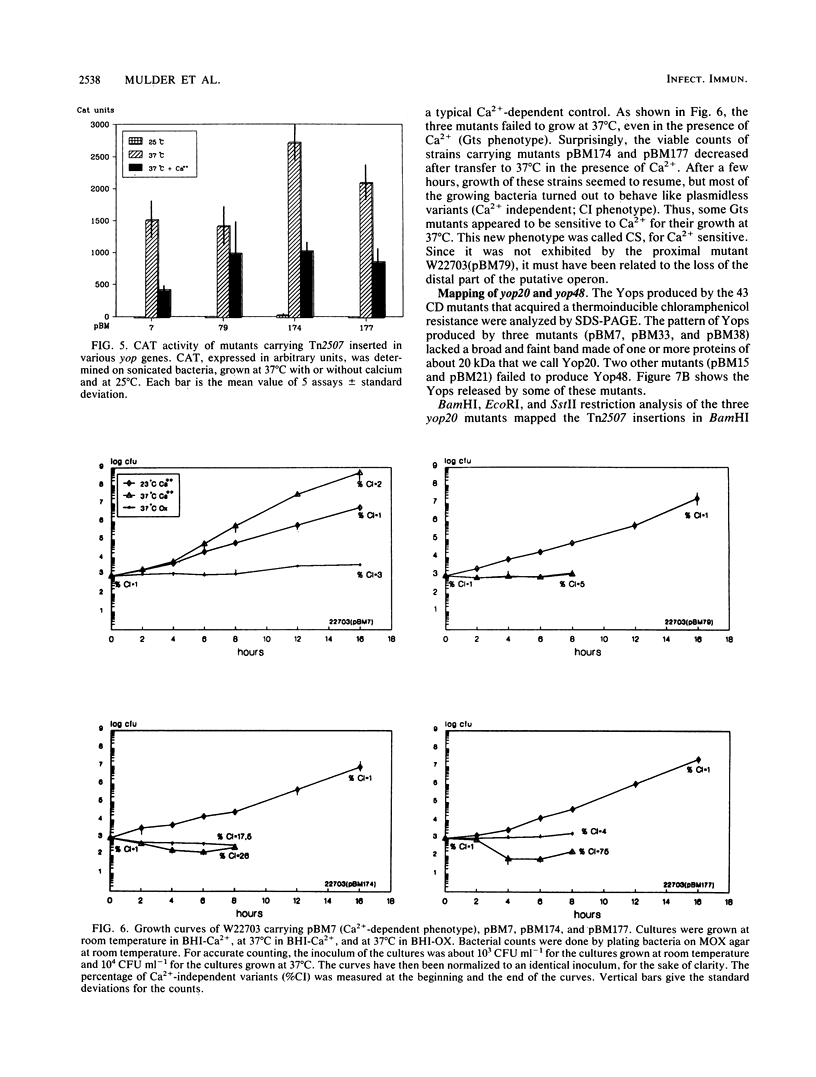
This paper describes the mutagenesis of the pYV plasmid from Yersinia enterocolitica W22703 (serotype O:9) with Tn2507, a new element generating operon fusions. Analysis of the mutants allowed the identification of an additional Yop protein called Yop20 and the mapping of yop20, yop44, yop48, and lcrV, the gene encoding the V antigen. The last gene appeared to be part of an operon that also may contain yop37 and yop44. At 37 degrees C, mutants affected in this operon grew poorly, irrespective of the presence of Ca2+, or they even died in the presence of Ca2+. This operon is thus involved in the regulation by Ca2+, and we called it car, for Ca2+ regulation. It is presumably the Y. enterocolitica counterpart of the lcrGVH operon of Yersinia pestis. Transcription of yop20 and of the car operon was strongly regulated by temperature and only slightly by calcium. Hence, these genes behaved like the other genes of the yop regulon. Mutants affected in yop20 or in yop48 were markedly less virulent for the desferrioxamine-treated mouse than was the parental strain. Yop20 and Yop48 thus probably are Yersinia virulence factors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACON G. A., BURROWS T. W. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br J Exp Pathol. 1956 Oct;37(5):481–493. [PMC free article] [PubMed] [Google Scholar]
- Balligand G., Laroche Y., Cornelis G. Genetic analysis of virulence plasmid from a serogroup 9 Yersinia enterocolitica strain: role of outer membrane protein P1 in resistance to human serum and autoagglutination. Infect Immun. 1985 Jun;48(3):782–786. doi: 10.1128/iai.48.3.782-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biot T., Cornelis G. R. The replication, partition and yop regulation of the pYV plasmids are highly conserved in Yersinia enterocolitica and Y. pseudotuberculosis. J Gen Microbiol. 1988 Jun;134(6):1525–1534. doi: 10.1099/00221287-134-6-1525. [DOI] [PubMed] [Google Scholar]
- Bölin I., Forsberg A., Norlander L., Skurnik M., Wolf-Watz H. Identification and mapping of the temperature-inducible, plasmid-encoded proteins of Yersinia spp. Infect Immun. 1988 Feb;56(2):343–348. doi: 10.1128/iai.56.2.343-348.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Portnoy D. A., Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985 Apr;48(1):234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Wolf-Watz H. The plasmid-encoded Yop2b protein of Yersinia pseudotuberculosis is a virulence determinant regulated by calcium and temperature at the level of transcription. Mol Microbiol. 1988 Mar;2(2):237–245. doi: 10.1111/j.1365-2958.1988.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Caron J., Coffield L. M., Scott J. R. A plasmid-encoded regulatory gene, rns, required for expression of the CS1 and CS2 adhesins of enterotoxigenic Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(3):963–967. doi: 10.1073/pnas.86.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalvignac M. A., Carniel E., Tram C., Joseph-Francois A., Mollaret H. H. In vitro expression of a 22-kilodalton Yersinia pestis polypeptide immunologically related to the 25-kilodalton plasmid-encoded protein of the three pathogenic Yersinia species. Infect Immun. 1988 Oct;56(10):2576–2580. doi: 10.1128/iai.56.10.2576-2580.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close T. J., Rodriguez R. L. Construction and characterization of the chloramphenicol-resistance gene cartridge: a new approach to the transcriptional mapping of extrachromosomal elements. Gene. 1982 Dec;20(2):305–316. doi: 10.1016/0378-1119(82)90048-8. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Colson C. Restriction of DNA in Yersinia enterocolitica detected by recipient ability for a derepressed R factor from Escherichia coli. J Gen Microbiol. 1975 Apr;87(2):285–291. doi: 10.1099/00221287-87-2-285. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Laroche Y., Balligand G., Sory M. P., Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987 Jan-Feb;9(1):64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Sluiters C., de Rouvroit C. L., Michiels T. Homology between virF, the transcriptional activator of the Yersinia virulence regulon, and AraC, the Escherichia coli arabinose operon regulator. J Bacteriol. 1989 Jan;171(1):254–262. doi: 10.1128/jb.171.1.254-262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G., Sory M. P., Laroche Y., Derclaye I. Genetic analysis of the plasmid region controlling virulence in Yersinia enterocolitica 0:9 by Mini-Mu insertions and lac gene fusions. Microb Pathog. 1986 Aug;1(4):349–359. doi: 10.1016/0882-4010(86)90067-7. [DOI] [PubMed] [Google Scholar]
- Cornelis G., Vanootegem J. C., Sluiters C. Transcription of the yop regulon from Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb Pathog. 1987 May;2(5):367–379. doi: 10.1016/0882-4010(87)90078-7. [DOI] [PubMed] [Google Scholar]
- Forsberg A., Bölin I., Norlander L., Wolf-Watz H. Molecular cloning and expression of calcium-regulated, plasmid-coded proteins of Y. pseudotuberculosis. Microb Pathog. 1987 Feb;2(2):123–137. doi: 10.1016/0882-4010(87)90104-5. [DOI] [PubMed] [Google Scholar]
- Forsberg A., Wolf-Watz H. The virulence protein Yop5 of Yersinia pseudotuberculosis is regulated at transcriptional level by plasmid-plB1-encoded trans-acting elements controlled by temperature and calcium. Mol Microbiol. 1988 Jan;2(1):121–133. [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980 Feb;27(2):682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goguen J. D., Yother J., Straley S. C. Genetic analysis of the low calcium response in Yersinia pestis mu d1(Ap lac) insertion mutants. J Bacteriol. 1984 Dec;160(3):842–848. doi: 10.1128/jb.160.3.842-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesemann J., Algermissen B., Laufs R. Genetically manipulated virulence of Yersinia enterocolitica. Infect Immun. 1984 Oct;46(1):105–110. doi: 10.1128/iai.46.1.105-110.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesemann J., Gross U., Schmidt N., Laufs R. Immunochemical analysis of plasmid-encoded proteins released by enteropathogenic Yersinia sp. grown in calcium-deficient media. Infect Immun. 1986 Nov;54(2):561–567. doi: 10.1128/iai.54.2.561-567.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Kapperud G., Namork E., Skurnik M., Nesbakken T. Plasmid-mediated surface fibrillae of Yersinia pseudotuberculosis and Yersinia enterocolitica: relationship to the outer membrane protein YOP1 and possible importance for pathogenesis. Infect Immun. 1987 Sep;55(9):2247–2254. doi: 10.1128/iai.55.9.2247-2254.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche Y., van Bouchaute M., Cornelis G. A restriction map of virulence plasmid pVYE439-80 from a serogroup 9 Yersinia enterocolitica strain. Plasmid. 1984 Jul;12(1):67–70. doi: 10.1016/0147-619x(84)90069-6. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Bhagwat A., Heffron F. Identification of a transposon Tn3 sequence required for transposition immunity. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6765–6769. doi: 10.1073/pnas.80.22.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Michiels T., Cornelis G. Nucleotide sequence and transcription analysis of yop51 from Yersinia enterocolitica W22703. Microb Pathog. 1988 Dec;5(6):449–459. doi: 10.1016/0882-4010(88)90006-x. [DOI] [PubMed] [Google Scholar]
- Michiels T., Cornelis G. Tn951 derivatives designed for high-frequency plasmid-specific transposition and deletion mutagenesis. Gene. 1986;43(3):175–181. doi: 10.1016/0378-1119(86)90205-2. [DOI] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. D., Haddix P., Atkins E. B., Soughers T. K., Straley S. C. Regulation of expression of V antigen and outer membrane proteins in Yersinia pestis. Contrib Microbiol Immunol. 1987;9:173–178. [PubMed] [Google Scholar]
- Perry R. D., Harmon P. A., Bowmer W. S., Straley S. C. A low-Ca2+ response operon encodes the V antigen of Yersinia pestis. Infect Immun. 1986 Nov;54(2):428–434. doi: 10.1128/iai.54.2.428-434.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins-Browne R. M., Prpic J. K. Effects of iron and desferrioxamine on infections with Yersinia enterocolitica. Infect Immun. 1985 Mar;47(3):774–779. doi: 10.1128/iai.47.3.774-779.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Bölin I., Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988 Aug;56(8):2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik M. Expression of antigens encoded by the virulence plasmid of Yersinia enterocolitica under different growth conditions. Infect Immun. 1985 Jan;47(1):183–190. doi: 10.1128/iai.47.1.183-190.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sory M. P., Cornelis G. Yersinia enterocolitica O:9 as a potential live oral carrier for protective antigens. Microb Pathog. 1988 Jun;4(6):431–442. doi: 10.1016/0882-4010(88)90028-9. [DOI] [PubMed] [Google Scholar]
- Straley S. C., Bowmer W. S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986 Feb;51(2):445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. G., Lee N., Fowler A. V. The araC gene of Escherichia coli: transcriptional and translational start-points and complete nucleotide sequence. Gene. 1980 Dec;12(3-4):179–190. doi: 10.1016/0378-1119(80)90100-6. [DOI] [PubMed] [Google Scholar]
- Yother J., Chamness T. W., Goguen J. D. Temperature-controlled plasmid regulon associated with low calcium response in Yersinia pestis. J Bacteriol. 1986 Feb;165(2):443–447. doi: 10.1128/jb.165.2.443-447.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yother J., Goguen J. D. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J Bacteriol. 1985 Nov;164(2):704–711. doi: 10.1128/jb.164.2.704-711.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]





