Abstract
Objective: The cytotoxic effect of berbamine on chronic myeloid leukemia (CML) cell line KU812 was evaluated, and the mechanisms of its action were explored. Methods: The effect of berbamine on the KU812 cell growth was determined by methyl thiazolyl tetrazolium (MTT) assay. Flow cytometry was used to profile cell cycle alteration upon berbamine treatment. Reverse transcription polymerase chain reaction (RT-PCR) was carried out to determine the transcripts of transforming growth factor-β (TGF-β) receptors (TβRs), Smad3, c-Myc, cyclin D1, p21Cip1(p21), and p27Kip1(p27). Changes in the protein levels of total Smad3, phosphorylated Smad3, the downstream targets of Smad3, and specific apoptosis-related factors were evaluated by Western blotting. Results: Berbamine inhibited KU812 cell proliferation in a dose- and time-dependent manner, and the half maximal inhibitory concentration (IC50) values for treatments of 24, 48, and 72 h were 5.83, 3.43, and 0.75 μg/ml, respectively. Berbamine induced G1 arrest as well as apoptosis in KU812 cells. Transcriptions of Smad3 and p21 were up-regulated, while those of TβRI, TβRII, c-Myc, cyclin D1 and p27 were not changed significantly. The protein levels of both total Smad3 and phosphorylated Smad3 were both up-regulated after berbamine treatment, together with decreased c-Myc and cyclin D1 and increased p21. Meanwhile, the levels of the anti-apoptotic proteins, such as Bcl-2 and Bcl-xL, were decreased, whereas pro-apoptotic Bax was increased. Conclusions: Berbamine suppresses KU812 cell proliferation through induction of cell cycle arrest in G1 and apoptosis. It activates Smad3 without additional stimulation of TGF-β, and alters the levels of the Smad3 downstream targets, including c-Myc, cyclin D1 and p21. Our findings suggest that berbamine is a promising drug in the treatment of advanced stage patients with CML.
Keywords: Berbamine, Apoptosis, Smad3, KU812 cells
1. Introduction
Chronic myeloid leukemia (CML) is a disorder arising from the transformation of pluripotent hematopoietic stem cells. Imatinib, a tyrosine-kinase inhibitor targeting oncogenic bcr/abl, has become a first-line option for CML management (Druker et al., 2006). Resistance to imatinib occurs in many patients carrying bcr/abl mutations (Engelman and Settleman, 2008). Strategies have been developed to overcome drug resistance to imatinib, for instance, dose escalation to achieve individual optimal levels, and introduction of the new generation bcr/abl kinase inhibitors Tasigna (nilotinib) or Sprycel (dasatinib) (Steinberg, 2007; An et al., 2010). However, prolonged exposure to these agents inevitably resulted in chemoresistance and refractory relapse with development of toxic side effects, and some patients eventually progressed with terminal stage of blast crisis. Therefore, it is necessary to identify novel therapeutic targets for CML treatment.
Berbamine, a small compound, naturally occurring in Berberis amurensis, has been shown to stimulate normal hematopoiesis and enhance immune function in cancer patients for decades. We previously showed that berbamine inhibits the growths of bcr/abl-positive K562 leukemia cells and primary CML cells through down-regulation of p210 bcr/abl, while little cytotoxicity is observed in normal blood cells (Xu et al., 2006; Wei et al., 2009b). Moreover, berbamine is able to reverse the resistance of K562-r cells to imatinib in vitro and in vivo (Wei et al., 2009a). It is known that most CML patients have abnormally high basophil counts in their peripheral blood or bone marrow, especially in those suffering blast crises. Therefore, KU812, a human basophilic leukemia cell line established from the peripheral blood of a patient in blast crisis of CML (Ozaki et al., 1989), is a useful model for the study of the blastic crisis of CML. Here we assess the effect of berbamine on KU812 CML cell growth and explore its anti-leukemic mechanisms.
2. Materials and methods
2.1. Leukemia cell culture
Human leukemia cell line KU812 was obtained from the Cancer Institute of Zhejiang University (China). KU812 cells were maintained in RPMI-1640 medium containing 10% (v/v) fetal bovine serum (FBS; Invirogen, USA) at 37 °C and in a humidified atmosphere of 5% CO2.
2.2. Cell proliferation and viability assay
Berbamine (M W 753.80, purity 99%), purchased from the Fushun Traditional Chinese Medicine Co. Ltd., was dissolved in phosphate buffered saline (PBS) at 1 mg/ml as stock solution. KU812 cells were seeded in 96-well plates and grown for 48 h after berbamine was added at the indicated concentration (0–32 μg/ml). Methyl thiazolyl tetrazolium (MTT) assay was used to measure cell viability.
2.3. Cell cycle analysis and assessment of apoptosis
Briefly, KU812 cells were cultured with berbamine of 4 μg/ml, and collected at 0, 6, 12, and 24 h after plating, respectively. DNA histogram was performed as described previously (Liang et al., 2009). To determine apoptosis, KU812 cells were collected after treatment with berbamine at 8 μg/ml for 24 h, and then assayed by the Annexin-V-FLUOS staining kit (Immunotech, France) following the manufacturer’s instruction.
Flow cytometry (FCM) was used to determine the effect of berbamine on the cell cycle and apoptosis. Briefly, KU812 cells were treated with 4 μg/ml berbamine, collected at 0, 6, 12, and 24 h, fixed in 70% cold ethanol overnight, stained with 50 μg/ml propidium iodide (PI; Sigma, USA) for 30 min at 4 °C, and then assessed with a FACSCan flow cytometer (Becton Dickinson). G0-G1, S, and G2-M indicate the different phases of the cell cycle. To determine the apoptosis of cells, KU812 cells were treated with 8 μg/ml berbamine for 24 h and double stained with annexin V-fluorescein isothiocyanate (FITC) and PI using the Annexin-V-FLUOS staining kit (Immunotech, France). Apoptosis was then quantified by FCM.
2.4. RNA extraction and reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNAs were extracted from KU812 cells receiving berbamine (8 μg/ml) treatment for indicated time by TRIzol reagent (Invitrogen, USA), and 2 μg of RNA was reverse transcribed. The primers are listed in Table 1 and β-actin was used as internal control. The PCR products were separated by electrophoresis on 1.5% (v/v) agarose gels, stained with ethidium bromide, and visualized under UV light.
Table 1.
Primers used for RT-PCR
| Primer | Product (bp) | Sequence | Anneal temperature (°C) | Cycle |
| TβRI | 252 | Forward: 5′-CGTGCTGACATCTATGCAAT-3′ | 55 | 30 |
| Reverse: 5′-AGCTGCTCCATTGGCATAC-3′ | ||||
| TβRII | 466 | Forward: 5′-GCACGTTCAGAAGTCGGTT-3′ | 55 | 35 |
| Reverse: 5′-AGATATGGCAACTCCCAGTGGT-3′ | ||||
| Smad3 | 253 | Forward: 5′-ACCATCCCCAGGTCCCTGGATGGCC-3′ | 60 | 35 |
| Reverse: 5′-AACTCGGCCGGGATCTCTGTGTGGCGT-3′ | ||||
| p21Cip1 | 238 | Forward: 5′-GGAAGACCATGTGGACCTGT-3′ | 55 | 35 |
| Reverse: 5′-AAGATGTAGAGCGGGCCTTT-3′ | ||||
| p27Kip1 | 451 | Forward: 5′-AATAAGGAAGCGACCTGCAA-3′ | 55 | 30 |
| Reverse: 5′-CCTCCCTTCCCCAAAGTTTA-3′ | ||||
| c-Myc | 338 | Forward: 5′-TCCAGCTTGTACCTGCAGGATCTGA-3′ | 60 | 30 |
| Reverse: 5′-CCTCCAGCAGAAGGTGATCCAGACT-3′ | ||||
| Cyclin D1 | 726 | Forward: 5′-CCCTCGGTGTCCTACTTCAAA-3′ | 58 | 30 |
| Reverse: 5′-CACCTCCTCCTCCTCCTCTTC-3′ |
2.5. Western blot analysis
After treatment with 8 μg/ml of berbamine for 0, 6, 12, and 24 h, respectively, KU812 cells were collected and whole cell lysates were prepared using mammalian protein extraction reagent (PIERCE, USA). Western blot was performed as described previously (Liang et al., 2009) with antibodies against Smad3, phospho-Smad3, cyclin D1, c-Myc, Bcl-2, Bax, Bid, p21, p27, β-actin (Santa Cruz, CA), and Bcl-xL (Cell Signaling, MA).
2.6. Statistical analysis
All experiments were performed at least twice and representative data are presented. The data are expressed as mean±standard deviation (SD) of several independent experiments, and the half maximal inhibitory concentration (IC50) was calculated via the OriginPro75 software.
3. Results
3.1. Berbamine induces growth inhibition of KU812 cells
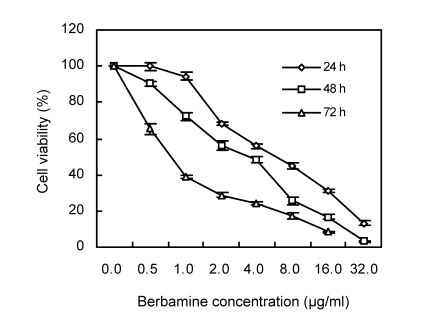
To determine the in vitro effect of berbamine on the viability of KU812 cells, both dose-response and time-response studies were performed. As shown in Fig. 1, the KU812 cell growth was inhibited by berbamine in a dose-dependent as well as time-dependent manner. For example, berbamine at a concentration of 8 μg/ml inhibited growth by (55.29±1.75)% in 24 h, (74.24±2.21)% in 48 h, and (82.78±1.54)% in 72 h, respectively. At 32 μg/ml, berbamine completely suppressed the growth of KU812 cells, and the IC50 values were 5.83, 3.43, and 0.75 μg/ml for 24, 48, and 72 h-treatments, respectively.
Fig. 1.
Effect of berbamine on the growth of KU812 cells
The percentage of cell viability was determined via MTT assay. Data represent mean (±SD) of quadruplicate cultures
3.2. Berbamine arrests cells at the G1 phase and induces apoptosis of KU812 cells
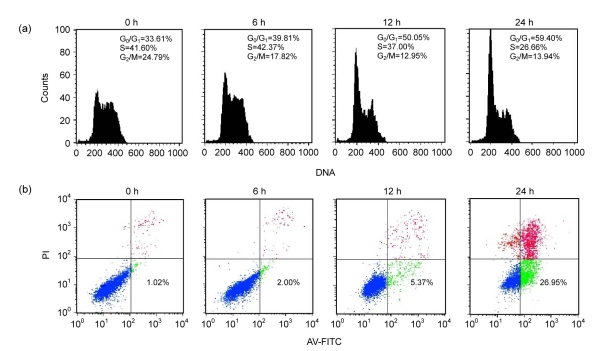
Next we tested whether growth inhibitory effect conferred by berbamine was due to induction of cell cycle arrest and/or apoptosis. FCM analysis was used to measure the percentage of different cell cycle phases 24 h after 4 μg/ml of berbamine treatment. We found that cells in G1 were markedly increased from 33.61% to 59.40%, while those in the S phase were decreased from 41.60% to 26.66% (Fig. 2a).
Fig. 2.
Cell cycle arrest and apoptotic effects of berbamine on KU812 cells
(a) Effect of berbamine on cell cycle arrest. KU812 cells were untreated or treated with 4 μg/ml berbamine for 0, 6, 12, or 24 h, after which the cells were stained with PI and analyzed for DNA content by FCM. (b) Apoptosis induction of berbamine. KU812 cells were untreated or treated with 8 μg/ml berbamine for 0, 6, 12, or 24 h. Percentages of apoptotic cells were determined by staining with annexin V and PI on FCM
The pro-apoptotic effect of berbamine on KU812 cells was analyzed by annexin V staining assay. As shown in Fig. 2b, KU812 cells with annexin V positive, which indicated apoptotic cells, were dramatically increased in a time-dependent manner when the cells were treated with 8 μg/ml berbamine for 24 h. The apoptotic cells increased from 5.37% at 12 h to 26.95% at 24 h after berbamine treatment. Together, these data suggested that berbamine induces G1 cell cycle arrest followed by apoptosis.
3.3. Berbamine mediates activation of Smad3 in KU812 cells
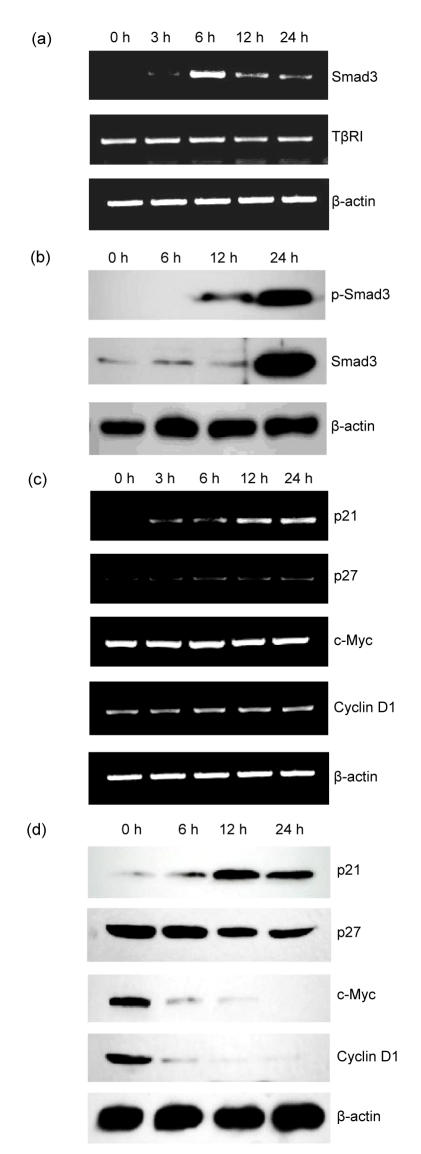
To explore the mechanism of its action on KU812 cells, we explored whether the transforming growth factor-β (TGF-β) signaling pathway was involved in KU812 cell growth inhibition and apoptosis induced by berbamine. RT-PCR was performed to detect mRNA levels of Smad3, TGF-β receptor I (TβRI), and TβRII in KU812 cells upon berbamine treatment for 0, 3, 6, 12, and 24 h, respectively. As presented in Fig. 3a, the mRNA level of Smad3 was remarkably increased after 6 h. However, no significant change on the mRNA level of TβRI was observed in KU812 cells after berbamine treatment. We also found TβRII mRNA was barely detectable in KU812 cells with/without berbamine treatment (data not shown). To further determine whether Smad3 protein was also altered by berbamine treatment, we examined the total Smad3 and phospho-Smad3 protein levels by Western blot. Total Smad3 protein remained at low levels in KU812 cells receiving 8 μg/ml of berbamine for 0, 6, and 12 h, and was distinctly increased after berbamine treatment for 24 h. Phospho-Smad3 protein was absent in untreated KU812 cells, and present after berbamine treatment for 12 h (Fig. 3b). These data indicate that berbamine induces KU812 cell apoptosis through Smad3 activation characterized by an increase in both mRNA and phosphoprotein levels.
Fig. 3.
Expressions of Smad3 and Smad3-regulated genes in KU812 cells after treatment with 8 μg/ml berbamine
(a) Effect of berbamine on transcriptions of Smad3 and TβRI genes in KU812 cells; (b) Effect of berbamine on proteins of total Smad3 and phosphor-Smad3; (c) Effect of berbamine on transcriptions of p21, p27, c-Myc, and cyclin D1 genes; (d) Effect of berbamine on proteins of p21, p27, c-Myc, and cyclin D1
3.4. Berbamine affects the expression of Smad3-regulated genes
The cell cycle regulators such as c-Myc, cyclin D1, p21 and p27 have been known to mediate Smad3-induced growth arrest (Claassen and Hann, 2000; Pardali et al., 2005). Thus, we performed semi-quantitative RT-PCR to detect mRNA levels of these genes in KU812 cells. As showed in Fig. 3c, after treatment with 8 μg/ml berbamine for 12 h, p21 mRNA was dramatically increased, whereas c-Myc, p27 and cyclin D1 mRNAs were not changed after 24-h berbamine treatment. We further evaluated the relevant protein levels by Western blot. Both c-Myc and cyclin D1 were down-regulated after berbamine treatment, and p21 protein was up-regulated and reached the highest level at 12 h, while the level of p27 protein showed no significant change (Fig. 3d).
3.5. Berbamine alters apoptosis-related proteins
Having demonstrated that berbamine induces KU812 cell apoptosis, we next evaluated its effect on protein expressions of several apoptosis mediators. Many reports have suggested that the molecular mechanism of TGF-β-Smad3-mediated apoptosis involves anti- and pro-apoptotic proteins including Bcl-2 family members. As showed in Fig. 4, the anti-apoptotic proteins Bcl-2, Bcl-xL and Bid were down-regulated, while pro-apoptotic Bax was up-regulated by berbamine treatment.
Fig. 4.
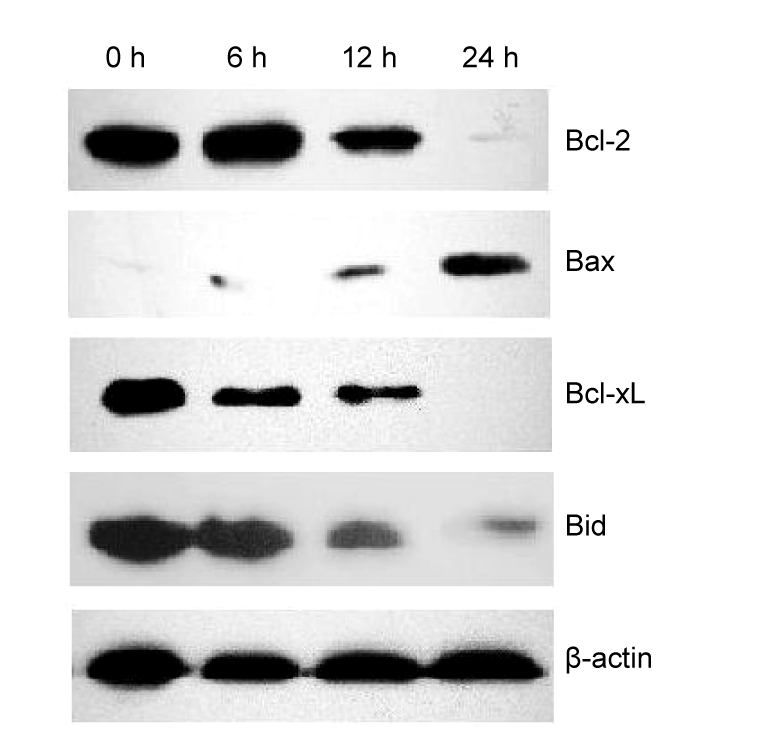
Western blot analysis of altered expressions of apoptosis-related Bcl-2 family proteins of KU812 cells after berbamine treatment
4. Discussion
TGF-β-Smad signaling pathway plays a crucial role in balancing between proliferation and differentiation activities in hematopoietic cells, and is likely the most potent endogenous negative regulator of hematopoiesis (Ruscetti et al., 2005). TGF-β ligand binds to its cell-surface serine/threonine kinase receptor TβRII, which leads to transphosphorylation of TβRI by TβRII. The activated heteromeric complex of TβRI and TβRII recruits and phosphorylates downstream Smad proteins generating an anti-proliferative signal (Elliott and Blobe, 2005; Chaudhury and Howe, 2009). TGF-β signaling is dependent on the presence of TβRI/TβRII heterodimeric receptor complex and loss of either receptor will lead to cell resistance to TGF-β-mediated growth inhibition (Zhu and Burgess, 2001). Many studies showed that the expression of TβRII is significantly low during each phase of CML comparing with the normal hematopoietic cells, which play an important role in the maintenance of the disease state (Rooke et al., 1999; Kim and Letterio, 2003). Our results show that the transcription of TβRII was hardly detected in KU812 cells and the transcription of TβRI showed no significant changes, while the mRNA level of Smad3 was significantly up-regulated and total Smad3 and phosphor-Smad3 proteins were both increased after berbamine treatment. These findings suggest that KU812 cells lacked the response to TGF-β signaling due to loss of TβRII, but berbamine could activate Smad3 through other signaling pathways.
Smad3, one of the intracellular Smad effectors, is the principal cytoplasmic intermediate involved in transmitting TGF-β signal from cell surface to the nucleus. The effects of Smad3 on hematopoiesis can be seen in that white blood-cell counts (WBCs) were significantly higher in Smad3-knockout mice than in wild-type mice. The bone marrow of Smad3-null mice was actively proliferative with granulocytosis and abnormal in hematopoietic differentiation (Zhang et al., 2003). Wolfraim et al. (2004) reported that Smad3 was detected at low level or barely detectable in some leukemia cells obtained from children patients without inactivating mutations in the Smad3 gene (MADH3), and they further showed that Smad3 worked in tandem with a loss of the cyclin-dependent kinase (CDK) inhibitor p27 to promote T-cell leukemogenesis by using a Smad3-deficient mouse model. During the blast crisis of CML, some proteins, such as EVI-1 and AML1-EVI-1, which are unexpressed in normal hematopoietic cells, block Smad3 DNA binding and recruit corepressors to abolish TGF-β signaling (Mitani, 2004). Some have suggested that while bcr-abl initiates leukemogenesis of CML, Smad3 plays a critical role during blastic transformation (Dong and Blobe, 2006).
The inhibitory mechanisms of activated Smad3 include the induction of the CDK inhibitors and suppression of the transcription regulator c-Myc. In early G1 phase, the transcription regulator c-Myc is rapidly down-regulated by a transcriptional complex of Smad3/E2F/DP-1 and the corepressor p107, which acts directly on the c-Myc promoter (Yagi et al., 2002). In later G1 phase, Smad3 can regulate activity of p21 or p27 to inhibit the progression to S phase (Kim and Letterio, 2003). In this study, we first found that Smad3 activity is involved in the berbamine-mediated G1 arrest in KU812 cells. Furthermore, our results show that c-Myc, cyclin D1 and p21, which are clearly involved in Smad3-induced G1 cell cycle arrest, were altered after berbamine treatment. The levels of c-Myc and cyclin D1 proteins were decreased, and the level of p21 protein was increased. Thus, we propose that Smad3 triggers the berbamine-mediated growth arrest through down-regulations of protein levels of c-Myc and cyclin D1 and up-regulations of both protein and mRNA levels of p21. Further work is needed to clarify the precise mechanism by which berbamine employs Smad3 protein to suppress cell development.
The mechanisms underlying Smad3-induced apoptosis in hematopoietic cells remain to be fully elucidated. Recent studies suggest that such process is associated with changes in the expressions and localizations of both anti-apoptotic and pro-apoptotic members including the Bcl-2 family (Richter and Duckett, 2000). The Bcl-2 family proteins act as regulators within the common pathway of apoptosis, consisting of anti-apoptotic (such as Bcl-2, Bid and Bcl-xL) and pro-apoptotic members (such as Bax). In this study, we also observed that the levels of the anti-apoptotic proteins, such as Bcl-2, Bid and Bcl-xL, were decreased and the level of pro-apoptotic protein Bax was increased in KU812 cells after berbamine treatment. Whether Smad3 directly affects Bcl-2 family in the berbamine-induced apoptosis is undetermined, but these results implicate a relationship between these molecules and Smad3.
In summary, this study found that berbamine suppressed the proliferation and induced apoptosis of KU812 leukemic cells by activating Smad3-mediated signaling pathway. G1 arrest and apoptosis of KU812 cells were induced through regulation of the downstream targets of Smad3, including c-Myc, cyclin D1 and p21. Further work is needed to clarify which is the upstream member of Smad3 in berbamine-related Smad3 activation, and berbamine may be promising in the development of novel anti-leukemia drugs.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30873095), and the Natural Science Foundation of Zhejiang Province, China (No. 491020-N20529)
References
- 1.An X, Tiwari AK, Sun Y, Ding PR. BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review. Leuk Res. 2010;34(10):1255–1268. doi: 10.1016/j.leukres.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhury A, Howe PH. The tale of transforming growth factor-β (TGFβ) signaling: a soigné enigma. IUBMB Life. 2009;61(10):929–939. doi: 10.1002/iub.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claassen GF, Hann SR. A role for transcriptional repression of p21CIP1 by c-Myc in overcoming transforming growth factor β-induced cell-cycle arrest. PNAS. 2000;97(17):9498–9503. doi: 10.1073/pnas.150006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong M, Blobe GC. Role of transforming growth factor-β in hematologic malignancies. Blood. 2006;107(12):4589–4596. doi: 10.1182/blood-2005-10-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Druker BJ, Guilhot F, O′Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 6.Elliott RL, Blobe GC. Role of transforming growth factor β in human cancer. J Clin Oncol. 2005;23(9):2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 7.Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr Opin Genet Dev. 2008;18(1):73–79. doi: 10.1016/j.gde.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Letterio J. Transforming growth factor-β signaling in normal and malignant hematopoiesis. Leukemia. 2003;17(9):1731–1737. doi: 10.1038/sj.leu.2403069. [DOI] [PubMed] [Google Scholar]
- 9.Liang Y, Xu RZ, Zhang L, Zhao XY. Berbamine, a novel nuclear factor κB inhibitor, inhibits growth and induces apoptosis in human myeloma cells. Acta Pharmacol Sin. 2009;30(12):1659–1665. doi: 10.1038/aps.2009.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitani K. Molecular mechanisms of leukemogenesis by AML1/EVI-1. Oncogene. 2004;23(24):4263–4269. doi: 10.1038/sj.onc.1207777. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki M, Kanemitsu N, Yasukawa M, Fujita S. Basophilic crisis of chronic myelogenous leukemia. Jpn J Med. 1989;28(1):67–71. doi: 10.2169/internalmedicine1962.28.67. [DOI] [PubMed] [Google Scholar]
- 12.Pardali K, Kowanetz M, Heldin CH, Moustakas A. Smad pathway-specific transcriptional regulation of the cell cycle inhibitor p21(WAF1/Cip1) J Cell Physiol. 2005;204(1):260–272. doi: 10.1002/jcp.20304. [DOI] [PubMed] [Google Scholar]
- 13.Richter BWM, Duckett CS. The IAP proteins: caspase inhibitors and beyond. Sci STKE. 2000;44:PE1. doi: 10.1126/stke.2000.44.pe1. [DOI] [PubMed] [Google Scholar]
- 14.Rooke HM, Vitas MR, Crosier PS, Crosier KE. The TGF-β type II receptor in chronic myeloid leukemia: analysis of microsatellite regions and gene expression. Leukemia. 1999;13(4):535–541. doi: 10.1038/sj.leu.2401384. [DOI] [PubMed] [Google Scholar]
- 15.Ruscetti FW, Akel S, Bartelmez SH. Autocrine transforming growth factor-β regulation of hematopoiesis: many outcomes that depend on the context. Oncogene. 2005;24(37):5751–5763. doi: 10.1038/sj.onc.1208921. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg M. Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia. Clin Ther. 2007;29(11):2289–2308. doi: 10.1016/j.clinthera.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Wei YL, Xu L, Liang Y, Xu XH, Zhao XY. Berbanine exhibits potent antitumor effects on imatinib-resistant CML cells in vitro and in vivo. Acta Pharmacol Sin. 2009;30(4):451–457. doi: 10.1038/aps.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei YL, Xu L, Zhao XY. Mechanism related to inhibition of leukemia K562 cells by berbamine. J Zhejiang Univ (Med Sci) 2009;38(4):387–391. (in Chinese) [PubMed] [Google Scholar]
- 19.Wolfraim LA, Fernandez TM, Mamura M, Fuller WL, Kumar R, Cole DE. Loss of Smad3 in acute T-cell lymphoblastic leukemia. N Engl J Med. 2004;351(6):552–559. doi: 10.1056/NEJMoa031197. [DOI] [PubMed] [Google Scholar]
- 20.Xu RZ, Dong QH, Yu YZ, Zhao XY, Gan XX, Wu D, Lu QH, Xu XH, Yu XF. Berbanine: a novel inhibitor of bcr/abl fusion gene with potent anti-leukemia activity. Leuk Res. 2006;30(1):17–23. doi: 10.1016/j.leukres.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 21.Yagi K, Furuhashi M, Aoki H, Goto D, Kuwano H, Sugamura K, Miyazono K, Mitsuyasu Kato M. C-Myc is a downstream target of the Smad pathway. J Biol Chem. 2002;277(1):854–861. doi: 10.1074/jbc.M104170200. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Sun Z, Shen AL, Ma L, Jiang XY, Ma GJ. The effects of the Smad3-knockout on the hematopoiesis of mouse. Chin J Biotechnol. 2003;19(4):428–432. (in Chinese) [PubMed] [Google Scholar]
- 23.Zhu HJ, Burgess AW. Regulation of transforming growth factor-β signaling. Mol Cell Biol Res Commun. 2001;4(6):321–330. doi: 10.1006/mcbr.2001.0301. [DOI] [PubMed] [Google Scholar]