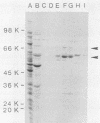
Abstract
Site-directed mutagenesis of the TGC codon in a cloned streptolysin O (SLO) gene exchanged the single Cys residue in SLO for either Ala or Ser. The parent wild-type SLO (SLO.Cys-530) and the SLO.Ala-530 and SLO.Ser-530 mutant toxins, expressed in Escherichia coli, were purified and analyzed. Wild-type SLO.Cys-530 and the SLO.Ala-530 mutant showed no significant differences in their specific hemolytic activities, while the SLO.Ser-530 mutant had a reduced (ca. 25%), but still considerable, specific hemolytic activity as compared with that of wild-type SLO. The parent and mutant toxins extracted from lysed erythrocyte membranes had similar sedimentation profiles on sucrose density gradients, suggesting that the mutations did not affect the ability of SLO to form oligomers in membranes. These results show that the widely held assumption that the in vitro cytolytic activity of SLO requires an essential Cys residue is not true.
Full text
PDF
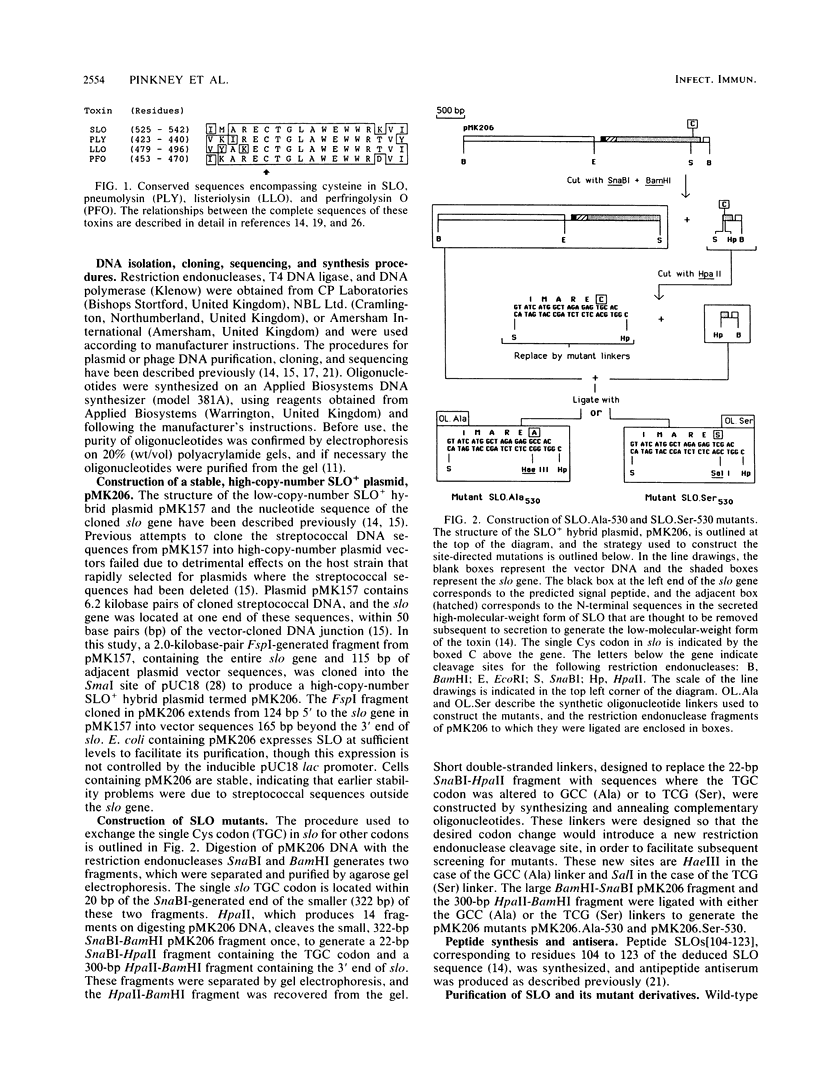




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol Ther. 1980;11(3):661–717. doi: 10.1016/0163-7258(80)90045-5. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Roth M., Sziegoleit A., Tranum-Jensen J. Isolation and identification of two hemolytic forms of streptolysin-O. Infect Immun. 1984 Nov;46(2):394–400. doi: 10.1128/iai.46.2.394-400.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Membrane damage by pore-forming bacterial cytolysins. Microb Pathog. 1986 Feb;1(1):5–14. doi: 10.1016/0882-4010(86)90027-6. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J., Sziegoleit A. Mechanism of membrane damage by streptolysin-O. Infect Immun. 1985 Jan;47(1):52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Duncan J. L., Buckingham L. Resistance to streptolysin O in mammalian cells treated with oxygenated derivatives of cholesterol. Cholesterol content of resistant cells and recovery of streptolysin O sensitivity. Biochim Biophys Acta. 1980 Dec 12;603(2):278–287. doi: 10.1016/0005-2736(80)90374-0. [DOI] [PubMed] [Google Scholar]
- Duncan J. L., Schlegel R. Effect of streptolysin O on erythrocyte membranes, liposomes, and lipid dispersions. A protein-cholesterol interaction. J Cell Biol. 1975 Oct;67(1):160–174. doi: 10.1083/jcb.67.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K., Geoffroy C., Alouf J. E. Binding of cholesterol by sulfhydryl-activated cytolysins. Infect Immun. 1980 Jan;27(1):97–101. doi: 10.1128/iai.27.1.97-101.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe M. A., Miller L., Walker J. A., Boulnois G. J. Nucleotide sequence of the streptolysin O (SLO) gene: structural homologies between SLO and other membrane-damaging, thiol-activated toxins. Infect Immun. 1987 Dec;55(12):3228–3232. doi: 10.1128/iai.55.12.3228-3232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe M., Timmis K. N. Cloning and expression in Escherichia coli of the streptolysin O determinant from Streptococcus pyogenes: characterization of the cloned streptolysin O determinant and demonstration of the absence of substantial homology with determinants of other thiol-activated toxins. Infect Immun. 1984 Mar;43(3):804–810. doi: 10.1128/iai.43.3.804-810.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leslie D., Fairweather N., Pickard D., Dougan G., Kehoe M. Phospholipase C and haemolytic activities of Clostridium perfringens alpha-toxin cloned in Escherichia coli: sequence and homology with a Bacillus cereus phospholipase C. Mol Microbiol. 1989 Mar;3(3):383–392. doi: 10.1111/j.1365-2958.1989.tb00183.x. [DOI] [PubMed] [Google Scholar]
- Mengaud J., Vicente M. F., Chenevert J., Pereira J. M., Geoffroy C., Gicquel-Sanzey B., Baquero F., Perez-Diaz J. C., Cossart P. Expression in Escherichia coli and sequence analysis of the listeriolysin O determinant of Listeria monocytogenes. Infect Immun. 1988 Apr;56(4):766–772. doi: 10.1128/iai.56.4.766-772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L., Burdett V., Poirier T. P., Gray L. D., Beachey E. H., Kehoe M. A. Conservation of protective and nonprotective epitopes in M proteins of group A streptococci. Infect Immun. 1988 Aug;56(8):2198–2204. doi: 10.1128/iai.56.8.2198-2204.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Saunders F. K., Mitchell T. J., Walker J. A., Andrew P. W., Boulnois G. J. Pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae, does not require a thiol group for in vitro activity. Infect Immun. 1989 Aug;57(8):2547–2552. doi: 10.1128/iai.57.8.2547-2552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweten R. K. Cloning and expression in Escherichia coli of the perfringolysin O (theta-toxin) gene from Clostridium perfringens and characterization of the gene product. Infect Immun. 1988 Dec;56(12):3228–3234. doi: 10.1128/iai.56.12.3228-3234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweten R. K. Nucleotide sequence of the gene for perfringolysin O (theta-toxin) from Clostridium perfringens: significant homology with the genes for streptolysin O and pneumolysin. Infect Immun. 1988 Dec;56(12):3235–3240. doi: 10.1128/iai.56.12.3235-3240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. A., Allen R. L., Falmagne P., Johnson M. K., Boulnois G. J. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect Immun. 1987 May;55(5):1184–1189. doi: 10.1128/iai.55.5.1184-1189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]